Abstract
A novel protein, MP73, was specifically found on the membrane of protein storage vacuoles of pumpkin seed. MP73 appeared during seed maturation and disappeared rapidly after seed germination, in association with the morphological changes of the protein storage vacuoles. The MP73 precursor deduced from the isolated cDNA was composed of a signal peptide, a 24-kD domain (P24), and the MP73 domain with a putative long α-helix of 13 repeats that are rich in glutamic acid and arginine residues. Immunocytochemistry and immunoblot analysis showed that the precursor-accumulating (PAC) vesicles (endoplasmic reticulum–derived vesicles responsible for the transport of storage proteins) accumulated proMP73, but not MP73, on the membranes. Subcellular fractionation of the pulse-labeled maturing seed demonstrated that the proMP73 form with N-linked oligosaccharides was synthesized on the endoplasmic reticulum and then transported to the protein storage vacuoles via PAC vesicles. Tunicamycin treatment of the seed resulted in the efficient deposition of proMP73 lacking the oligosaccharides (proMP73ΔΨ) into the PAC vesicles but no accumulation of MP73 in vacuoles. Tunicamycin might impede the transport of proMP73ΔΨ from the PAC vesicles to the vacuoles or might make the unglycosylated protein unstable in the vacuoles. After arrival at protein storage vacuoles, proMP73 was cleaved by the action of a vacuolar enzyme to form a 100-kD complex on the vacuolar membranes. These results suggest that PAC vesicles might mediate the delivery of not only storage proteins but also membrane proteins of the vacuoles.
INTRODUCTION
Protein bodies are widely distributed in the dry seed of higher plants. They are membrane-bound organelles that store various seed proteins. Protein bodies are formed from protein storage vacuoles at the late stage of seed maturation in association with the desiccation of pumpkin seed (Hara-Nishimura et al., 1987). Conversely, after seed imbibition, protein bodies swell, start to fuse with one another, and are converted into a large central vacuole (Hara-Nishimura et al., 1991b). The dynamic morphological transformation (i.e., from protein storage vacuoles to protein bodies and then to lytic vacuoles) occurs in the seed cells during seed desiccation and imbibition. Protein bodies can be regarded as a desiccation form of protein storage vacuoles, which are present in dry seed.
The membranes of the protein storage vacuoles and the lytic vacuoles have been isolated from dry seed and seedlings of pumpkin, respectively (Inoue et al., 1995a, 1995b). A spin label electron paramagnetic resonance experiment with the isolated membranes showed that the membranes of the protein storage vacuoles are less fluid and have more restricted motional freedom than the membranes of the lytic vacuoles of pumpkin (Strzalka et al., 1995). One of the factors contributing to the changes in membrane fluidity during the transformation of protein storage vacuoles into lytic vacuoles may be a reduction of the ratio of membrane proteins to lipids. The organellar transformation is accompanied by the breakdown and synthesis of the membrane proteins.
A seed-specific water channel, αTIP, that is widely conserved among plants is abundant on membranes of protein storage vacuoles (Mäder and Chrispeels, 1984; Johnson et al., 1989). αTIP is synthesized and accumulated on the membranes of protein storage vacuoles during seed maturation and then is degraded after seed germination (Melroy and Herman, 1991; Inoue et al., 1995b). In contrast to the degradation of αTIP, another water channel γTIP is newly synthesized and incorporated into the vacuolar membranes after seed germination (Maeshima et al., 1994). Previously, we reported that other membrane proteins, MP27 and MP32, of pumpkin also are synthesized during seed maturation and then rapidly degraded after seed germination, as αTIP is (Inoue et al., 1995a). As minor components, vacuolar H+-pyrophosphatase and H+-ATPase are localized on the membranes in dry seed, and their amounts are increased after seed germination (Maeshima et al., 1994).
To elucidate the mechanisms responsible for organellar transformation, it is necessary to clarify what kinds of membrane proteins are specifically expressed and how they are localized on the membranes of protein storage vacuoles during seed maturation. Previously, we found that precursor-accumulating (PAC) vesicles are responsible for the transport of storage proteins directly to protein storage vacuoles (Hara-Nishimura et al., 1998b). The PAC vesicles with diameters of 200 to 400 nm contain an electron-dense core of storage proteins surrounded by an electron-translucent layer, and some vesicles also contain small vesicle-like structures. The protein cores are derived from numerous aggregates of storage proteins formed within the rough endoplasmic reticulum (RER). The PAC vesicles might mediate a Golgi-independent transport pathway for insoluble aggregates of storage proteins directly to protein storage vacuoles (Hara-Nishimura et al., 1998b).
At present, it is unclear whether the PAC vesicles are involved in the delivery of proteins other than storage proteins, such as membrane proteins. In this study, we characterized a novel 73-kD membrane protein with a long α-helix of a large number of hydrophilic residues. We also demonstrated that the precursor of the membrane protein is delivered to protein storage vacuoles via PAC vesicles.
RESULTS
Molecular Characterization of a Novel Membrane Protein, MP73, of Protein Storage Vacuoles
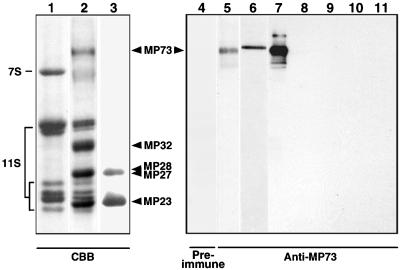
To characterize membrane components of protein storage vacuoles, protein storage vacuoles were purified from dry pumpkin seed and their membranes were isolated. Five membrane proteins, designated MP23, MP27, MP28, MP32, and MP73, were enriched in the final preparation, although there was some contamination by storage proteins 11S globulin and 7S globulin, as shown in Figure 1 (lanes 1 and 2). Both MP23 and MP28, which were shown previously to be types of αTIP water channels (Inoue et al., 1995b), were not extractable from the membranes with an alkaline solution (Figure 1, lane 3). MP27 and MP32 were shown previously to be peripheral membrane proteins (Inoue et al., 1995a). The remaining membrane protein, MP73, is the largest one, and its function is unknown.
Figure 1.
MP73 Is a Peripheral Membrane Protein of Protein Storage Vacuoles of Pumpkin and Castor Bean.
Protein storage vacuoles from pumpkin seed (lane 1), the vacuolar membranes (lane 2), and the alkaline-washed membranes (lane 3) were subjected to SDS-PAGE with Coomassie blue staining (CBB). Five major proteins, MP23, MP27, MP28, MP32, and MP73, are indicated. MP73, MP32, and MP27 were extracted with alkali, whereas two αTIPs, MP23 and MP28, were not (lane 3). Immunoblots with preimmune serum (lane 4) and anti-MP73 antibodies (lanes 5 to 11) are shown. The lanes show the membranes of protein storage vacuoles of pumpkin (lane 4, 50 μg of total proteins; lane 5, 1 μg of total proteins), the membranes of protein storage vacuoles of castor bean (lane 6, 1 μg of total proteins), and each organ of pumpkin (50 μg of total proteins): maturing seed (lane 7), 10-day-old seedlings (lane 8), roots (lane 9), stems (lane 10), and mature leaves (lane 11). 7S, 7S globulin; 11S, 11S globulin.
To clarify the seed-specific expression of MP73, immunoblotting was performed with antibodies raised against purified MP73 (Figure 1, right). Preimmune serum gave no signal on the blot of 50 μg of total proteins of the isolated membranes of protein storage vacuoles (lane 4). Anti-MP73 antibodies specifically detected MP73 in the membrane fraction (1 μg of total proteins; lane 5) as well as in the isolated membranes of protein storage vacuoles of castor bean (1 μg of total proteins; lane 6). On the immunoblots of various organs (50 μg of total proteins), MP73 was detected in the maturing seed (lane 7) but not in the 10-day-old seedlings (lane 8), the roots (lane 9), the stems (lane 10), or the mature leaves (lane 11). These results suggest that MP73, like αTIPs, is a membrane protein that is unique in protein storage vacuoles of various seed.
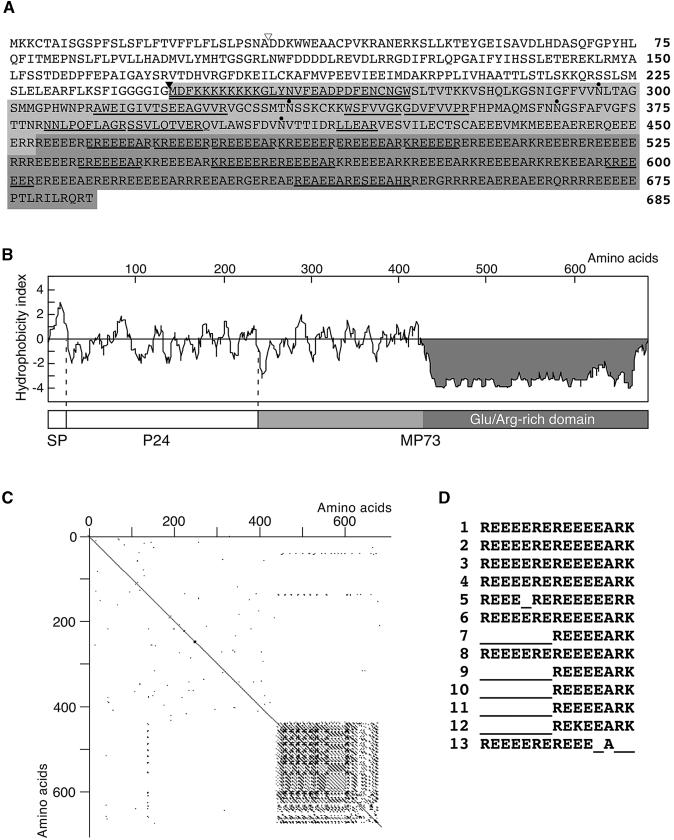
MP73 cDNAs were isolated from the cDNA library of maturing pumpkin cotyledons. The primary structure of the MP73 precursor (685 amino acids) was deduced from the complete nucleotide sequence, as shown in Figure 2A. Figure 2B shows its hydropathy plot. The N-terminal hydrophobic sequence has the features of a putative signal peptide, expected to be cleaved off the C-terminal side of Ala-31 (Figure 2A, open triangle). The sequence of the N-terminal 29 amino acid residues of purified MP73 corresponded to the sequence from Met-245 to Trp-273 (Figure 2A, double underline). Thus, post-translational processing might occur on the C-terminal side of Gly-244 (Figure 2A, closed triangle) that is located in the hydrophilic region of the precursor (Figure 2B). The sequences of 15 peptide fragments obtained from a trypsin digest of purified MP73 were found in the region after Gly-244 (Figure 2A, underlined). These results indicate that the MP73 precursor is composed of a possible 31–amino acid signal peptide for cotranslational insertion into the RER, a 213–amino acid propeptide domain (expected molecular mass, 24 kD [P24]), and a 54-kD domain corresponding to the mature MP73. The calculated molecular mass (54 kD) of the mature molecule is much lower than the 73 kD that was estimated by SDS-PAGE (discussed below).
Figure 2.
Molecular Characterization of MP73.
(A) Deduced primary sequence of a precursor of MP73 (preproMP73). The mature MP73 domain is shaded. A Glu/Arg-rich domain of the C-terminal half of MP73 is indicated by darker shading. The determined N-terminal sequence of MP73 is double underlined, and the determined internal sequences are underlined. Open triangle, putative cleavage site of a signal peptide; closed triangle, post-translational processing site; dots, possible glycosylation sites.
(B) Hydropathy profile of preproMP73 and its domain structure. A Glu/Arg-rich domain indicated by darker shading is extremely hydrophilic. SP, putative signal peptide; P24, 24-kD domain.
(C) Homology plot of preproMP73 showing that the Glu/Arg-rich domain of the C-terminal half is composed of homologous repeat sequences.
(D) Alignment of the 13 homologous repeats in the Glu/Arg-rich domain.
MP73 has no hydrophobic region sufficient to form a transmembrane domain (Figure 2B), indicating that it is a peripheral membrane protein. This was supported by the result that MP73 was extractable with alkali (Figure 1, lane 3). The C-terminal half of MP73 is a Glu/Arg-rich domain (indicated by the darker shading in Figures 2A and 2B) that is highly hydrophilic and acidic (pI = 4.53). A homology plot shows that 13 repeats are found in the Glu/Arg-rich domain (Figure 2C). A typical repeat sequence is Arg-(Glu)4-Arg-Glu-Arg-(Glu)4-Ala-Arg-Lys (Figure 2D). The Glu/Arg-rich domain is predicted to form a long α-helix.
ProMP73 Is Localized on the Membranes of PAC Vesicles, whereas MP73 Is Found on the Membranes of Protein Storage Vacuoles
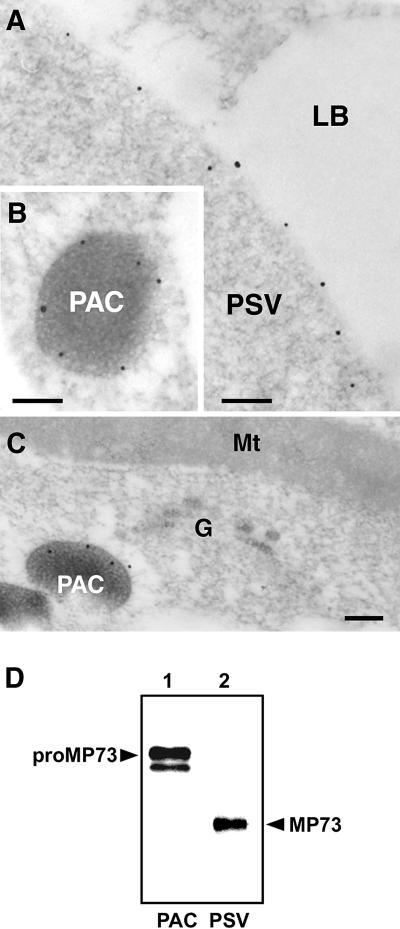
PAC vesicles have been shown to be responsible for the transport of a large amount of precursors of seed proteins to protein storage vacuoles in maturing pumpkin seed (Hara-Nishimura et al., 1998b; Yamada et al., 1999). To determine whether PAC vesicles also are responsible for the transport of vacuolar membrane proteins, we performed immunocytochemistry with specific anti-MP73 antibodies and maturing pumpkin seed. MP73 was localized on the membranes of both protein storage vacuoles (Figure 3A) and PAC vesicles (Figure 3B). On the other hand, MP73 was not localized on the Golgi complex (Figure 3C). To clarify the molecular forms of MP73 on these membranes, immunoblotting was performed with the purified PAC vesicles and protein storage vacuoles. The 100-kD precursor, proMP73, was accumulated on the membranes of the PAC vesicles, whereas the 73-kD mature MP73 was localized on the membranes of the protein storage vacuoles (Figure 3D).
Figure 3.
MP73 Is Localized on the Membranes of Protein Storage Vacuoles and ProMP73 Is Localized on the Membranes of the PAC Vesicles in Maturing Pumpkin Seed.
(A) Immunocytochemistry with anti-MP73 antibodies showing gold particles on the membrane of a protein storage vacuole. LB, lipid body. Bar = 250 nm.
(B) Immunocytochemistry with anti-MP73 antibodies showing gold particles on the membrane of a PAC vesicle. Bar = 250 nm.
(C) Immunocytochemistry with anti-MP73 antibodies showing gold particles on the membrane of a PAC vesicle but not on a Golgi complex. G, Golgi complex; Mt, mitochondrion. Bar = 200 nm.
(D) Immunoblots of the isolated PAC vesicles and protein storage vacuoles with anti-MP73 antibodies. ProMP73 was detected in the isolated PAC vesicles (lane 1), whereas MP73 was found in the isolated protein storage vacuoles (lane 2, PSV).
PSV, protein storage vacuole.
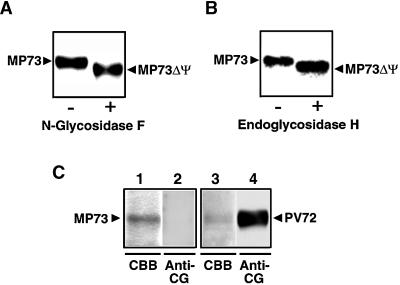
Four potential N-glycosylation sites were found at positions 296, 334, 366, and 408 of the proMP73 polypeptide (Figure 2A, dots). N-glycosylation is a cotranslational process that occurs in the lumen of the RER. To determine whether proMP73 is glycosylated or not, the purified membranes of the protein storage vacuoles were treated with glycosidases. Treatment of MP73 with either N-glycosidase F or endoglycosidase H reduced its molecular mass as determined by SDS-PAGE, as shown in Figures 4A and 4B. The sensitivity to endoglycosidase H with a substrate specificity toward high mannose oligosaccharides showed that MP73 has high mannose oligosaccharides. An immunoblot with anti–complex glycan antibodies showed no signal for the purified MP73 (Figure 4C, lanes 1 and 2) but a positive signal for the purified PV72 that is known as a glycosylated vacuolar sorting receptor (Figure 4C, lanes 3 and 4). It seems likely that MP73 has no complex glycan. N-glycosylation indicated that MP73 is associated with the lumenal side of the protein storage vacuoles.
Figure 4.
MP73 Has High Mannose Oligosaccharides but No Complex Glycan.
(A) Purified MP73 was incubated in the absence (−) or presence (+) of N-glycosidase F at 37°C for 18 hr and then subjected to SDS-PAGE with Coomassie blue staining.
(B) Purified MP73 was incubated in the absence (−) or presence (+) of endoglycosidase H at 37°C for 18 hr and then subjected to SDS-PAGE with Coomassie blue staining.
(C) Purified MP73 (lanes 1 and 2) and purified PV72, a vacuolar sorting receptor of pumpkin (lanes 3 and 4), were subjected to SDS-PAGE followed by either staining with Coomassie blue (CBB) or immunoblotting with anti–complex glycan antibodies (anti-CG).
MP73ΔΨ, unglycosylated MP73.
PAC Vesicles Mediate the Delivery of Glycosylated ProMP73 to Protein Storage Vacuoles
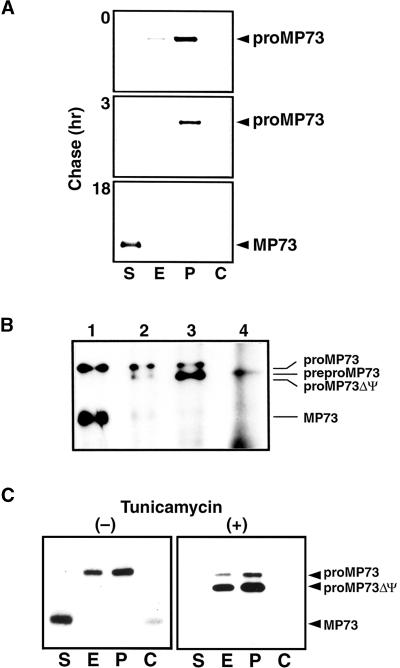
To demonstrate the intracellular transport of proMP73, subcellular fractionation of pulse-chase–labeled maturing seed of castor bean was performed. After the pulse for 30 min, the labeled proMP73 was distributed in the endoplasmic reticulum (ER) and the PAC vesicle fractions (Figure 5A). The proMP73 disappeared during the 18-hr chase, and the mature MP73 appeared in the supernatant fraction (the S fraction), which contained both the 13% (w/w) sucrose fraction and the interface between 13% (w/w) sucrose and 20% (w/w) sucrose (Figure 5A). Most MP73 protein storage vacuoles were recovered in the S fraction, whereas the crystalloids of protein storage vacuoles were recovered in the pellet fraction (the C fraction). The protein storage vacuoles were ruptured easily by centrifugation because of crystalloids inside the vacuoles (see Figure 8C, top). The membrane fragments from the broken vacuoles might be recovered in the S fraction. A slight amount of MP73 was detected in the C fraction that might contain a few intact vacuoles (Figure 5C, left). These results indicated that proMP73 synthesized on the ER membranes was transported to the PAC vesicle membranes and then to the membranes of protein storage vacuoles, where the precursor might be converted to the mature form.
Figure 5.
PAC Vesicle–Mediated Transport of ProMP73 and the Effect of Tunicamycin on Transport.
(A) Maturing castor bean seed were pulse labeled with 35S-methionine for 30 min and then chased with unlabeled methionine for 0, 3, or 18 hr. Each subcellular fraction of the labeled tissues was immunoprecipitated with anti-MP73 antibodies, and the immunoprecipitates were subjected to SDS-PAGE and fluorography.
(B) Maturing castor bean seed were incubated without tunicamycin (lane 1) or in the presence of 3 μg/mL (lane 2) or 30 μg/mL (lane 3) tunicamycin for 3 hr before a 4-hr pulse labeling with 35S-methionine. Each homogenate was immunoprecipitated with anti-MP73 antibodies, and the immunoprecipitates were subjected to SDS-PAGE and fluorography. In vitro translation products directed by poly(A) RNA from the maturing seed also were immunoprecipitated to show the preproMP73 (lane 4).
(C) Maturing castor bean seed were incubated in the absence (−) or presence (+) of 30 μg/mL tunicamycin for 3 hr before a 4-hr pulse labeling with 35S-methionine. The labeled seed were applied to subcellular fractionation. Immunoprecipitates of each fraction with anti-MP73 antibodies were subjected to SDS-PAGE and fluorography.
S,13% (w/w) sucrose fraction and the 13 to 20% (w/w) interface fraction containing vacuolar membranes; E, ER fraction; P, PAC vesicle fraction; C, crystalloids of protein storage vacuoles; proMP73ΔΨ, unglycosylated form of proMP73.
Figure 8.
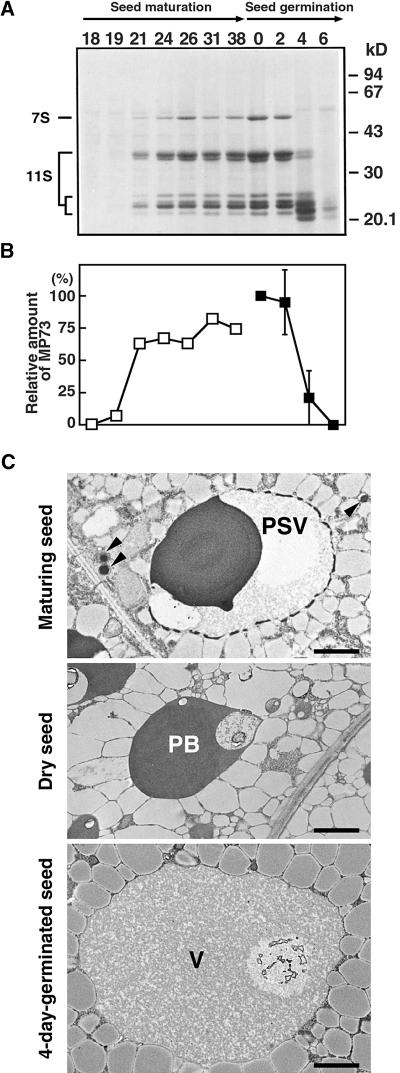
Developmental Changes in the Level of MP73 in Pumpkin Cotyledons during Seed Maturation, Germination, and Subsequent Seedling Growth.
(A) Pumpkin cotyledons were harvested 18, 19, 21, 24, 26, 31, and 38 days after anthesis (Seed maturation) and 0, 2, 4, and 6 days after germination (Seed germination). The homogenate from each 75 μg (fresh weight) of the cotyledon was subjected to SDS-PAGE with
The effect of tunicamycin on the biosynthesis of MP73 was investigated in the maturing seed. The pulse-labeled seed gave two positive bands corresponding to proMP73 and MP73 (Figure 5B, lane 1). The maturing seed that had been treated with tunicamycin accumulated only the unglycosylated proMP73 (proMP73ΔΨ), which has a smaller molecular mass than proMP73 (Figure 5B, lanes 2 and 3). The molecular mass of preproMP73, an in vitro translation product that had a signal peptide but no oligosaccharide, was smaller than that of proMP73 and larger than that of proMP73ΔΨ (Figure 5B, lanes 3 and 4).
To clarify the effect of tunicamycin on the intracellular transport of proMP73, subcellular fractionation of the pulse-labeled maturing seed that had been incubated in the absence or presence of tunicamycin was performed. After the pulse of tunicamycin-untreated seed, the labeled proMP73 was distributed in the ER and the PAC vesicle fractions, whereas the labeled MP73 was localized in the S fraction and slightly in the C fraction (Figure 5C, left). On the other hand, tunicamycin treatment of the maturing seed resulted in the efficient deposition of proMP73ΔΨ into the PAC vesicles but no accumulation of MP73 in the vacuoles (Figure 5C, right). In contrast to the efficient transport from the ER to the PAC vesicles, tunicamycin might impede the transport of proMP73ΔΨ from the PAC vesicles to the vacuoles or might make the unglycosylated protein unstable in the vacuoles.
A Soluble Proteinase Is Involved in the Processing of ProMP73 to Form a 100-kD Complex in Protein Storage Vacuoles
The absence of the mature MP73 in the PAC vesicles (Figures 3D, 5A, and 5C) suggested that the conversion of proMP73 into MP73 occurred in the vacuoles. To determine whether the processing activity is localized in the vacuoles, we performed an in vitro processing experiment with the soluble vacuolar fraction. The labeled proMP73, which had been prepared from the PAC vesicles of the pulse-labeled maturing seed, was completely converted into the mature MP73 by the action of the soluble fraction of the isolated protein storage vacuoles, as shown in Figure 6. This result indicated that the enzyme responsible for the maturation of MP73 is localized in the protein storage vacuoles.
Figure 6.
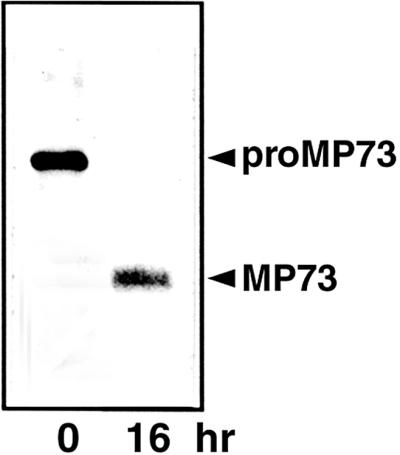
In Vitro Conversion of ProMP73 to MP73.
PAC vesicles from the pulse-labeled maturing castor bean seed were incubated with an extract of protein storage vacuoles at 30°C for 0 or 16 hr, followed by immunoprecipitation with anti-MP73 antibodies. The immunoprecipitate was subjected to SDS-PAGE and fluorography. ProMP73 was converted to MP73.
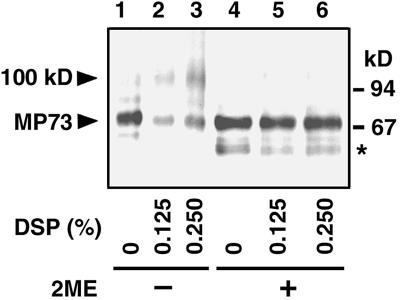
To clarify the higher structure of MP73, the isolated membranes of the protein storage vacuoles were incubated with a cross-linking reagent, dithiobissuccinimidylpropionate (DSP). As shown in Figure 7, the level of a 100-kD complex was increased in parallel with the increased concentration of DSP on the immunoblot with anti-MP73 antibodies (lanes 1 to 3). When the cross-linkage of the complex was broken with a reducing agent, only MP73 was detected on the blot (lanes 4 to 6). This result indicates that MP73 binds to an unknown molecule to form a 100-kD complex on the membranes of protein storage vacuoles. Because the molecular mass of the complex was similar to that of proMP73, it is possible that the 24-kD domain (P24) remains associated with MP73 after post-translational processing.
Figure 7.
Oligomeric Structure of MP73.
The membranes of protein storage vacuoles were treated with 0, 0.125, and 0.250% DSP. Aliquots of the reaction mixture were subjected to SDS-PAGE under nonreducing (lanes 1 to 3) or reducing (lanes 4 to 6) conditions with 2-mercaptoethanol (2ME) and subsequently were subjected to immunoblotting with anti-MP73 antibodies. A 100-kD complex was detected on the blot only under nonreducing conditions (lanes 2 and 3). The molecular mass of each marker protein is given at right in kD. The asterisk indicates degradation products of MP73.
MP73 on the Membranes of Protein Storage Vacuoles Appeared during Seed Maturation and Disappeared after Seed Germination, in Association with the Morphological Changes of Protein Storage Vacuoles
Figure 8A shows the developmental change in the levels of the storage proteins (7S and 11S globulin) during seed maturation followed by seed germination. The levels of storage proteins increased from 18 to 38 days after anthesis and reached a maximum in the dry seed. A drastic degradation of the storage proteins occurred between 2 and 4 days after germination. Figure 8B shows the change in the level of MP73. The results show that MP73 is transiently accumulated in protein storage vacuoles specifically during seed desiccation and is degraded after seed germination. Protein storage vacuoles accumulating storage proteins are converted into the desiccation form (protein bodies) after seed desiccation (Hara-Nishimura et al., 1987), as shown in Figure 8C. The protein bodies are fused to each other and give rise to lytic vacuoles (Hara-Nishimura et al., 1991b). The increase in the abundance and decline of MP73 on the membranes is associated with the morphological change of the organelles, as shown in Figure 8C.
DISCUSSION
PAC Vesicles Are Responsible for the Transport of a Membrane Protein Together with Storage Proteins to Protein Storage Vacuoles
Previously, we demonstrated that various seed storage proteins form an aggregate within the lumen of the ER and that the insoluble aggregate is transported directly to protein storage vacuoles via PAC vesicles (Hara-Nishimura et al., 1998b). Our results indicate that the PAC vesicles are involved in the delivery of not only storage proteins but also a membrane protein (Figures 3 and 5). When the seed were treated with tunicamycin, an inhibitor of N-glycosylation on ER membranes, we found efficient deposition of the unglycosylated proMP73 (proMP73ΔΨ) in the PAC vesicles (Figure 5C). In contrast, we found no accumulation of MP73 in the vacuoles (Figure 5C). The failure of the accumulation of the mature MP73 suggests two possibilities. One is that proMP73ΔΨ is not delivered from the PAC vesicles to the vacuoles. Another is that proMP73ΔΨ is unstable enough to be degraded in the vacuoles.
Two other peripheral membrane proteins, MP27 and MP32, share a similar sequence with MP73 (discussed below). It is possible that they are transported to the membranes of protein storage vacuoles in a PAC-dependent pathway, as is MP73. Jiang and Rogers (1998) reported that a reporter protein connected with a cytosolic tail of αTIP is transported to vacuoles in a Golgi-independent manner. On the other hand, Hinz et al. (1999) reported a Golgi-dependent transport of both αTIP and 11S globulin in maturing pea cotyledons. Further analysis is required to elucidate the involvement of the PAC vesicles in the transport of the integral membrane proteins αTIPs. It cannot be excluded that integral and peripheral membrane proteins are transported to protein storage vacuoles by distinct pathways.
Processing and Oligomerization of MP73 on the Vacuolar Membranes
We have reported that vacuolar processing enzyme (VPE) is responsible for the maturation of various seed proteins, including 11S globulin and 2S albumin (Hara-Nishimura and Nishimura, 1987; Hara-Nishimura et al., 1991a, 1993b, 1995, 1998a; Hiraiwa et al., 1993, 1997a, 1997b; Shimada et al., 1994; Yamada et al., 1999). We have also reported that VPE-dependent processing produces different functional proteins, such as a proteinase inhibitor, cytotoxic proteins, and 7S globulin from PV100, in the protein storage vacuoles (Yamada et al., 1999). VPE cleaves the peptide bonds at the C-terminal sides of asparagine residues exposed on the surface of these seed proteins (Hara-Nishimura et al., 1993a). It has been shown that the membrane proteins MP27 and MP32 are synthesized as a single precursor. The precursor protein is post-translationally processed at the C-terminal side of Asn-278 to produce MP27 and MP32 (Inoue et al., 1995a). The manner of processing is consistent with the substrate specificity of VPE. It seems likely that VPE is involved in the maturation of these membrane proteins.
In contrast to MP27 and MP32, proMP73 was post-translationally processed at the C-terminal side of Gly-244 (Figure 2A), which is not consistent with the substrate specificity of VPEs. We found that there was processing activity to convert proMP73 to MP73 in a vacuolar subcellular fraction (Figure 6). Thus, another proteinase in the vacuoles is responsible for the maturation of MP73 on the membranes. It is possible that after processing at Gly-244, the N-terminal P24 domain is still attached to the MP73 domain to form a 100-kD complex and that the P24 domain exerts some functions on the membranes of protein storage vacuoles after being cleaved off from the precursor protein. MP27 and MP32 are produced similarly from a single precursor, and they form a complex on vacuolar membranes (K. Inoue, M. Nishimura, and I. Hara-Nishimura, unpublished data).
Molecular Characterization of the Glu/Arg-Rich Domain of MP73
MP73 has an extremely hydrophilic Glu/Arg-rich domain composed of 13 repeats (Figure 2). The Chou and Fasman model (Chou and Fasman, 1974) predicts that the Glu/Arg-rich domain is a long α-helix (data not shown). The repeats of Arg-(Glu)4-Arg-Glu-Arg-(Glu)4-Ala-Arg-Lys might form a cluster of positive charges on the negatively charged surface of the α-helix. The cluster of basic amino acids in Raf-1 kinase is known to be critical for interaction with phosphatidylserine in the membranes (Improta-Brears et al., 1999). The Glu/Arg-rich α-helix domain of MP73 might interact with membrane phospholipids to bind MP73 to the vacuolar membranes.
The molecular mass of the MP73 polypeptide was deduced to be 53,911 D. The molecular mass of the fully glycosylated MP73 could be ∼64 kD, which is much lower than the value of 73 kD estimated from SDS-PAGE (Figure 1, lane 2). One explanation for this discrepancy is that the Glu/Arg-rich domain causes a reduction of the mobility of MP73 on SDS gels. A similar acidic sequence is found in MP32 (Inoue et al., 1995a) and calnexin (Wada et al., 1991). MP32 has a C-terminal domain rich in glutamic acids. The calculated molecular mass of 25 kD is much lower than the estimated value of 32 kD on SDS gels (Inoue et al., 1995a). Calnexin also has a glutamic acid–rich C-terminal domain. The calculated 66 kD is lower than the estimated value of 90 kD on SDS gels. These findings suggest that such acidic sequences reduce the mobility of proteins on SDS gels.
MP73 Functions on the Membranes of Protein Storage Vacuoles of Seed during Desiccation and Imbibition
The five major membrane proteins of the protein storage vacuoles of pumpkin are classified into two types. One type includes peripheral membrane proteins with an extremely hydrophilic domain, such as MP73, MP32, and MP27. These novel proteins are expressed transiently in seed; they appear during desiccation of seed and disappear after seed imbibition (Figure 8). During this period, seed cells are exposed to rapid water loss and gain. It is possible that the membrane proteins function to maintain membrane integrity and to increase the desiccation tolerance of the vacuoles by keeping water on the charged surface of their highly hydrophilic domains. The other type of membrane proteins includes water channels of the αTIP family (Melroy and Herman, 1991; Inoue et al., 1995b). These proteins appear to be necessary to promote water transport across the vacuolar membranes during seed desiccation and imbibition.
Late embryogenesis–abundant proteins have been implicated in the acquisition of desiccation tolerance of maturing seed (Ingram and Bartels, 1996). They are hydrophilic proteins and are expressed transiently during seed maturation. MP73 and late embryogenesis–abundant proteins might share a physiological role in desiccation tolerance in seed, despite their low sequence homology.
MP73 is a novel membrane protein, but a limited region of 59 amino acids from Asn-259 to Val-317 shows high homology with a part of pumpkin MP32 (49%) and proteins with unknown function of carrot (GenBank accession number U47078; 53%) and Arabidopsis (GenBank accession number Z48554; 53%). In contrast, P24 exhibits very low homology with the corresponding N-terminal domains of MP27 (23%), the carrot protein (23%), and the Arabidopsis protein (30%). Further analysis with a knockout mutant of Arabidopsis will help to clarify the function of such peripheral membrane proteins on the membranes.
METHODS
Plant Materials
Seed of pumpkin (Cucurbita maxima cv Kurokawa Amakuri Nankin) were purchased from Aisan Shubyo Seed Co. (Nagoya, Japan), and seed of castor bean (Ricinus communis) were a gift from Ito Oil Co. (Yokkaichi, Japan). The seed were used directly for isolation of protein storage vacuoles. To obtain germinating seed and growing seedlings, the seed were soaked in water for 16 hr and then grown in the dark at 25°C for up to 6 days for pumpkin. To obtain immature seed, pumpkin seed were grown at the experimental farm of the National Institute for Basic Biology during the summer season. Cotyledons from maturing seed were freshly harvested at 18 to 38 days after anthesis to use for experimentation. Castor bean seed also were grown in a greenhouse, and endosperm from maturing seed were freshly harvested at 20 days after anthesis. Whole homogenates of pumpkin seed at various stages of seed development and germination were subjected to SDS-PAGE and subsequent immunoblot analysis, as were the homogenates of roots, stems, and mature leaves, as described previously (Hatano et al., 1997).
Isolation of Protein Storage Vacuoles and Preparation of Protein Storage Vacuole Membranes
Protein storage vacuoles were isolated from dry seed of either pumpkin or castor bean by a nonaqueous method, as described previously (Hara-Nishimura et al., 1982). The isolated protein storage vacuoles were inspected with a microscope (Olympus BH; Olympus, Tokyo, Japan) to show that they were not contaminated with other cellular components.
The vacuolar membranes were prepared from the isolated protein storage vacuoles of pumpkin and castor bean, as described previously (Mettler and Beevers, 1979; Inoue et al., 1995b). The crude membranes were suspended with 10 mM Tris-Mes, pH 6.4, and applied to a linear density gradient of 15 to 60% (w/w) sucrose in 10 mM Tris-Mes, pH 6.4. After centrifugation at 80,000g at 4°C for 5 hr in an SW28 rotor (Beckman Instruments, Tokyo, Japan), each fraction (0.6 mL) was used to determine both the sucrose concentration with a refractometer and the protein content with a Protein Assay Kit (Bio-Rad, Hercules, CA). An aliquot of each fraction was subjected to SDS-PAGE and subsequent staining with Coomassie Brilliant Blue R 250.
To detect integral membrane proteins, the membranes of the isolated protein storage vacuoles were extracted with alkali as described previously (Mäder and Chrispeels, 1984). A solution of NaOH was added to the isolated membrane fraction to give a final concentration of 10 mM. The sample was kept at 4°C for 16 hr and then centrifuged at 100,000g for 1 hr. The pellet was suspended in distilled water, and the remaining proteins in the membranes were subjected to SDS-PAGE.
Isolation of Precursor-Accumulating Vesicles
Precursor-accumulating (PAC) vesicles were isolated from pumpkin cotyledons at the middle stage of seed maturation as described previously (Hara-Nishimura et al., 1998b).
Determination of Both N-Terminal and Internal Amino Acid Sequences
The membrane proteins of protein storage vacuoles of pumpkin were subjected to SDS-PAGE and then transferred electrophoretically to a polyvinylidene fluoride (PVDF) membrane (0.22 μm; Nihon Millipore, Tokyo, Japan). After staining proteins on the blot with Coomassie Brilliant Blue R 250, the band corresponding to MP73 was cut from the blot and subjected to automatic Edman degradation on a peptide sequencer (model 492; Applied Biosystems, Foster City, CA).
To determine the internal sequences, the band corresponding to MP73 on an SDS gel with Coomassie Brilliant Blue R 250 was subjected to in-gel digestion as described by Hellman et al. (1995). The gel piece was incubated with 1 μg of trypsin at 37°C for 16 hr. The trypsin digest solution of MP73 was filtered through a membrane (0.45 μm; Nihon Millipore) and then applied to a reverse phase column (μRPC C2/C18 SC 2.1/10) on a SMART system (Amersham Pharmacia Biotech) with monitoring absorbance of 214 nm. The digests were eluted by increasing the concentration of acetonitrile to 80% (v/v). Elution was performed with a gradient from 0.065% trifluoroacetic acid in distilled water to 0.05% trifluoroacetic acid in acetonitrile. N-terminal sequences of the separated peptides were determined as described above.
Isolation of MP73 cDNAs and Determination of Nucleotide Sequences
A cDNA library in pBluescript II SK+ (Stratagene, La Jolla, CA) was constructed with the poly(A) RNA from maturing pumpkin cotyledons, as described previously (Hara-Nishimura et al., 1993b). Two degenerate primers, 5′-ATGGA(C/T)TT(C/T)AA(A/G)AA(A/G)AA(A/G)A-A(A/G)AA(A/G)AA-3′ and 5′-AC(A/G/T)AT(A/C/G/T)CC(A/G/T)AT(C/T)T- CCCA(A/C/G/T)GC-3′, were designed on the basis of the N-terminal and internal amino acid sequences of MP73 (MDFKKKKKK and AWEIGIV, respectively) and were synthesized on a DNA synthesizer (model 394; Applied Biosystems). Polymerase chain reaction was performed using a set of the primers and the cDNA library as a template. The 221-bp amplified DNA was labeled with α-32P-dCTP and a Megaprime DNA labeling system (Amersham Pharmacia Biotech). The cDNA library was screened by colony hybridization using the 32P-labeled DNA as a probe.
The two isolated cDNAs of 1061 and 1012 bp did not cover the entire sequence of MP73. Subsequently, we amplified DNAs covering the 5′ and 3′ regions of MP73 cDNA using a 5′-Full RACE (rapid amplification of cDNA ends) Core Set and a 3′-Full RACE Core Set (Takara, Tokyo, Japan), respectively. The 5′-RACE gave a 202-bp DNA that contained an initiation codon. The 3′-RACE gave a 978-bp DNA that contained a poly(A) tail.
DNA sequencing was performed with a DNA sequencer (model 377; Applied Biosystems) and 21M13 forward and M13 reverse fluorescent primers according to the manufacturer's directions. The nucleotide and deduced amino acid sequences were analyzed with DNA analytical software (GeneWorks; IntelliGenetics, Mountain View, CA). The hydropathy profile of the amino acid sequence was computed by application of the algorithms of Kyte and Doolittle (1982) with a window size of 10 residues. The homology plot was computed with the PAM-250 algorithm (Pearson and Lipman, 1988), and the secondary structure was computed according to the Chou and Fasman model (Chou and Fasman, 1974).
Specific Antibodies
The purified membranes of protein storage vacuoles of pumpkin were subjected to SDS-PAGE and subsequent staining with Coomassie Brilliant Blue R 250. The protein band corresponding to MP73 was cut from the gel and emulsed with complete Freund's adjuvant. The homogenate was injected subcutaneously into a rabbit. After 3 weeks, two booster injections of the protein with incomplete adjuvant were given at 7-day intervals. One week after the booster injections, blood was drawn and the antiserum was prepared. We confirmed the specificity of the antibodies by immunoblot analysis with a crude extract from pumpkin seed. Specific antibodies against either complex glycan (Hara-Nishimura et al., 1998b) or castor bean MP73 homolog (Inoue et al., 1995a) were used for the experiments.
Immunoblot Analysis
The separated proteins on the gels were transferred electrophoretically to a GVHP membrane (0.22 μm; Nihon Millipore). The membrane blot was incubated for 1 hr with either anti-MP73 antibodies (diluted 20,000-fold) or anti–complex glycan antibodies (diluted 1000-fold) in a solution of 50 mM Tris-HCl, pH 7.5, 0.15 M NaCl, 0.05% (v/v) Tween 20, and 3% (w/v) skim milk. Horseradish peroxidase–conjugated donkey antibodies against rabbit IgG (diluted 10,000-fold; Amersham Pharmacia Biotech) were used as second antibodies. MP73 was immunologically detected with an enhanced chemiluminescence kit (an ECL system; Amersham Pharmacia Biotech).
Electron Microscopic Analysis by High Pressure Freezing, Freeze Substitution, and Immunogold Labeling
For high pressure freezing and freeze substitution, both the maturing pumpkin cotyledons and the isolated PAC vesicles were frozen with a high pressure freezing machine (model HPM010; Bal-Tec, Balzers, Liechtenstein), as described by Craig and Staehelin (1998). The subsequent procedures were essentially the same as those described previously (Hayashi and Ueda, 1987). The frozen samples were treated with acetone for 2 days at −85°C and then embedded in Spurr's resin.
Immunogold labeling procedures were essentially the same as those described previously (Nishimura et al., 1993; Hara-Nishimura et al., 1998b) except for the use of the appropriate antibodies raised against MP73. Ultrathin sections (80 nm in thickness) were mounted on Formvar-coated nickel grids. The sections were treated with blocking solution (0.3% BSA in PBS) for 30 min and then incubated with either anti-MP73 antibodies or preimmune serum (diluted 1:10,000) for 10 hr at 4°C. After washing with PBS, sections were incubated for 1 hr at room temperature with a solution of protein A–gold (15 nm; Amersham Pharmacia Biotech) that had been diluted 1:30 in the blocking solution. After washing with 0.1% BSA and distilled water, sections were stained with a solution of 4% uranyl acetate and lead citrate. After staining, all sections were examined with a transmission electron microscope (model 1200EX; JEOL) at 80 kV. No gold particles were detected when preimmune serum was used (data not shown).
Procedures for ultrastructural studies of chemically fixed samples, the maturing seed, dry seed, and 4-day-old seed of pumpkin were essentially the same as those described previously (Hara-Nishimura et al., 1993a) except that the material was postfixed with 1% osmium tetroxide in 0.1 M cacodylate buffer, pH 7.4, at 4°C for 2 hr.
In Vivo Pulse-Chase Labeling
One pair of the endosperms of maturing castor bean seed (20 days after anthesis) was pulse labeled with 35S-methionine (1.9 MBq/half endosperm, 37.8 TBq/mmol; ICN Biomedicals Inc., Costa Mesa, CA) for 30 min as described previously (Hara-Nishimura et al., 1985). The tissues were rinsed three times with a 10-mM solution of unlabeled methionine and placed on filter paper moistened with water to chase the label. After a chase period, the labeled endosperms were homogenized by chopping with a razor blade in a solution of 150 mM Tricine-KOH, pH 6.5, 1 mM EDTA, and 13% (w/w) sucrose. The homogenate was filtered through one layer of cheesecloth. The filtrate was loaded on a stepwise sucrose gradient consisting of 1.3 mL of 55% (w/w) sucrose, 1.3 mL of 35% (w/w) sucrose, and 1.3 mL of 20% (w/w) sucrose. The gradient solution was then centrifuged for 1 hr in an ultracentrifuge (Beckman) using an SW65Ti rotor at 60,000g and 4°C, as described by Hara-Nishimura and Nishimura (1987). Endoplasmic reticulum (ER) was recovered in the interface between 20% (w/w) and 35% (w/w) sucrose, the PAC vesicles were recovered in the interface between 35% (w/w) and 55% (w/w) sucrose, the crystalloids were recovered in the pellet fraction, and the membranes were recovered in the 13% (w/w) sucrose fraction and in the interface between 13% (w/w) and 20% (w/w) sucrose. Each fraction of ER, PAC vesicles, crystalloids, and vacuolar membranes was immunoprecipitated with anti–castor bean MP73 homolog antibodies and protein A–Sepharose (Amersham Pharmacia Biotech). The precipitate was subjected to SDS-PAGE and fluorography.
To examine the effect of tunicamycin on the glycosylation of proMP73, maturing castor bean seed were incubated in the absence or presence of 3 or 30 μg/mL tunicamycin for 3 hr before a 4-hr pulse labeling, as described above. The labeled proteins were immunoprecipitated with anti–castor bean MP73 homolog antibodies and subjected to SDS-PAGE and fluorography. For subcellular fractionation, maturing castor bean seed were incubated in the absence or presence of 30 μg/mL tunicamycin for 3 hr before a 4-hr pulse labeling. The tissues were subjected to subcellular fractionation as described above. Each fraction was immunoprecipitated with anti–castor bean MP73 homolog antibodies and protein A–Sepharose. The precipitate was subjected to SDS-PAGE and fluorography.
In Vitro Translation
Poly(A) RNA prepared from maturing pumpkin cotyledons was translated in vitro in a reticulocyte lysate cell-free system in the presence of 35S-methionine (ICN Biomedicals), as described previously (Hara-Nishimura et al., 1985, 1993a). The labeled precursor was immunoprecipitated with anti–castor bean MP73 homolog and protein A–Sepharose (Amersham Pharmacia Biotech), and it was subjected to SDS-PAGE and subsequent fluorography.
In Vitro Conversion of ProMP73 to MP73
The PAC vesicles were prepared from maturing castor bean seed that had been pulse labeled with 35S-methionine for 40 min as described above and then frozen and thawed three times. The labeled proMP73 in the fraction was incubated with a soluble extract from the isolated protein storage vacuoles for up to 16 hr at 30°C and then immunoprecipitated with anti–castor bean MP73 homolog antibodies. The immunoprecipitates were subjected to SDS-PAGE followed by fluorography.
Treatment with N-Glycosidase F and Endoglycosidase H
The membranes of protein storage vacuoles prepared from dry pumpkin seed were incubated with either 1 unit of N-glycosidase F (Boehringer Mannheim) in 20 mM Tris-HCl, pH 7.5, at 37°C for 18 hr or 0.05 unit of endoglycosidase H (Boehringer Mannheim) in 20 mM Na-phosphate, pH 5.0, at 37°C for 18 hr.
Cross-Linking with Dithiobissuccinimidylpropionate
The membranes of protein storage vacuoles prepared from dry pumpkin seed were suspended with 10 mM Na-phosphate, pH 7.2, and treated with a cross-linker reagent, dithiobissuccinimidylpropionate (DSP), as described by Koumoto et al. (1999). The samples were subjected to SDS-PAGE under nonreducing and reducing conditions with 2-mercaptoethanol, followed by immunoblot analysis with anti-MP73 antibodies.
Acknowledgments
We thank Chiyeko Namba for growing castor bean and pumpkin plants. We are grateful to Yumiko Makino for helpful support with peptide sequencing. We also thank Yuka Takeuchi and Maki Kondo for helpful support with electron microscopy. This work was supported by Grants-in-Aid for the Human Frontier Science Program (RG0018/2000-M 103) and the Research for the Future Program (JSPS-RFTF96L60407) and by the Japan Society for the Promotion of Science and for Scientific Research (Grant No. 12304049) from the Ministry of Education, Science, Sports, and Culture of Japan.
References
- Chou, P.Y., and Fasman, G.D. (1974). Prediction of protein conformation. Biochemistry 13, 222–245. [DOI] [PubMed] [Google Scholar]
- Craig, S., and Staehelin, L.A. (1998). High pressure freezing of intact plant tissues: Evaluation and characterization of novel features of the endoplasmic reticulum and associated membrane systems. Eur. J. Cell Biol. 46, 80–93. [PubMed] [Google Scholar]
- Hara-Nishimura, I., and Nishimura, M. (1987). Proglobulin processing enzyme in vacuoles isolated from developing pumpkin cotyledons. Plant Physiol. 85, 440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Nishimura, I., Nishimura, M., Matsubara, H., and Akazawa, T. (1982). Suborganellar localization of proteinase catalyzing the limited hydrolysis of pumpkin globulin. Plant Physiol. 70, 699–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Nishimura, I., Nishimura, M., and Akazawa, T. (1985). Biosynthesis and intracellular transport of 11S globulin in developing pumpkin cotyledons. Plant Physiol. 77, 747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Nishimura, I., Hayashi, M., Nishimura, M., and Akazawa, T. (1987). Biogenesis of protein bodies by budding from vacuoles in developing pumpkin cotyledons. Protoplasma 136, 49–55. [Google Scholar]
- Hara-Nishimura, I., Inoue, K., and Nishimura, M. (1991. a). A unique vacuolar processing enzyme responsible for conversion of several proprotein precursors into the mature forms. FEBS Lett. 294, 89–93. [DOI] [PubMed] [Google Scholar]
- Hara-Nishimura, I., Inoue, K., and Nishimura, M. (1991b). Reversible transformation between vacuoles and protein bodies in pumpkin cotyledons. In Molecular Approaches to Compartmentation and Metabolic Regulation, A. Huang and L. Taiz, eds (American Society of Plant Physiologists, Baltimore, MD), pp. 245–246.
- Hara-Nishimura, I., Takeuchi, Y., Inoue, K., and Nishimura, M. (1993. a). Vesicle transport and processing of the precursor to 2S albumin in pumpkin. Plant J. 4, 793–800. [DOI] [PubMed] [Google Scholar]
- Hara-Nishimura, I., Takeuchi, Y., and Nishimura, M. (1993. b). Molecular characterization of a vacuolar processing enzyme related to a putative cysteine proteinase of Schistosoma mansoni. Plant Cell 5, 1651–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Nishimura, I., Shimada, T., Hiraiwa, N., and Nishimura, M. (1995). Vacuolar processing enzyme responsible for maturation of seed proteins. J. Plant Physiol. 145, 632–640. [Google Scholar]
- Hara-Nishimura, I., Kinoshita, T., Hiraiwa, N., and Nishimura, M. (1998. a). Vacuolar processing enzymes in protein-storage vacuoles and lytic vacuoles. J. Plant Physiol. 152, 668–674. [Google Scholar]
- Hara-Nishimura, I., Shimada, T., Hatano, K., Takeuchi, Y., and Nishimura, M. (1998. b). Transport of storage proteins to protein-storage vacuoles is mediated by large precursor-accumulating vesicles. Plant Cell 10, 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano, K., Shimada, T., Hiraiwa, N., Nishimura, M., and Hara-Nishimura, I. (1997). A rapid increase in the level of binding protein (BiP) is accompanied by synthesis and degradation of storage proteins in pumpkin cotyledons. Plant Cell Physiol. 38, 344–351. [DOI] [PubMed] [Google Scholar]
- Hayashi, Y., and Ueda, K. (1987). Localization of mannose, N-acetylglucosamine and galactose in the Golgi apparatus, plasma membranes and cell walls of Scenedesmus acuminatus. Plant Cell Physiol. 28, 1357–1362. [Google Scholar]
- Hellman, U., Wernstedt, C., Góñez, J., and Heldin, C.-H. (1995). Improvement of an “in-gel” digestion procedure for the micropreparation of internal protein fragments for amino acid sequencing. Anal. Biochem. 224, 451–455. [DOI] [PubMed] [Google Scholar]
- Hinz, G., Hilmer, S., Baumer, M., and Hohl, I. (1999). Vacuolar storage proteins and the putative vacuolar sorting receptor BP-80 exit the Golgi apparatus of developing pea cotyledons in different transport vesicles. Plant Cell 11, 1509–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraiwa, N., Takeuchi, Y., Nishimura, M., and Hara-Nishimura, I. (1993). A vacuolar processing enzyme in maturing and germinating seeds: Its distribution and associated changes during development. Plant Cell Physiol. 34, 1197–1204. [Google Scholar]
- Hiraiwa, N., Kondo, M., Nishimura, M., and Hara-Nishimura, I. (1997. a). An aspartic proteinase is involved in the maturation of storage proteins in concert with the vacuolar processing enzyme. Eur. J. Biochem. 246, 133–141. [DOI] [PubMed] [Google Scholar]
- Hiraiwa, N., Nishimura, M., and Hara-Nishimura, I. (1997. b). Expression and activation of the vacuolar processing enzyme in Saccharomyces cerevisiae. Plant J. 12, 819–829. [DOI] [PubMed] [Google Scholar]
- Improta-Brears, T., Ghosh, S., and Bell, R.M. (1999). Mutational analysis of Raf-1 cysteine rich domain: Requirement for a cluster of basic amino acids for interaction with phosphatidylserine. Mol. Cell. Biochem. 198, 171–178. [DOI] [PubMed] [Google Scholar]
- Ingram, J., and Bartels, D. (1996). The molecular basis of dehydration tolerance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 377–403. [DOI] [PubMed] [Google Scholar]
- Inoue, K., Motozaki, A., Takeuchi, Y., Nishimura, M., and Hara-Nishimura, I. (1995. a). Molecular characterization of proteins in protein-body membranes that disappear most rapidly during transformation of protein bodies into vacuoles. Plant J. 7, 235–243. [DOI] [PubMed] [Google Scholar]
- Inoue, K., Takeuchi, Y., Nishimura, M., and Hara-Nishimura, I. (1995. b). Characterization of two integral membrane proteins located in the protein bodies of pumpkin seeds. Plant Mol. Biol. 28, 1089–1101. [DOI] [PubMed] [Google Scholar]
- Jiang, L., and Rogers, J.C. (1998). Integral membrane protein sorting to vacuoles in plant cells: Evidence for two pathways. J. Cell Biol. 143, 1183–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, K.D., Herman, E.M., and Chrispeels, M.J. (1989). An abundant, highly conserved tonoplast protein in seeds. Plant Physiol. 91, 1006–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumoto, Y., Shimada, T., Kondo, M., Hara-Nishimura, I., and Nishimura, M. (1999). Chloroplast Cpn20 forms a tetrameric structure in Arabidopsis thaliana. Plant J. 17, 467–477. [DOI] [PubMed] [Google Scholar]
- Kyte, J., and Doolittle, R.F. (1982). A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157, 105–132. [DOI] [PubMed] [Google Scholar]
- Mäder, M., and Chrispeels, M.J. (1984). Synthesis of an integral membrane protein of the protein-body membrane in Phaseolus vulgaris cotyledons. Planta 160, 330–340. [DOI] [PubMed] [Google Scholar]
- Maeshima, M., Hara-Nishimura, I., Takeuchi, Y., and Nishimura, M. (1994). Accumulation of vacuolar H+-pyrophosphatase and H+-ATPase during reformation of the central vacuole in germinating pumpkin seeds. Plant Physiol. 106, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melroy, D.L., and Herman, E.M. (1991). TIP, an integral membrane protein of the protein-storage vacuoles of the soybean cotyledon, undergoes developmentally regulated membrane accumulation and removal. Planta 184, 113–122. [DOI] [PubMed] [Google Scholar]
- Mettler, I.J., and Beevers, H. (1979). Isolation and characterization of the protein body membrane of castor beans. Plant Physiol. 64, 506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura, M., Takeuchi, Y., De Bellis, L., and Hara-Nishimura, I. (1993). Leaf peroxisomes are directly transformed to glyoxysomes during senescence of pumpkin cotyledons. Protoplasma 175, 131–137. [Google Scholar]
- Pearson, W.R., and Lipman, D.J. (1988). Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 85, 2444–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada, T., Hiraiwa, N., Nishimura, M., and Hara-Nishimura, I. (1994). Vacuolar processing enzyme of soybean that converts proprotein to the corresponding mature forms. Plant Cell Physiol. 35, 713–718. [DOI] [PubMed] [Google Scholar]
- Strzalka, K., Hara-Nishimura, I., and Nishimura, M. (1995). Changes in physical properties of vacuolar membrane during transformation of protein bodies into vacuoles in germinating pumpkin seeds. Biochim. Biophys. Acta 1239, 103–110. [DOI] [PubMed] [Google Scholar]
- Wada, I., Rindress, D., Cameron, P.H., Ou, W.-J., Doherty, J.J., II, Louvard, D., Bell, A.W., Dignard, D., Thomas, D.Y. and Bergeron, J.J.M. (1991). SSRa and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J. Biol. Chem. 266, 19599–19610. [PubMed] [Google Scholar]
- Yamada, K., Shimada, T., Kondo, M., Nishimura, M., and Hara-Nishimura, I. (1999). Multiple functional proteins are produced by cleaving Asn-Gln bonds of a single precursor by vacuolar processing enzyme. J. Biol. Chem. 274, 2563–2570. [DOI] [PubMed] [Google Scholar]