Abstract
Legumes form a mutualistic symbiosis with bacteria collectively referred to as rhizobia. The bacteria induce the formation of nodules on the roots of the appropriate host plant, and this process requires the bacterial signaling molecule Nod factor. Although the interaction is beneficial to the plant, the number of nodules is tightly regulated. The gaseous plant hormone ethylene has been shown to be involved in the regulation of nodule number. The mechanism of the ethylene inhibition on nodulation is unclear, and the position at which ethylene acts in this complex developmental process is unknown. Here, we used direct and indirect ethylene application and inhibition of ethylene biosynthesis, together with comparison of wild-type plants and an ethylene-insensitive supernodulating mutant, to assess the effect of ethylene at multiple stages of this interaction in the model legume Medicago truncatula. We show that ethylene inhibited all of the early plant responses tested, including the initiation of calcium spiking. This finding suggests that ethylene acts upstream or at the point of calcium spiking in the Nod factor signal transduction pathway, either directly or through feedback from ethylene effects on downstream events. Furthermore, ethylene appears to regulate the frequency of calcium spiking, suggesting that it can modulate both the degree and the nature of Nod factor pathway activation.
INTRODUCTION
The interaction between legumes and rhizobial bacteria results in the formation of nodules on the roots of the host plant. The nodule is a specialized symbiotic organ that provides the environmental conditions required by the bacteria to fix atmospheric nitrogen (Mylona et al., 1995). Although much is known about the bacterial genes required for the establishment of the early events in this interaction, less is known about the plant genes involved. The plant genetic components required for this symbiosis are being identified by analysis of the model legumes Lotus japonicus and Medicago truncatula. In this study, we focus on M. truncatula, host for the bacterial symbiont Sinorhizobium meliloti (Cook, 1999).
The first macroscopic event in M. truncatula after inoculation with S. meliloti is the deformation of root hairs. The majority of root hairs show contorted growth and branching. A few root hairs form a “shepherd's crook,” whereby the tip of the root hair curls upon itself, enclosing a colony of S. meliloti in the center (Kijne, 1992). From this point is initiated a plant-derived infection thread that allows the invasion of the bacteria into the cortex of the root. The inner cortical cells of the root immediately below the infection thread divide, eventually forming a nodule primordium (Mylona et al., 1995). The infection thread allows the invasion of bacteria from the outside of the root into the nodule primordium and ultimately the release of bacteria into membrane-enclosed compartments within the cells of the nodule.
Signaling between the plant and the bacteria is critical for the establishment of the symbiosis (Long and Staskawicz, 1993). The first stages of this interaction involve plant signals, predominantly flavonoids, that induce the production of lipochitooligosaccharide molecules, termed Nod factors, in the bacteria through the inducible expression of the nod genes. The interaction between the plant and the bacteria involves a high degree of specificity, with much of this specificity being encoded in the structure of the Nod factor (Long, 1996). Isolated Nod factor on the appropriate plant host can induce many of the responses observed during the infection of the plant by the bacteria.
The earliest responses to Nod factor observed in the epidermal root cells of alfalfa are depolarization of and ion flux across the plasma membrane (Ehrhardt et al., 1992; Felle et al., 1998, 1999a, 1999b; Cardenas et al., 2000; Kurkdjian et al., 2000). These events occur 1 to 3 min after treatment with Nod factor. After these responses, with a delay of 3 to 30 min, is the induction of calcium spiking (Ehrhardt et al., 1996). Nod factors also activate the expression of genes such as RIP1 (Cook et al., 1995) and ENOD11 (Gamas et al., 1996; Journet et al., 2001) in the epidermis of the root within a few hours of treatment. Although none of these responses have been shown to be involved directly in the activation of nodule development, all are induced by Nod factor with the same structural specificity required for other nodulation responses.
The gaseous plant hormone ethylene has many effects on plant development and has been shown to act as a secondary signal in the induction of plant defenses (Ecker, 1995). Application of ethylene during the interaction between some legumes and their rhizobial symbionts inhibits the number of nodules that form (Peters and Crist Estes, 1989; Lee and LaRue, 1992b). It has been proposed that the inhibition of nodule formation could be a result of developmental effects that ethylene has on the plant. For example, ethylene is required for the elongation of root hairs (Tanimoto et al., 1995; Pitts et al., 1998), a function clearly involved in the invasion of rhizobia, but simultaneously inhibits cell division (Goodlass and Smith, 1979), a function that would be detrimental to nodule formation. Recently, however, it was shown that the induction of root hair growth by Nod factor occurs independently of ethylene (Heidstra et al., 1997). Furthermore, Heidstra et al. (1997) showed that the ethylene biosynthetic enzyme 1-aminocyclopropane-1-carboxylic acid (ACC) oxidase is activated by Nod factor in the root cortex in a pattern that suggests a possible role for ethylene in defining the position at which nodule primordia are initiated.
Ethylene appears to regulate many aspects of the interaction between the plant and the bacteria: it regulates the formation of the infection thread (Penmetsa and Cook, 1997), inhibits the maintenance of calcium spiking after induction by Nod factor (D.W. Ehrhardt, K.E. Wilson, J.A. Downie, and S.R. Long, unpublished data), and regulates the maintenance of the nodule meristem in Sesbania rostrata that distinguishes an indeterminate from a determinate nodule (Fernandez-Lopez et al., 1998). The mechanism of ethylene inhibition and the position in the pathway leading to nodule formation where ethylene functions is unknown. Ethylene could regulate the placement of the nodule primordium in the root by inhibiting cell division; it also could have a direct effect on the Nod factor perception pathway that defines the nodule number.
Genetic studies in M. truncatula have been initiated to identify genes involved in this symbiosis. sickle (skl), a mutant of M. truncatula, shows a supernodulation phenotype, with a 10-fold increase in the number of nodules that form relative to wild-type plants (Penmetsa and Cook, 1997). Further study of this mutant revealed that skl shows phenotypes characteristic of ethylene-insensitive mutants of Arabidopsis. The supernodulation phenotype cosegregates with the ethylene-insensitive phenotypes (Penmetsa and Cook, 1997). The identification of skl provided genetic evidence for ethylene's involvement in the negative regulation of nodulation.
Experimental manipulation of ethylene can be accomplished by direct application of the hormone, by supply of biosynthetic precursors, by the application of biosynthesis or perception inhibitors, and by exploitation of plant mutants that affect ethylene physiology. Ethylene is synthesized from ACC by the activity of an abundant enzyme, ACC oxidase, and it is thought that the conversion of ACC to ethylene is a very rapid step (Yang and Hoffman, 1984; Theologis, 1992). Application of ACC to the plant leads to an increase in ethylene production (Cameron et al., 1979; Lurssen et al., 1979). The generation of ACC by ACC synthase has been shown to be the rate-limiting step in the ethylene biosynthetic pathway and is found to be a target in many cases of ethylene regulation (Yang and Hoffman, 1984; Theologis, 1992). ACC synthase is inhibited by l-α-(2-aminoethoxyvinyl)-glycine (AVG) and α-aminoisobutyric acid, which block ethylene production in the plant (Amrhein and Wenker, 1979).
In this work, we used ACC, AVG, ethylene, and the ethylene-insensitive mutant skl to study the effects of varying ethylene levels on nodulation responses in M. truncatula. We show that ethylene can inhibit many aspects of the plant's response to S. meliloti and Nod factor. The earliest event assayed that was inhibited by ethylene was calcium spiking. Ethylene appears to establish the threshold concentration of Nod factor that is required to activate the calcium-spiking response. We propose that ethylene inhibits nodulation by modulating a component of the Nod factor signal transduction pathway at or upstream of calcium spiking.
RESULTS
Ethylene Regulates Nodule Number and Infection Frequency
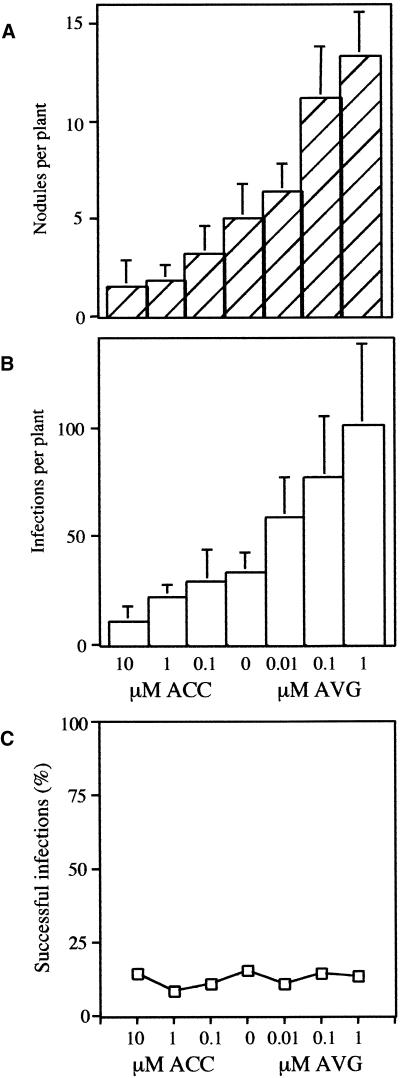
To assess the effect of ethylene on nodulation and infection, we grew M. truncatula on 10, 1, 0.1, and 0 μM ACC and 1, 0.1, and 0.01 μM AVG. We assume that the ACC and AVG treatments provide a range of ethylene levels, with plants being exposed to the highest ethylene levels at 10 μM ACC and the lowest ethylene levels at 1 μM AVG. The number of nodules per plant was assessed 14 days after inoculation with S. meliloti strain Rm1021 (pXLGD4). The average number of nodules gradually increased as ethylene levels decreased (Figure 1A), consistent with a role for ethylene as a negative regulator of nodulation. Rm1021 (pXLGD4) constitutively expressed lacZ from a hemA promoter, allowing the bacteria to be stained in planta, which aids in the visualization of infection threads. Four days after inoculation, roots were removed from plants and stained for lacZ activity. The number of infection events was counted. Included in this analysis were shepherd's crooks with a blue spot of bacteria in the center but no infection threads visible. Figure 1B shows that infection events also increased with decreasing levels of ethylene. This finding suggests that ethylene inhibits the initiation of infection threads and therefore the ethylene block must be upstream or at the point of infection thread formation.
Figure 1.
Ethylene Regulates the Number of Nodules and Infection Events Induced by S. meliloti.
Plants were grown on differing concentrations of ACC or AVG and inoculated with Rm1021 (pXLDG4). It is assumed that ethylene levels are highest in plants grown on 10 μM ACC and lowest in plants grown on 1 μM AVG.
To ensure that the AVG effect on nodulation was a function of its inhibition of ACC synthase, we treated plants with 0.1 μM AVG and 10 μM ACC and compared the number of nodules generated with the number found in plants grown with either 0.1 μM AVG or 10 μM ACC. Plants grown in the presence of both AVG and ACC showed nodulation equivalent to plants grown on ACC alone (data not shown), indicating that the addition of ACC can overcome AVG effects. Therefore, the AVG effects on nodulation are ethylene specific. skl plants showed a 30.5-fold increase in the number of nodules and a 21.7-fold increase in the number of infection events compared with wild-type plants grown in the absence of ACC or AVG (data not shown), further implying a role for ethylene in inhibiting the number of infections initiated.
The ratio of nodule number to infection events for each AVG or ACC concentration did not change significantly (Figure 1C). This finding suggests that, under these experimental conditions, the regulation of nodule number is a result of ethylene inhibition at or before the initiation of infections, as opposed to inhibition upon the expansion of infection threads. If nodule number were additionally regulated at the level of infection thread expansion, we would expect to see an increase in the ratio of nodule number to infection events as ethylene levels decrease.
skl Shows an Increase in the Root Hair Deformation Response
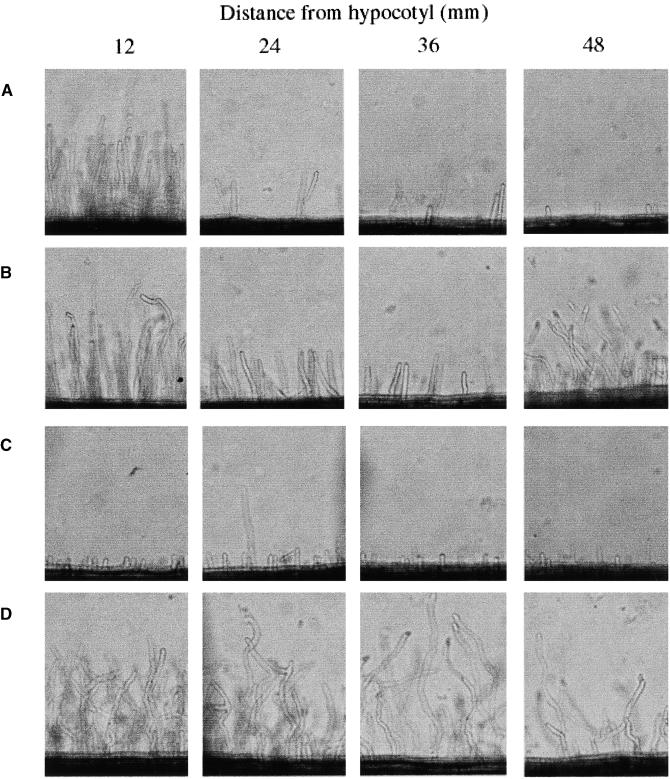
Because the ethylene block appeared to be upstream of infection thread formation, we analyzed the effect of ethylene on root hair deformation, the morphological response that precedes infection thread formation. The degree and position of root hair deformation were compared for wild-type and skl plants. In this assay, roots were sandwiched between thin slabs of solid medium containing either 0.1 μM AVG or 0.1 μM AVG and 10 pM Nod factor. The roots were allowed to grow through the medium slabs for up to 8 days. Root hairs were assessed for deformation at 12 mm intervals from the hypocotyl/root junction, as defined by the initial point of root hair emergence. The root hairs 12 mm below this point were initiated after the plants were transferred to the agar slabs. If AVG was not present in this assay, the roots grew out of the agar slabs. Hence, we were unable to assess the effect of either the absence of AVG or the presence of ACC in this root hair deformation assay. We believe this problem of root growth is associated with ethylene accumulation, because skl roots continued to grow between the agar slabs when AVG was not present (data not shown).
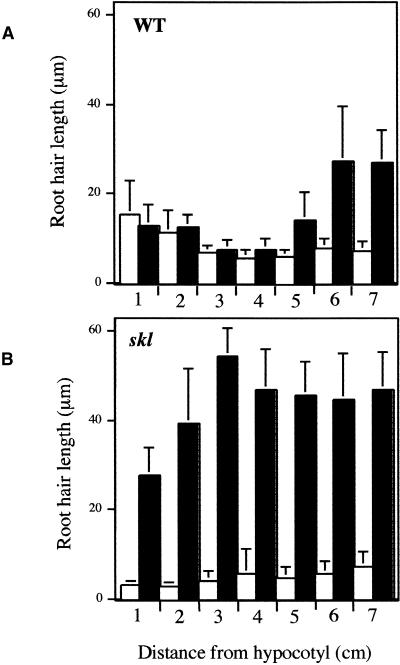
In the absence of Nod factor, root hair growth and root hair number gradually decreased (Figures 2A and 3A), which could be a function of AVG inhibition, since ethylene is required for the elongation of root hairs (Tanimoto et al., 1995; Pitts et al., 1998). In the presence of Nod factor, root hair deformation was visible starting 48 mm below the hypocotyl/root junction (Figure 2B). This response was characterized by an increase in root hair growth, an uneven pattern of polar growth, and root hair branching. To quantify this response, the length of the root hairs was measured at 1-cm intervals down the root from the hypocotyl/root junction and compared between plants treated with AVG and plants treated with both AVG and Nod factor. Root hair length was unchanged in response to Nod factor to 5 cm down the root (Figure 3A). At this point, there was a clear difference in length between untreated root hairs and those treated with Nod factor. The position at which root hair length increased in response to Nod factor was consistent with the position on the root at which root hair branching occurred.
Figure 2.
Root Hair Deformation in Response to Nod Factor Reveals a Stronger Response in skl Relative to Wild-Type Plants.
Roots were allowed to grow between two thin slabs of medium containing AVG and in some cases Nod factor for 4 days. The root hairs were imaged at set distances along the root from the hypocotyl/root junction.
(A) Wild type, 0.1 μM AVG.
(B) Wild type, 0.1 μM AVG plus 10 pM Nod factor.
(C) skl, 0.1 μM AVG.
(D) skl, 0.1 μM AVG plus 10 pM Nod factor.
Figure 3.
The Root Hair Response to Nod Factor Is Regulated by Ethylene.
The length of the root hairs was assayed at set distances on the root from the hypocotyl/root junction.
(A) Wild type (WT).
(B) skl.
Open bars represent plants treated with 0.1 μM AVG; closed bars represent plants treated with 0.1 μM AVG and 10 pM Nod factor. Data points represent the average root hair length at each distance along the root for three plants per treatment. Error bars show ±sd.
Unlike wild-type plants, skl showed immediate root hair deformation in response to Nod factor (Figures 2C, 2D, and 3B). Furthermore, the degree of root hair growth in response to Nod factor was much more dramatic in skl relative to wild-type plants (Figure 3B). In these experiments, we assume that the increased response in skl reflects the fact that skl was at a lower perceived ethylene status than wild-type plants grown in 0.1 μM AVG. We could not duplicate the skl phenotype in wild-type plants by increasing the amount of AVG, because AVG concentrations greater than 1 μM resulted in inhibition of root growth. These results imply that ethylene regulates the degree and possibly the position of root hair deformation in response to Nod factor.
In this assay, there was a delay before deformation was observed in wild-type plants. The roots grew approximately 1.5 cm/day. Therefore, the 5-cm region of the wild-type root at which no root hair deformation was observed relates to ∼3 days of growth in the presence of Nod factor. This is a surprising result, because in Nod factor flood assays for root hair deformation there is a root hair response that is visible within a few hours (Heidstra et al., 1994; de Ruijter et al., 1998). The 3-day block in root hair deformation likely is dependent on ethylene because skl plants showed an immediate response. The reason for the differing results in the assay used in this study and in Nod factor flood assays may be an amplification of the local levels of ethylene caused by an inhibition of gas flow in the agar. At 3 days, the levels of ethylene or potentially an additional molecule must change in such a way as to allow root hair deformation. This could reflect the mechanisms of AVG inhibition or a change in some aspect of the metabolic status of the plant.
Ethylene Inhibits the Inducible Expression of RIP1 and ENOD11
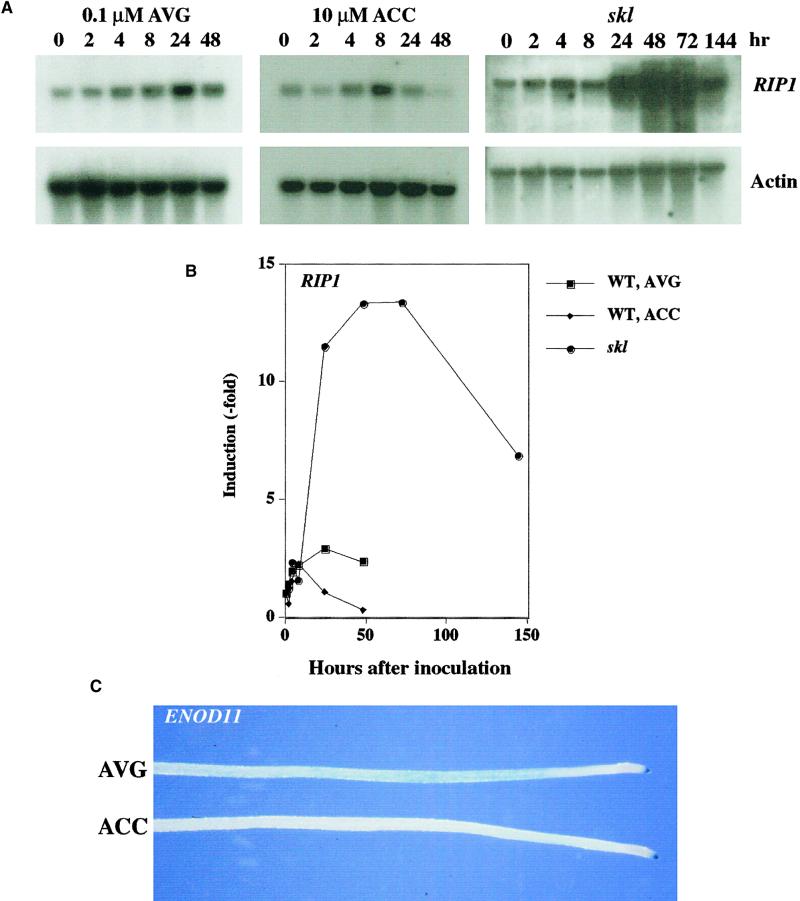
A number of genes have been identified in M. truncatula that are activated by Nod factor and S. meliloti. Two of the earliest genes to be induced are RIP1 and ENOD11 (Cook et al., 1995; Journet et al., 2001). These genes show increased expression in the epidermis of the root within ∼3 hr after inoculation with bacteria (Cook et al., 1995; Gamas et al., 1996; Journet et al., 2001). To assess the effect of ethylene on RIP1 expression, we grew wild-type plants on 0.1 μM AVG or 10 μM ACC and skl plants in the absence of either AVG or ACC. The expression of RIP1 was assessed by RNA gel blot hybridization using samples collected at times after S. meliloti inoculation. In plants grown on AVG, RIP1 was induced at 4 hr after inoculation, and expression increased up to 24 hr after inoculation and then decreased slightly at 48 hr (Figures 4A and 4B). This expression pattern is consistent with previously published work (Cook et al., 1995).
Figure 4.
Ethylene Modulates Gene Expression Activated by S. meliloti or Nod Factor.
(A) Wild-type plants were grown on 0.1 μM AVG or 10 μM ACC, and skl plants were grown in the absence of either AVG or ACC and inoculated with S. meliloti Rm1021 (pXLGD4) at OD600 = 0.1. Root tissue was collected at set times after inoculation (hr). RNA gel blots were probed with a region of exon 2 of RIP1 and secondarily with actin to assess loading.
(B) The expression levels of RIP1 in (A) were standardized using the actin loading control and quantified as the fold induction of the levels before inoculation with S. meliloti for each treatment. WT, wild type.
(C) M. truncatula plants, stably transformed with the ENOD11 promoter driving the expression of GUS, were treated with either 0.1 μM AVG or 10 μM ACC and secondarily treated with 1 nM Nod factor for 6 hr. The photograph shows representative roots from each treatment. Note the nonsymbiotic root cap expression of ENOD11-GUS present in both treatments. The inducible expression of ENOD11-GUS in the epidermis of the root, behind the root tip, is present only in plants treated with AVG.
Plants grown on 10 μM ACC showed a similar induction pattern up to 8 hr after inoculation. However, at 24 hr, the expression of RIP1 decreased, and by 48 hr after inoculation, the expression of RIP1 decreased to uninoculated levels (Figures 4A and 4B). skl showed normal induction patterns for the first 8 hr, but by 24 hr, the expression of RIP1 was much higher than in wild-type plants grown on AVG. Furthermore, skl showed a longer period of maximal expression: RIP1 in skl reached a maximum at 48 hr after inoculation and was still at the maximum expression level at 72 hr after inoculation (Figures 4A and 4B). These results demonstrate that ethylene can regulate the level and timing of RIP1 expression.
To analyze the expression of ENOD11, we used M. truncatula plants stably transformed with β-glucuronidase (GUS) transcriptionally regulated by the ENOD11 promoter (Journet et al., 2001). Plants were grown on medium containing 0.1 μM AVG for 5 days and then transferred to liquid medium containing 0.1 μM AVG, 10 μM ACC, or medium alone. After 24 hr in liquid medium, the plants were treated with 1 nM Nod factor for 6 hr. The roots were tested for GUS activity. In the absence of Nod factor, only the root cap showed GUS activity, indicated by blue staining, in plants treated with both ACC and AVG (data not shown). This is consistent with previous work that showed nonsymbiotic root cap expression of ENOD11 (Journet et al., 2001). The induction of ENOD11 by Nod factor in the epidermis of the root was observed in plants treated with AVG but not in plants treated with ACC (Figure 4C). In both cases, nonsymbiotic root cap expression was observed. ENOD11 epidermal expression in plants treated with Nod factor alone was similar but reduced slightly in comparison with that in plants treated with Nod factor and 0.1 μM AVG (data not shown). However, studies in Vicia sativa have shown that plants whose ethylene status is not controlled externally display highly variable responses to Nod factor (D.W. Ehrhardt, K.E. Wilson, J.A. Downie, and S.R. Long, unpublished data); therefore, ENOD11 expression in the absence of external ethylene regulation also may be variable. The results with RIP1 and ENOD11 suggest that ethylene can negatively regulate early gene expression induced by Nod factor.
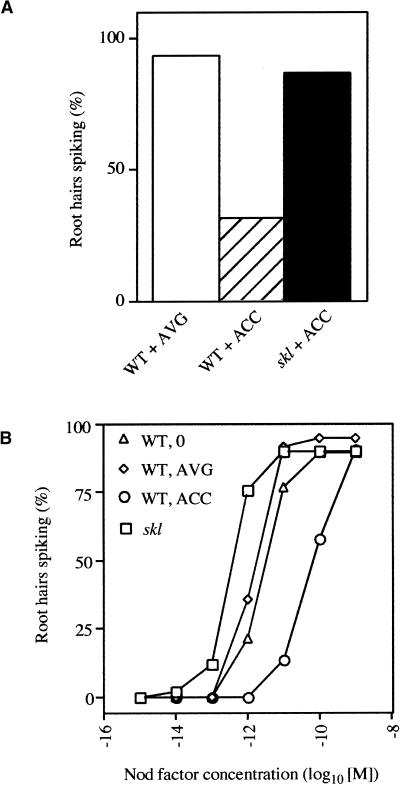
Ethylene Regulates the Threshold Concentration of Nod Factor Required for the Induction of Calcium Spiking in Root Hair Cells
Changes in gene expression occur within hours after treatment with Nod factor. We were interested in whether the effects of ethylene could be detected before gene expression. Calcium spiking is one of the earliest plant responses and is induced with a lag of 3 to 30 min after the application of Nod factor (Ehrhardt et al., 1996). To determine the effect of ethylene on the initiation of calcium spiking, we grew wild-type seedlings on either 0.1 μM AVG or 10 μM ACC and analyzed the percentage of root hair cells that showed calcium spiking in response to 10 pM Nod factor. A total of 93% of root hair cells showed calcium spiking in plants grown on 0.1 μM AVG. In contrast, only 31% of root hair cells showed calcium spiking in plants treated with 10 μM ACC (Figure 5A, Table 1). To ensure that the inhibition of calcium spiking by ACC was a precursor to ethylene production, we tested the effect of ACC on the initiation of calcium spiking in skl plants. skl grown on 10 μM ACC initiated calcium spiking in 86.9% of root hair cells when treated with 10 pM Nod factor (Figure 5A). This finding shows that ACC has an effect on calcium spiking only as a precursor of ethylene production, because if there were additional ethylene-independent effects of ACC on the plants, these should be active in skl.
Figure 5.
Ethylene Regulates the Number of Root Hairs Activated for Calcium Spiking in Response to Nod Factor.
(A) Wild-type plants (WT) were grown on either 0.1 μM AVG or 10 μM ACC, and skl plants were grown on 10 μM ACC. Data points represent the percentage of root hairs that were induced for calcium spiking 60 min after the addition of 10 pM Nod factor.
(B) Wild-type plants (WT) were grown either on medium alone or on 0.1 μM AVG or 10 μM ACC, and skl plants were grown in the absence of either AVG or ACC. The concentration of Nod factor was increased 10-fold every 30 min, starting at 1 fM and ending at 1 nM. The number of root hairs induced for calcium spiking at each concentration was assessed.
Table 1.
Number of Plants and Cells Analyzed in the Calcium Spiking Experiments
| Figure | Genotype | ACC/AVG Concentration |
Number of Plants Analyzed |
Number of Cells Analyzed |
|---|---|---|---|---|
| 5A | WTa | 0.1 μM AVG | 9 | 58 |
| WT | 10 μM ACC | 8 | 58 | |
| skl | 10 μM ACC | 8 | 61 | |
| 5B | WT | 0 | 5 | 50 |
| WT | 0.1 μM AVG | 5 | 56 | |
| WT | 10 μM ACC | 4 | 52 | |
| skl | 0 | 5 | 49 | |
| 7 | WT | 0.1 μM AVG | 4 | 14 |
| skl | 0.1 μM AVG | 5 | 13 |
a WT, wild type.
From this experiment, we could not determine whether ethylene acts as an absolute inhibitor of calcium spiking or defines the sensitivity of the plant to Nod factor. In an attempt to differentiate between these two possibilities, we assessed whether increasing the concentration of Nod factor could overcome the ethylene block on calcium spiking. In these experiments, root hair cells were analyzed for their response to a range of Nod factor concentrations. Plants were exposed to increasing concentrations of Nod factor starting at 1 fM and increasing in 10-fold increments every 30 min up to a maximum concentration of 1 nM. The number of root hairs that showed calcium spiking was tallied for each Nod factor dose (Figure 5B, Table 1). The Nod factor concentration at which 50% of the root hairs initiate calcium spiking was ∼4.5 × 10−12 M for wild-type plants grown in the absence of AVG or ACC, ∼2 × 10−12 M for wild-type plants grown on 0.1 μM AVG, and ∼8 × 10−11 M for wild-type plants grown on 10 μM ACC. The threshold concentration for skl plants grown in the absence of either AVG or ACC was 3 × 10−13 M (Figure 5B). These findings suggests that the block on calcium spiking by ACC is not total and can be overcome with high enough concentrations of Nod factor. Furthermore, ethylene regulated the sensitivity of the plant to Nod factor and defined the threshold concentration of Nod factor that is required for a response.
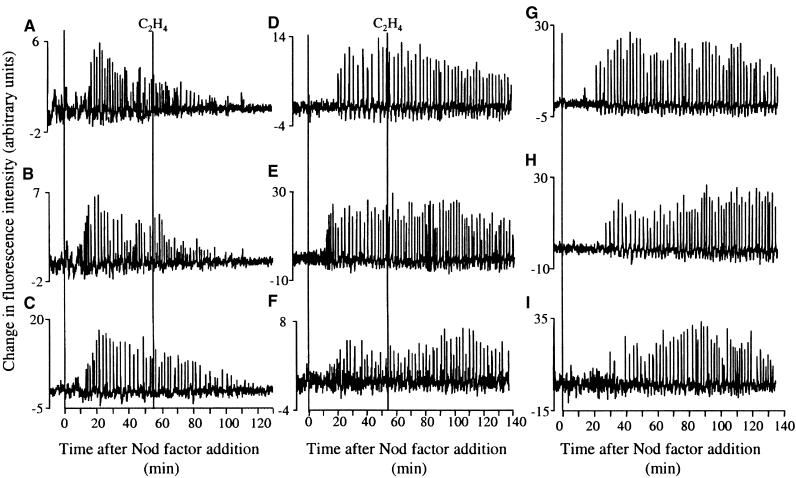
Ethylene Inhibits the Maintenance of Calcium Spiking
We have demonstrated that ethylene regulates the initiation of calcium spiking. It also has been shown that ethylene inhibits calcium spiking after its initiation in V. sativa (vetch), completely blocking calcium spiking within 30 min after application, with the first effects visible within 15 min (D.W. Ehrhardt, K.E. Wilson, J.A. Downie, and S.R. Long, unpublished data). To determine whether this is also true for M. truncatula, we treated wild-type and skl plants with 10 pM Nod factor and then added medium containing 10 pM Nod factor and ∼0.4 or ∼0.07% ethylene or 100 μM ACC 30 min after the initiation of calcium spiking. Seventeen of 20 cells on five wild-type plants showed a complete inhibition of calcium spiking, with a lag of 57.1 ± 14.5 min after the addition of 0.4% ethylene (Figures 6A to 6C). A concentration of 0.07% ethylene was insufficient to inhibit calcium spiking (data not shown). Three wild-type plants were tested with 100 μM ACC: 14 of 17 cells showed an inhibition of calcium spiking with a lag of 58.96 ± 18.36 min after ACC addition (data not shown).
Figure 6.
Ethylene Can Block the Maintenance of Calcium Spiking.
The traces show the change in calcium levels of individual cells on the same wild-type or skl plant. Calcium spiking was activated using 10 pM Nod factor and 0.4% ethylene added ∼30 min after the initiation of spiking.
(A) to (C) Wild type.
(D) to (F) skl.
(G) to (I) Wild type, no ethylene.
The y axis represents the change in fluorescence after treatment of the raw fluorescence data with the equation Y = X(n+1) − X(n). This transformation reduces the level of background fluctuations and amplifies rapid changes in calcium levels, thus aiding in the identification of calcium spikes.
Cells undergoing spiking on skl plants were unaffected by the addition of either ethylene (Figures 6D to 6F) or ACC: 12 of 12 cells on three skl plants showed continued calcium spiking after treatment with 0.4% ethylene, and eight of nine cells on two skl plants showed continued calcium spiking after treatment with 100 μM ACC (data not shown). Wild-type plants that were allowed to undergo calcium spiking for 2 hr, the approximate length of these experiments, with only a continuous supply of 10 pM Nod factor did not show calcium spiking inhibition (Figures 6G to 6I): 20 of 21 cells on four plants were still spiking 2 hr after initiation. The ∼30 minute difference in the timing before inhibition between the experiments undertaken with ethylene in V. sativa (D.W. Ehrhardt, J.A. Downie, S.R. Long, and K.E. Wilson, unpublished data) and M. truncatula could be a function of either slight differences in the experimental designs or the different plants studied.
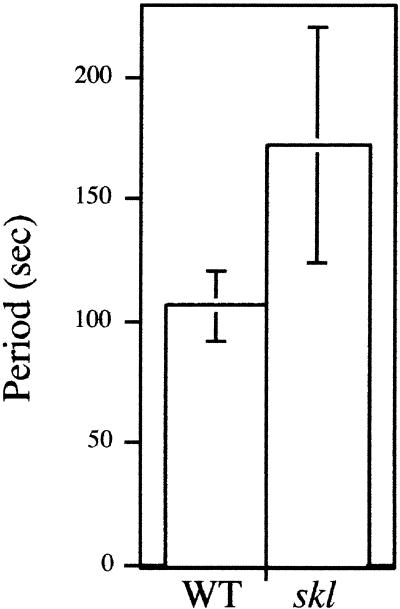
skl Shows a Decreased Frequency of Calcium Spiking
In a number of animal systems in which ligand-induced calcium spiking occurs, the frequency of spiking is modulated by the concentration of the signaling molecule (Fewtrell, 1993). It has been suggested, and in a few cases demonstrated, that the frequency of calcium spiking encodes information affecting downstream responses such as gene expression (Dolmetsch et al., 1997, 1998; Li et al., 1998). In legumes, the spiking frequency does not appear to be affected by the concentration of Nod factor (D.W. Ehrhardt, K.E. Wilson, J.A. Downie, and S.R. Long, unpublished data). Nod factor–induced calcium spiking is typified by an average period between spikes of 106 ± 14 sec. skl appears to have a consistently reduced frequency and therefore a longer period between spikes relative to wild-type plants. To calculate the average period between calcium spikes, the initial 30 min of calcium spiking were examined from wild-type and skl plants grown overnight on 0.1 μM AVG. skl showed an average period of 172 ± 48 sec, which represented a 1.62-fold increase in the period between spikes compared with wild type (Figure 7, Table 1). This finding suggests that ethylene not only affects the activation of calcium spiking but also may modulate aspects of the calcium spiking itself.
Figure 7.
skl Shows an Increased Period between Calcium Spikes Compared with Wild-Type (WT) Plants.
For each cell, the average period was calculated from 30 min of spiking induced by 10 pM Nod factor. The data points represent the average of the average period from multiple cells ±sd.
DISCUSSION
By modulating ethylene levels in the plant and comparing the behavior of wild-type plants with an ethylene-insensitive mutant, we have shown that ethylene has an inhibitory effect on nodule formation, infection thread initiation, root hair deformation, early gene expression, and calcium spiking in the plant's response to S. meliloti or Nod factor. The inhibition of calcium spiking demonstrates that ethylene regulates a component of the Nod factor signaling pathway at or upstream of calcium spiking. Nod factor is required to initiate many of the plant's responses to S. meliloti (Ardourel et al., 1994; Relic et al., 1994; Kozik et al., 1995; Denarie et al., 1996; Walker and Downie, 2000). Therefore, this single early point of inhibition could explain the multiple ethylene-regulated effects in the developmental pathway leading to nodule formation. An alternative explanation, however, is that ethylene's effects on multiple steps in the nodulation process arise because multiple components in the nodulation pathway are inhibited directly and independently by ethylene. Ethylene appears to define the sensitivity of the plant to Nod factor. Interestingly, ethylene has a similar effect on the germination of Arabidopsis seed in response to abscisic acid, with an ethylene-insensitive mutant showing increased abscisic acid sensitivity and an ethylene constitutive response mutant showing decreased abscisic acid sensitivity (Beaudoin et al., 2000).
Ethylene production in legumes is induced by infection with symbiotic rhizobia and has been proposed to be involved in the nitrate regulation of nodulation (Ligero et al., 1986, 1991; Herrera et al., 1987; Lee and LaRue, 1992b; Suganuma et al., 1995; van Spronsen et al., 1995). Therefore, ethylene may act as a secondary signal regulating nodulation in response to the nitrogen status of the plant and as a negative feedback regulator of rhizobial infection. By inhibiting both the early and late developmental stages of symbiosis, ethylene can act as a dynamic inhibitor, blocking the initiation as well as the maintenance of individual infection events.
In this work, we show that ethylene can block infection thread initiation. It also has been shown to block infection thread growth after initiation (Penmetsa and Cook, 1997). Here, we found that the ethylene effect on nodule number could be accounted for without invoking a specific ethylene regulation of infection thread growth. However, we do not believe that this experimental design disproves the possibility that ethylene inhibition of infection thread growth could function in the regulation of nodulation. In our experiments, the ethylene levels of the plants were constant. In situations in which ethylene levels fluctuate, later points of ethylene regulation, such as infection thread growth, may play an important role. The activation of ethylene production by infection with S. meliloti may regulate the number of successful infection events by inhibiting further initiation of infections and blocking the extension of existing infection threads. Indeed, in the wild-type plant, the majority of infection threads do not lead to infected nodules (Penmetsa and Cook, 1997), and this may be a function of ethylene inhibition.
In a number of animal systems that show ligand-induced calcium spiking, the frequency of calcium spikes increases as the concentration of the signaling ligand increases (Woods et al., 1986; Fewtrell, 1993). An effect on spiking frequency is of particular interest, because in animal systems the frequency of calcium spikes has been shown to affect gene expression both qualitatively and quantitatively (Dolmetsch et al., 1997, 1998; Li et al., 1998). Faster frequencies up to a maximum level increase the level of gene expression (Dolmetsch et al., 1998; Li et al., 1998).
In legume root hairs, calcium spiking frequency appears to be independent of Nod factor concentration (D.W. Ehrhardt, K.E. Wilson, J.A. Downie, and S.R. Long, unpublished data). Our observations in skl suggest either that the skl mutation has pleiotropic effects on spiking frequency independent of its ethylene effects or that ethylene has the capacity to modulate the frequency of calcium spikes. If the latter is the case, then an interesting comparison would involve how skl affects calcium spiking frequency and gene expression. We found a decrease in calcium spiking frequency but an increase in RIP1 expression in skl compared with wild-type plants. This finding appears to be inconsistent with the calcium spiking frequency/gene expression relationships documented in animal systems. However, because the role of calcium spiking in plants is unknown, there are no easy predictions for the effect of calcium spiking frequency on downstream events. For example, it is feasible that lower frequency spiking results in lower expression of negative regulators of RIP1. Furthermore, it is difficult to determine the combinatorial effects of ethylene on calcium spiking frequency and on the quantity of cells activated for calcium spiking. Studies of gene expression and calcium spiking frequency may best be completed at the single cell level.
The relationship between calcium spiking and gene expression can be further explored by the common influence of ethylene on both. From our studies in wild-type plants, it appears that the ethylene status defines the threshold of Nod factor concentration required to activate calcium spiking. Furthermore, the Nod factor–inducible expression of RIP1 and ENOD11 was regulated by ethylene. Interestingly, the ethylene status of the plant appears to regulate not only the level of RIP1 expression but also the maintenance of the expression. Based on the link between gene expression and calcium spiking in animal systems (Dolmetsch et al., 1998; Li et al., 1998), it is tempting to speculate that the effects on gene expression are a direct result of the inhibition of calcium spiking by ethylene. However, because we have not yet defined the role of calcium spiking in nodulation or the exact point in the pathway at which ethylene acts, we cannot distinguish between the possibility that ethylene acts independently on calcium spiking and gene expression and the possibility that calcium spiking is causal.
Recent work has shown that mutants blocked for most Nod factor responses were also blocked for calcium spiking, suggesting a role for calcium spiking in the Nod factor signal transduction pathway (Wais et al., 2000; Walker et al., 2000). However, the events downstream of calcium spiking have yet to be characterized. In this study, we show that ethylene can inhibit the Nod factor induction of calcium spiking, gene expression, and root hair deformation. The correlations between the inhibition of calcium spiking and downstream events provides further support for a role of calcium spiking in Nod factor signal transduction.
Because the identities of the molecular components involved in Nod factor signal transduction are unknown, it is difficult to propose how ethylene may regulate this pathway. It is possible that ethylene acts on the calcium pumps or channels that are involved directly in the calcium spiking behavior (Figure 8). Alternatively, ethylene may inhibit a component of the signal transduction pathway upstream of calcium spiking (Figure 8). According to this hypothesis, the levels of ethylene and Nod factor would act in concert to define the degree to which the signaling pathway is activated and ultimately the downstream outcome. At high concentrations of ethylene and low concentrations of Nod factor, the signaling pathway would be blocked completely. If the ethylene levels were decreased or the Nod factor levels increased, the signaling pathway would be activated, but the level of the activation may be dependent on the ratio between these two components. A more complex possibility is that early Nod factor perception is subject to feedback from downstream nodulation behavior, and the effects of ethylene on this downstream behavior thus have an indirect effect on the earlier stages of the pathway.
Figure 8.
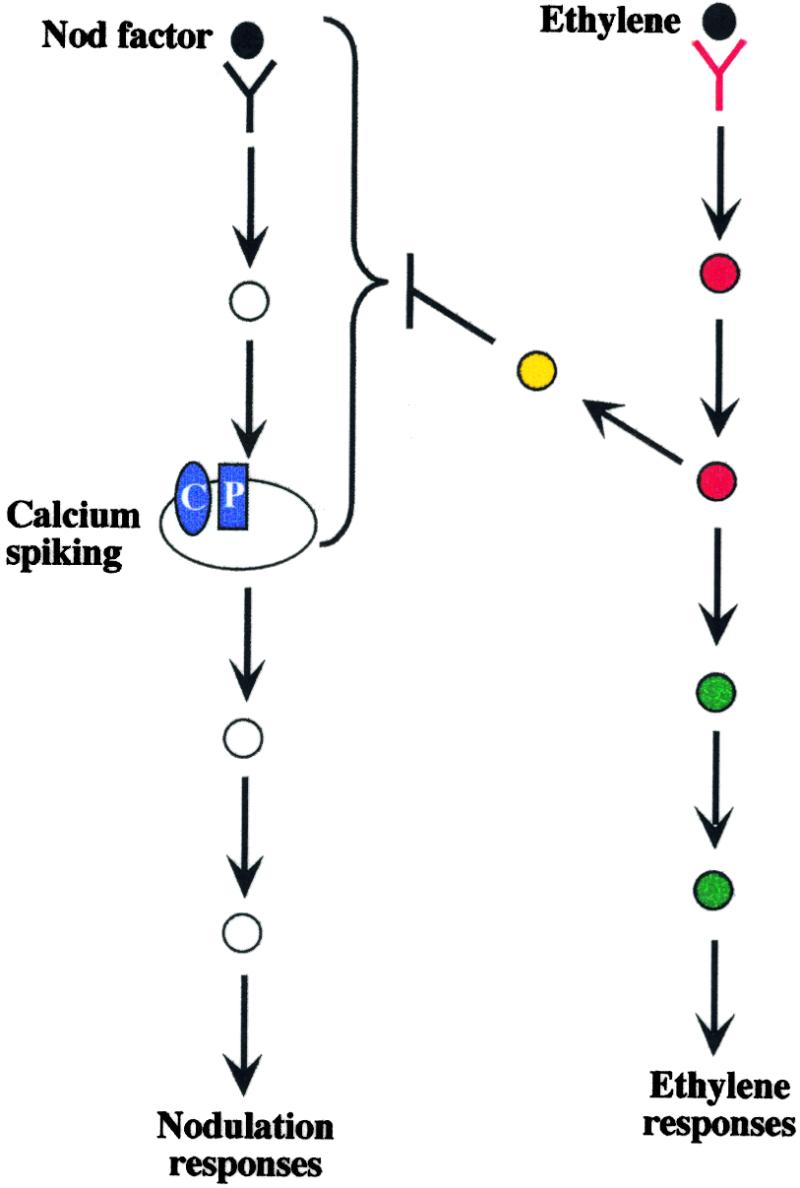
A Model Representing the Communication between the Ethylene Perception Pathway and the Nod Factor Perception Pathway.
In this model, it is assumed that calcium spiking is part of the Nod factor perception pathway immediately upstream of Nod factor responses. However, the responses downstream of calcium spiking have not been characterized. A protein in the ethylene perception pathway is involved in the inhibition of the Nod factor perception pathway at or upstream of calcium spiking. Additional proteins may be involved in the communication (yellow). Not shown is a possibility discussed in the text whereby ethylene might affect a downstream step of the Nod factor pathway that has feedback to a step upstream of spiking. Mutations in proteins of the ethylene perception pathway at or upstream of the communication will show a loss of ethylene sensitivity for both ethylene-regulated developmental responses and nodulation responses (red). skl must represent one of these proteins. Mutations in proteins downstream of this point will be ethylene insensitive for general ethylene responses but sensitive for nodulation-specific ethylene effects (green). Mutations in proteins involved in communication between the two pathways will be ethylene sensitive for ethylene-regulated developmental responses but insensitive to ethylene for the nodulation-specific ethylene effects (yellow). C, calcium channels; P, calcium pumps.
Although much is known about the ethylene perception pathway in Arabidopsis (Ecker, 1995), very little is known about this pathway in legumes. In V. sativa, gaseous ethylene has the capacity to inhibit calcium spiking within 30 min, with the first effects visible within 15 min after application (D.W. Ehrhardt, K.E. Wilson, J.A. Downie, and S.R. Long, unpublished data). This work has been repeated here in M. truncatula. These results suggest that calcium spiking requires continuous input from the Nod factor perception pathway and that ethylene can rapidly inhibit activation of the pathway. The rapidity of the ethylene effect implies that it is unlikely that the mechanism of the ethylene block involves a change in gene expression as a downstream result of ethylene perception. More likely, the mechanism of the ethylene block is communication between components of the ethylene perception pathway and the Nod factor perception pathway.
Figure 8 shows a model of how this communication may occur. A component of the ethylene perception pathway may act directly on a component of the Nod factor perception pathway, inhibiting the pathway at or upstream of calcium spiking. Alternatively, secondary components may be involved in the communication between the two pathways. skl must lie either at the point or upstream of the position in the ethylene perception pathway where the communication occurs (proteins marked red in Figure 8). The model predicts the possibility of mutants that are ethylene insensitive for developmental consequences of ethylene but ethylene sensitive for nodulation (proteins marked green in Figure 8). Conversely, it may be possible to identify mutations that are ethylene sensitive for development but ethylene insensitive for nodulation (proteins marked yellow in Figure 8). Such mutants could represent proteins involved in the communication between the two pathways.
Ethylene inhibits multiple Nod factor– and rhizobia–induced responses and defines the sensitivity of the plant to Nod factor by modulating a component of the Nod factor signal transduction pathway at or upstream of calcium spiking. In the absence of ethylene, the plant is unable to down regulate its response to S. meliloti, leading to the supernodulation phenotype observed in the ethylene-insensitive mutant skl (Penmetsa and Cook, 1997). Furthermore, ethylene also may regulate the specificity of downstream responses by modulating the frequency of calcium spikes. Nod factor appears to merely activate calcium spiking. The nature of the calcium spiking response and the degree to which the plant responds seem to be functions of the ethylene status of the plant. In this way, ethylene may delineate the level to which the plant responds to S. meliloti as a function of the environmental state and the symbiotic status of the plant.
METHODS
Plant Growth Conditions and Bacterial Strains
Sterilized Medicago truncatula seedlings germinated overnight were plated onto buffered nodulation medium (BNM) (Ehrhardt et al., 1992) with 1.2% agar and allowed to grow for a minimum of 3 days before inoculation. Seed of cv Jemalong were used as the wild type. Plants were grown in a growth chamber at 20°C with 16 hr of light per day. Sinorhizobium meliloti strain Rm1021 (pXLGD4) was grown in liquid Luria broth overnight in the presence of 500 μg/mL streptomycin selection. Bacteria were pelleted at 23,340g for 10 min and resuspended in 10 mM MgSO4. This bacterial suspension was diluted 1:50 in 10 mM MgSO4 and inoculated onto plants by flooding the roots with the inoculum. Where appropriate, the bacterial inoculum was diluted to a specific concentration after quantitation at OD600. Nod factor preparations were isolated as described by Ehrhardt et al. (1996). Strain Rm1021 (pXLGD4) was used to visualize infection threads. This strain carries a tetracycline-selectable plasmid containing lacZ driven by the hemA promoter (Penmetsa and Cook, 1997).
Assessment of Nodulation and Infections
To quantify the number of infection events, plants were grown on BNM with differing concentrations of 1-aminocyclopropane-1-carboxylic acid (ACC) or l-α-(2-aminoethoxyvinyl)-glycine (AVG) and inoculated with Rm1021 (pXLGD4). At 4 days after inoculation, roots from six plants per treatment were fixed for 1 hr in 1.25% glutaraldehyde and 200 mM sodium cacodylate, washed twice in 200 mM sodium cacodylate, and stained overnight in 200 mM sodium cacodylate, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, and 0.08% 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside (X-Gal). Roots were destained in a 1:5 dilution of household bleach for 5 min and imaged using a Nikon (Tokyo, Japan) Optiphot microscope. The total number of infection events was counted. Blue spots of bacteria in the center of a shepherd's crook before infection thread formation were counted as infection events. The total number of nodules was assessed on six plants per treatment at 14 days after inoculation.
Root Hair Deformation Assay
To assess the deformation of root hairs, plants were sandwiched between two thin slabs of BNM containing 1.2% agar and 0.1 μM AVG and in some cases 10 pM Nod factor. Plants were allowed to grow through the medium for up to 8 days. The roots stayed between the slabs of medium provided there was AVG present. In the absence of AVG or in the presence of ACC, the roots emerged out of the agar slabs. The hypocotyl/root junction was chosen as the standard point from which to measure root hair position. At distances from this hypocotyl/root junction, the root hairs were imaged using a Nikon Diaphot inverted microscope (Technical Instruments, San Francisco, CA) set for Nomarski differential interference contrast optics and a Nikon ×10 fluor objective. The length of the root hairs was assayed from the root epidermis to the tip of each root hair. Three plants were assessed per treatment.
RNA Gel Blot Analysis
Wild-type plants were grown on BNM with 0.1 μM AVG or 10 μM ACC, and skl plants were grown on BNM alone for 6 days and inoculated with S. meliloti at OD600 = 0.1. At set times after inoculation, roots from 20 plants were isolated, frozen in liquid nitrogen, and stored at −80°C. RNA was isolated using TRIZOL Reagent (Life Technologies, Rockville, MD) according to the manufacturer's protocol. RNA samples were separated on a 1.2% agarose gel containing 6.2% formaldehyde. The RNA was transferred to a nylon membrane (Hybond N; Amersham Pharmacia Biotech, Buckinghamshire, UK) and hybridized with a 280-bp fragment from exon 2 of RIP1 (Cook et al., 1995) and secondarily with a 720-bp fragment that represents a full length cDNA clone of an actin homolog identified from the M. truncatula expressed sequence tag database (Covitz et al., 1998).
β-Glucuronidase Assay
Plants carrying the ENOD11 promoter driving the expression of β-glucuronidase (GUS) were grown on medium containing 0.1 μM AVG for 4 days. Three plants were transferred to liquid medium containing either 0.1 μM AVG or 10 μM ACC or medium alone and left for 24 hr in the dark. They were then treated with 1 nM Nod factor for 6 hr in the liquid medium. The plants were fixed in 0.3% formaldehyde and 0.1 M potassium phosphate, pH 7.0, for 1 hr on ice. GUS staining was performed overnight at 37°C with 1 mM 5-bromo-4-chloro-3-indolyl- β-glucuronic acid, 5 mM EDTA, 0.5 mM potassium ferrocyanide, 0.5 mM potassium ferricyanide, and 0.1 M potassium phosphate, pH 7.0. The experiments were repeated three times with similar results.
Calcium Spiking Experiments
Analysis of calcium spiking was performed as described by Ehrhardt et al. (1996), with slight modifications as described by Wais et al. (2000). To assess the effect of ethylene on the percentage of cells spiking, seedlings were grown overnight on BNM containing either 0.1 μM AVG or 10 μM ACC. For experiments involving ACC, seedlings were transferred to liquid BNM containing 10 μM ACC, whereas plants grown on AVG were transferred to liquid BNM alone. The tip of the root was removed to avoid disturbances caused by growth of the root during imaging. Root hairs were injected with the calcium-sensitive dye Oregon green–dextran (Molecular Probes, Eugene, OR), aiming for the maximum number of root hairs that could be imaged (∼15 cells).
Root hairs on two plants were injected for each experiment. Cells were allowed to recover from the injections for ∼30 min before imaging. Root hair cells were imaged for 10 min under perfusion with liquid BNM containing either 10 μM ACC or alone at a perfusion rate of 0.2 mL/min. After 10 min, the plants were perfused with BNM containing either 10 μM ACC and 10 pM Nod factor or just 10 pM Nod factor. The cells on the first plant were imaged for 60 min after the addition of Nod factor. After this 60-min period, the cells on the second plant in the experiment were imaged. To aid in the identification of spiking, the raw fluorescence data were transformed by the equation Y = X(n+1) − X(n), which accentuates rapid changes in the calcium levels. Root hair cells that spike within ∼60 min after the addition of Nod factor were considered positive for calcium spiking.
Plants were treated in a similar way for analysis of the responsiveness of cells to different concentrations of Nod factor. However, for these tests, a single plant was imaged throughout the experiment. The plants were perfused with BNM alone or BNM containing 10 μM ACC. After 10 min, the perfusion solution was switched to BNM with or without ACC and 1 fM Nod factor. Every 30 min, the concentration of Nod factor in the perfusion solution was increased 10-fold up to a maximum of 1 nM. The number of cells spiking at each concentration of Nod factor was assessed.
Separate experiments were performed to assess the period between calcium spikes. In these experiments, approximately five cells per plant were injected. Calcium spiking was initiated with perfusion of 10 pM Nod factor at a rate of 0.2 mL/min. The period was calculated using the first 30 min of spiking not including the first few spikes that were initiated. A spike was identified as an increase of at least twice the standard deviation for background fluctuations of calcium before the addition of Nod factor.
The effect of ethylene and ACC on the maintenance of calcium spiking was analyzed by activating calcium spiking under perfusion using 10 pM Nod factor. Approximately 30 min after the initiation of calcium spiking, the perfusion solution was changed to BNM containing 10 pM Nod factor and ethylene or 100 μM ACC. Ethylene was introduced into the solution by two methods. In the first method, 15 mL of 50 mL of liquid BNM in a 60-mL syringe was exchanged with 1% ethylene. The syringe was capped, shaken vigorously, and left to stand for ∼2 hr. The solution was perfused onto the plant with the 15 mL of gas still present in the syringe. In the second method, a solution of BNM was allowed to equilibrate for 5 days in a chamber supplied continuously with 1% ethylene. The medium was agitated during this time using a stir plate. The ethylene concentrations in solution were quantified using 5-μL samples in a gas chromatograph. The first method led to a 0.4 ± 0.6% solution, whereas the second method led to a 0.07 ± 0.05% solution. We believe that these different concentrations may reflect different pressures applied in the two methods.
Acknowledgments
We thank Rebecca Wais and David Ehrhardt for critical reading of the manuscript and advice on the calcium spiking experiments. This work was supported in part by the Howard Hughes Medical Institute.
References
- Amrhein, N., and Wenker, D. (1979). Novel inhibitors of ethylene production in higher plants. Plant Cell Physiol. 20, 1635–1642. [Google Scholar]
- Ardourel, M., Demont, N., Debelle, F., Maillet, F., de Billy, F., Prome, J.C., Denarie, J., and Truchet, G. (1994). Rhizobium meliloti lipooligosaccharide nodulation factors: Different structural requirements for bacterial entry into target root hair cells and induction of plant symbiotic developmental responses. Plant Cell 6, 1357–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin, N., Serizet, C., Gosti, F., and Giraudat, J. (2000). Interactions between abscisic acid and ethylene signaling cascades. Plant Cell 12, 1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron, A.C., Fenton, C.A.L., Yu, Y.B., Adams, D.O., and Yang, S.F. (1979). Increased production of ethylene by plant tissues treated with 1-aminocyclopropane-1-carboxylic acid. HortScience 14, 178–180. [Google Scholar]
- Cardenas, L., Holdaway-Clarke, T.L., Sanchez, F., Quinto, C., Feijo, J.A., Kunkel, J.G., and Hepler, P.K. (2000). Ion changes in legume root hairs responding to Nod factors. Plant Physiol. 123, 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, D., Dreyer, D., Bonnet, D., Howell, M., Nony, E., and Vandenbosch, K. (1995). Transient induction of a peroxidase gene in Medicago truncatula precedes infection by Rhizobium meliloti. Plant Cell 7, 43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, D.R. (1999). Medicago truncatula: A model in the making. Curr. Opin. Plant Biol. 2, 301–304. [DOI] [PubMed] [Google Scholar]
- Covitz, P.A., Smith, L.S., and Long, S.R. (1998). Expressed sequence tags from a root-hair-enriched Medicago truncatula cDNA library. Plant Physiol. 117, 1325–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denarie, J., Debelle, F., and Prome, J.-C. (1996). Rhizobium lipo-chitooligosaccharide nodulation factors: Signaling molecules mediating recognition and morphogenesis. Annu. Rev. Biochem. 65, 503–535. [DOI] [PubMed] [Google Scholar]
- de Ruijter, N.C.A., Rook, M.B., Bisseling, T., and Emons, A.M.C. (1998). Lipochito-oligosaccharides re-initiate root hair tip growth in Vicia sativa with high calcium and spectrin-like antigen at the tip. Plant J. 13, 341–350. [Google Scholar]
- Dolmetsch, R.E., Lewis, R.S., Goodnow, C.C., and Healy, J.I. (1997). Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386, 855–858. [DOI] [PubMed] [Google Scholar]
- Dolmetsch, R.E., Xu, K., and Lewis, R.S. (1998). Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392, 933–936. [DOI] [PubMed] [Google Scholar]
- Ecker, J.R. (1995). The ethylene signal transduction pathway in plants. Science 268, 667–675. [DOI] [PubMed] [Google Scholar]
- Ehrhardt, D.W., Atkinson, E.M., and Long, S.R. (1992). Depolarization of alfalfa root hair membrane potential by Rhizobium meliloti Nod factors. Science 256, 998–1000. [DOI] [PubMed] [Google Scholar]
- Ehrhardt, D.W., Wais, R., and Long, S.R. (1996). Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell 85, 673–681. [DOI] [PubMed] [Google Scholar]
- Felle, H.H., Kondorosi, E., Kondorosi, A., and Schultze, M. (1998). The role of ion fluxes in Nod factor signalling in Medicago sativa. Plant J. 13, 455–463. [Google Scholar]
- Felle, H.H., Kondorosi, E., Kondorosi, A., and Schultze, M. (1999. a). Elevation of the cytosolic free [Ca2+] is indispensable for the transduction of the Nod factor signal in alfalfa. Plant Physiol. 121, 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle, H.H., Kondorosi, E., Kondorosi, A., and Schultze, M. (1999. b). Nod factors modulate the concentration of cytosolic free calcium differently in growing and non-growing root hairs of Medicago sativa. Planta 209, 207–212. [DOI] [PubMed] [Google Scholar]
- Fernandez-Lopez, M., Goormachtig, S., Gao, M., D'Haeze, W., Van Montagu, M., and Holsters, M. (1998). Ethylene-mediated phenotypic plasticity in root nodule development on Sesbania rostrata. Proc. Natl. Acad. Sci. USA 95, 12724–12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewtrell, C. (1993). Ca2+ oscillations in non-excitable cells. Annu. Rev. Physiol. 55, 427–454. [DOI] [PubMed] [Google Scholar]
- Gamas, P., Niebel, F.D.C., Lescure, N., and Cullimore, J.V. (1996). Use of a subtractive hybridization approach to identify new Medicago truncatula genes induced during root nodule development. Mol. Plant-Microbe Interact. 9, 233–242. [DOI] [PubMed] [Google Scholar]
- Goodlass, G., and Smith, K.A. (1979). Effects of ethylene on root extension and nodulation of pea (Pisum sativum L.) and white clover (Trifolium repens L.). Plant Soil 51, 387–395. [Google Scholar]
- Heidstra, R., Geurts, R., Franssen, H., Spaink, H.P., van Kammen, A., and Bisseling, T. (1994). Root hair deformation activity of nodulation factors and their fate on Vicia sativa. Plant Physiol. 105, 787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidstra, R., Yang, W.C., Yalcin, Y., Peck, S., Emons, A.M., van Kammen, A., and Bisseling, T. (1997). Ethylene provides positional information on cortical cell division but is not involved in Nod factor–induced root hair tip growth in Rhizobium–legume interaction. Development 124, 1781–1787. [DOI] [PubMed] [Google Scholar]
- Herrera, M.A., Bedmar, E.J., and Olivares, J. (1987). Effects of nitrate and light intensity on photosynthesis and nitrogen fixation in alfalfa plants. J. Plant Physiol. 128, 467–472. [Google Scholar]
- Journet, E.-P., El-Gachtouli, N., Vernoud, V., de Billy, F., Pichon, M., Dedieu, A., Arnould, C., Morandi, D., Barker, D.G., and Gianinazzi-Pearson, V. (2001). Medicago truncatula ENOD11: A novel RPRP-encoding early nodulin gene is expressed in mycorrhizal arbuscule-containing cells. Mol. Plant-Microbe Interact., in press. [DOI] [PubMed]
- Kijne, J.W. (1992). The Rhizobium infection process. In Biological Nitrogen Fixation, G. Stacey, R.H. Burris, and H.J. Evans, eds (New York: Chapman and Hall), pp. 349–398.
- Kozik, A., Heidstra, R., Horvath, B., Kulikova, O., Tikhonovich, I., Ellis, T.H.N., Van Kammem, A., Lie, T.A., and Bisseling, T. (1995). Pea lines carrying sym1 or sym2 can be nodulated by Rhizobium strains containing nodX; sym1 and sym2 are allelic. Plant Sci. 108, 41–49. [Google Scholar]
- Kurkdjian, A., Bouteau, F., Pennarun, A.-M., Convert, M., Cornel, D., Rona, J.-P., and Bousquet, U. (2000). Ion currents involved in early Nod factor response in Medicago sativa root hairs: A discontinuous single-electrode voltage-clamp study. Plant J. 22, 9–17. [DOI] [PubMed] [Google Scholar]
- Lee, K.H., and LaRue, T.A. (1992. a). Ethylene as a possible mediator of light and nitrate-induced inhibition of nodulation of Pisum sativum L. cv Sparkle. Plant Physiol. 100, 1334–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K.H., and LaRue, T.A. (1992. b). Exogenous ethylene inhibits nodulation of Pisum sativum L. cv Sparkle. Plant Physiol. 100, 1759–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W., Llopis, J., Whitney, M., Zlokarnik, G., and Tsien, R.Y. (1998). Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature 392, 936–941. [DOI] [PubMed] [Google Scholar]
- Ligero, F., Lluch, C., and Olivares, J. (1986). Evolution of ethylene from roots of Medicago sativa plants inoculated with Rhizobium meliloti. J. Plant Physiol. 125, 361–366. [Google Scholar]
- Ligero, F., Caba, J.M., Lluch, C., and Olivares, J. (1991). Nitrate inhibition of nodulation can be overcome by the ethylene inhibitor aminoethoxyvinylglycine. Plant Physiol. 97, 1221–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, S.R. (1996). Rhizobium symbiosis: Nod factors in perspective. Plant Cell 8, 1885–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, S.R., and Staskawicz, B.J. (1993). Prokaryotic plant parasites. Cell 73, 921–935. [DOI] [PubMed] [Google Scholar]
- Lurssen, K., Naumann, K., and Schroder, R. (1979). 1-Amino-cyclopropane-1-carboxylic acid: An intermediate of the ethylene biosynthesis in higher plants. Pflanzenphysiologie 92, 285–294. [Google Scholar]
- Mylona, P., Pawlowski, K., and Bisseling, T. (1995). Symbiotic nitrogen fixation. Plant Cell 7, 869–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penmetsa, R.V., and Cook, D.R. (1997). A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science 275, 527–530. [DOI] [PubMed] [Google Scholar]
- Peters, N.K., and Crist Estes, D.K. (1989). Nodule formation is stimulated by the ethylene inhibitor aminoethoxyvinylglycine. Plant Physiol. 91, 690–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts, R.J., Cernac, A., and Estelle, M. (1998). Auxin and ethylene promote root hair elongation in Arabidopsis. Plant J. 16, 553–560. [DOI] [PubMed] [Google Scholar]
- Relic, B., Perret, X., Estrada-Garcia, M.T., Kopcinska, J., Golinowski, W., Kirshnan, H.B., Pueppke, S.G., and Broughton, W.J. (1994). Nod factors of Rhizobium are a key to the legume door. Mol. Microbiol. 13, 171–178. [DOI] [PubMed] [Google Scholar]
- Suganuma, N., Yamauchi, H., and Yamamoto, K. (1995). Enhanced production of ethylene by soybean roots after inoculation with Bradyrhizobium japonicum. Plant Sci. 111, 163–168. [Google Scholar]
- Tanimoto, M., Roberts, K., and Dolan, L. (1995). Ethylene is a positive regulator of root hair development in Arabidopsis thaliana. Plant J. 8, 943–948. [DOI] [PubMed] [Google Scholar]
- Theologis, A. (1992). One rotten apple spoils the whole bushel: The role of ethylene in fruit ripening. Cell 70, 181–184. [DOI] [PubMed] [Google Scholar]
- van Spronsen, P.C., van Brussel, A.A.N., and Kijne, J.W. (1995). Nod factors produced by Rhizobium leguminosarum biovar viciae induce ethylene-related changes in root cortical cells of Vicia sativa ssp. nigra. Eur. J. Cell Biol. 68, 463–469. [PubMed] [Google Scholar]
- Wais, R.J., Galera, C., Oldroyd, G., Catoira, R., Penmetsa, R.V., Cook, D., Gough, C., Denarie, J., and Long, S.R. (2000). Genetic analysis of calcium spiking responses in nodulation mutants of Medicago truncatula. Proc. Natl. Acad. Sci. USA 97, 13407–13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, S.A., and Downie, J.A. (2000). Entry of Rhizobium leguminosarum bv. viciae into root hairs requires minimal nod factor specificity, but subsequent infection thread growth requires nodO and nodE. Mol. Plant-Microbe Interact. 13, 754–762. [DOI] [PubMed] [Google Scholar]
- Walker, S.A., Viprey, V., and Downie, J.A. (2000). Dissection of nodulation signaling using pea mutants defective for calcium spiking induced by Nod factors and chitin oligomers. Proc. Natl. Acad. Sci. USA 97, 13413–13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, N.M., Cuthbertson, K.S.R., and Cobbold, P.H. (1986). Repetitive transient rises in cytoplasmic free calcium in hormone stimulated hepatocytes. Nature 319, 600–602. [DOI] [PubMed] [Google Scholar]
- Yang, S.F., and Hoffman, N.E. (1984). Ethylene biosynthesis and its regulation in higher plants. Annu. Rev. Plant Physiol. 35, 155–189. [Google Scholar]