Abstract
Sitosterolemia (MIM 210250) is a rare genetic disorder caused by disruption of the normal mechanisms that regulate dietary cholesterol absorption and prevent the accumulation of noncholesterol sterols. As a result of this defect, affected individuals accumulate high concentrations of plant sterols in plasma and tissues. They present clinically with tendon or tuberoeruptive xanthomas, premature coronary artery disease, and/or hemolytic anemia. Two genes, ABCG5 and ABCG8, compose the STSL locus, and complete mutation in either, but not both, results in disease. The expression of these genes is confined to the intestine and liver. They are thought to function as sterol efflux pumps. It is not clear which organ, liver or intestine, is of greater importance in maintaining sterol balance with respect to noncholesterol sterols. We report here a case of a patient who presented with “chronic active liver disease” and was found to have sitosterolemia by chance and subsequently underwent orthotopic liver transplantation. Following transplantation, the grossly elevated pretransplantation serum plant sterol levels decreased to values only slightly higher than those of the patient’s heterozygous father. This case highlights 2 important features: (1) The liver functions as the predominant organ for maintaining noncholesterol sterol balance (because the intestinal defect was not altered), and (2) a new clinical feature of undiagnosed sitosterolemia may be “idiopathic” liver disease. Because the diagnosis of sitosterolemia is based on specialized plasma analyses, we would propose that some consideration to this diagnosis should be given in appropriate cases.
Schoenheimer observed more than 70 years ago that herbivores eat plants (which do not contain significant cholesterol but are high in plant sterols), yet their bodies only contain cholesterol and not plant sterols. 1 It is presumed that mammals have evolved exquisite mechanisms to exclude these “noncholesterol” sterols from the body. The basis for this important physiology remained a mystery, until the seminal discovery of 2 sisters who presented with tendon xanthomas, arthralgias, and near normal plasma cholesterol but were found to have elevated plant sterol levels in the plasma.2 Bhattacharyya and Connor2 surmised that this disease state must be caused by a genetic defect resulting in the disruption of the normal physiologic pathway that restricts the entry and/or rapid elimination of these sterols from the body. Subsequent reports have shown that sitosterolemia is an autosomal recessive inherited disorder of sterol metabolism.3 Clinically, the disease is characterized by markedly increased plasma plant sterol levels (predominately sitosterol) and can be associated with tendon or tuberoeruptive xanthomata, chronic hemolysis with splenomegaly, arthritis/arthralgias, and premature atherosclerosis, with fatal or nonfatal cardiovascular events.4-6 Hepatic transaminase levels (aspartyl aminotransferase [AST] and alanine aminotransferase [ALT]) can be mildly elevated, but no actual disturbances in liver function or chronic liver disease have been ascribed to this disease. A body of evidence has accumulated that suggests that the liver, via biliary excretion, and the intestine, by restricting entry, play a critical role in regulating both cholesterol and noncholesterol sterol balance. Studies of sitosterolemic individuals suggest that they hyperabsorb all dietary sterols and have an impaired ability to secrete biliary sterols.5-7 Following the mapping of the STSL locus on human chromosome 2p21, it was found that the genetic defect that causes disease is a mutation in one, but not both, of the 2 genes composing this locus.8-10 The STSL locus is composed of 2 genes, ABCG5 and ABCG8, oriented in a head-to-head configuration. These genes are members of the larger ATP-binding cassette gene family. To date, no phenotypic difference has been described to differentiate a mutation in ABCG5 or ABCG8. The genetic organization of the 2 genes suggests that the proteins encoded by ABCG5 and ABCG8 may function as obligate heterodimers. Studies in animal models and tissue culture show that ABCG5 and ABCG8 are apically expressed in the intestine and the hepatobiliary system.11-15 Additionally, data from animal models suggest that these proteins can heterodimerize or potentially homodimerize and are extremely important in biliary sterol secretion.15,16 What is not clear is whether the major organ important in this physiologic process to regulate dietary noncholesterols is the hepatobiliary system or the intestine.
The case described in this paper is of a patient who presented with chronic hepatitis and cirrhosis and who worsened during a 7-year period of follow-up, ultimately leading to liver transplantation. The diagnosis of sitosterolemia was made by a serendipitous examination of his plasma during the course of his disease. Interestingly, he did not have any features that are classical for sitosterolemia (xanthomas, ischemic heart disease, or others), although he did have mild hemolysis. Mutational analyses confirmed the diagnosis of sitosterolemia. Following transplantation, his sitosterolemia unexpectedly resolved. His plasma plant sterol levels fell to the same levels seen in unaffected individuals. This unforeseen correction of his sitosterolemia suggests that correction of the genetic defect only in the liver is sufficient to reverse this disorder. Because the transplantation liver most likely restored biliary secretion of sterols but could not affect the genetic defect at the intestinal level, this case report suggests that the primary organ involved in determining the plasma steady state of noncholesterol sterol levels is the liver. Additionally, screening for sitosterolemia in patients with cirrhosis from unknown causes should be considered as part of the differential diagnosis.
Materials and Methods
Clinical
The patient had reportedly been healthy for the first 18 years of his life. At 19 years of age, he started to experience fevers, joint pains, and swelling in the knees and ankles. Additionally, he experienced weight loss, pneumonias, bouts of diarrhea, and skin rash. Physical examination revealed that he was jaundiced with appreciable splenomegaly, but no xanthomata were seen. Routine hematologic testing showed thrombocytopenia, leucopenia, and mild hemolysis. Total and direct bilirubin levels were approximately 3 times above the upper limits of normal. Liver transaminase levels, AST, ALT, and alkaline phosphatase were found to be 2-3 times above the upper limits of normal. Additional laboratory testing showed that he had hypergammaglobulinemia (high IgG and IgM), positive antinuclear antibodies, positive smooth muscle cell antibodies, and reduced thromboplastin time (TT-SPA). Serologies for chronic viral hepatitis viruses (hepatitis B and C) were undetectable.
The patient then underwent a liver biopsy that revealed chronic active hepatitis with signs of cirrhosis. Subsequent endoscopic gastroduodenoscopy showed the presence of esophageal varices, and endoscopic retrograde cholangiopancreatography suggested a diagnosis compatible with sclerosing cholangitis.
It was initially assumed that the patient had “autoimmune” chronic hepatitis, and this was the reason why he developed cirrhosis. However, 3 years after the first clinical signs of disease, a diagnosis of sitosterolemia was made, based on the findings of elevated serum plant sterol levels by gas-liquid chromatography (GLC). Owing to further worsening of his liver disease, orthotopic liver transplantation was performed in 1999. The study protocol was approved by the Institutional Review Board at the University of Helsinki, and informed consent was obtained from all subjects prior to entering the study.
Sterol Measurements, Mutational Analyses, and Immunohistochemistry
Hematologic, immunologic, and liver function studies were performed using routine clinical laboratory methods. Serum total and high-density lipoprotein (HDL) cholesterol and triglycerides were measured with commercial kits according to manufacturers’ instructions (Boehringer Diagnostica and Wako Chemicals, Germany). Very-low-density lipoprotein (VLDL), intermediate density lipoprotein (IDL), low-density lipoprotein (LDL), and HDL were separated with ultracentrifugation, and lipids were analyzed using the same commercial kits. Cholesterol and noncholesterol sterols, extracted as nonsaponifiable lipid fractions of serum, lipoprotein, or fecal samples were measured by GLC using a 50-meter-long SE-30 capillary column (Ultra 2 column, Hewlett-Packard, Palo Alto, CA). The internal standard used for normalization of extraction efficiency was 5α-cholestane, as previously described.17
Mutational analyses of ABCG5 and ABCG8 were performed as previously described.18 The nucleotide and peptide numbering is also as previously reported. Briefly, all exons and boundary intronic sequences were amplified, and both strands of the polymerase chain reaction (PCR) products were sequenced by an automated capillary sequencer (Beckman CEQ8000, Palo Alto, CA) and compared with wild-type sequences.
Immunohistochemistry was performed on “normal” liver and the patient’s liver as previously described.14 For colocalization, anti-human MRP2 was used to show apical expression.
Results
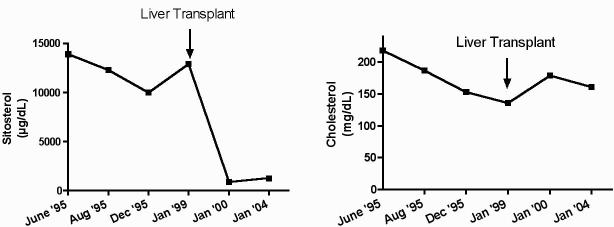
Figure 1 shows the plasma sitosterol and cholesterol levels in our patient over time. At the time of diagnosis in 1995, his untreated plasma sitosterol levels were 0.36 mmol/L (∼14 mg/dL) (normal <0.01 mmol/L, <0.5 mg/dL), with a relatively normal cholesterol level of ∼5.17 mmol/dL (∼200 mg/dL) and a total sterol level of ∼5.7 mmol/L (∼220 mg/dL). Although therapy with cholestyramine was instituted (standard therapy at that time for sitosterolemia), he had a minimal response. The addition of stanol ester margarine consumption led to a further lowering of his plant sterol levels, without a significant increase in plasma sitostanol levels. Table 1 summarizes the proband’s pre- and post-transplantation cholesterol and plant sterol levels. Note that his lathosterol level, a marker of endogenous cholesterol synthesis, was low. This is in keeping with previous reports of reduced cholesterol synthesis in affected individuals.5-7 Pretransplantation serum plant sterol levels were up to 50 times higher than that of controls and fell significantly following the transplantation. In keeping with other case reports of sitosterolemic patients, the cholesterol levels in our proband were not elevated and were actually lower than that of healthy controls.
Figure 1.
Plasma sitosterol and cholesterol levels of the proband. The sitosterol level falls somewhat after treatment with cholestyramine, but, following liver transplantation, (A) sitosterol levels decline significantly. Cholesterol levels trend down, (B) likely because of worsening liver failure, but remain relatively stable throughout follow up.
Table 1.
Serum Sterols in a Patient With Sitosterolemia and Cirrhosis, the Proband's Family, and Healthy Controls
| Time/source | Cholesterol (mmol/L) | Sitosterol (μmol/L) | Campesterol (μmol/L) | Squalene (μmol/L) | Lathosterol (μmol/L) |
|---|---|---|---|---|---|
| Proband preoperation | 3.5 | 310.9 | 215.9 | 4.6 | 1.9 |
| Proband postoperation | 4.6 | 21.6 | 37 | 1.7 | 5.4 |
| VLDL preoperation | 0.3 | 17.5 | 15.5 | 0.7 | 0.3 |
| VLDL postoperation | 0.3 | 0.8 | 2 | 0.5 | 0.5 |
| LDL preoperation | 1.7 | 156.4 | 108.3 | 1.1 | 0.9 |
| LDL postoperation | 2.5 | 11.3 | 20.3 | 0.5 | 3.1 |
| HDL preoperation | 0.6 | 56.5 | 37.2 | 0.6 | 0.5 |
| HDL postoperation | 1.2 | 6.1 | 9.5 | 0.4 | 1.2 |
| Father | 7 | 15.5 | 34.7 | 4.7 | 6.7 |
| Mother | 5.8 | 7.4 | 13 | 4.3 | 8 |
| Brother | 3.9 | 5 | 7.9 | 2.6 | 7.6 |
| Healthy controls | 6.5 | 5.8 | 9 | — | 3.7 |
NOTE. Conversion factors: Cholesterol (mmol/L), multiply by 38.7 for mg/dL; sitosterol (μmol/L), multiply by 41.5 for μg/dL; campesterol (μmol/L), multiply by 40 for μg/dL; squalene (μmol/L), multiply by 41.1 for μg/dL; lathosterol (μmol/L), multiply by 38.6 for μg/dL.
Sterol balance studies were performed on the proband by techniques previously described.17 Cholesterol absorption was found to be low at 33%. However, bile acid synthesis rate (182 mg/day) and cholesterol production rate (2.86 mmol/day, 1107 mg/day) were found to be normal. Without the patient being on antibiotics, intestinal bacterial action was lacking because no secondary product of cholesterol or bile acids was seen.
Analyses of the proband’s family members showed serum plant sterol and cholesterol that were increased only modestly. The proband’s father showed the highest values, and his brother had similar values to those of healthy controls (Table 1).
Genetic analyses of DNA from the proband showed that he carried the following 2 mutations: W361X (G1173A) and E423D (G1359T) in ABCG8. No mutations in ABCG5 were detected. Although the W361X mutation has been previously reported, the E423D has not been described and is novel.18 In more than 100 normal alleles examined, E423D was not detected. The latter mutation maps to the putative first transmembrane domain, and one would predict that the glutamine to aspartic acid amino acid substitution would not alter the topology or folding of this protein because this is a conserved change in amino acid charge and size.
Surprisingly, the ABCG8 protein was detected by immunohistochemistry using an anti-ABCG8 peptide antibody as previously described.14 Colocalization studies were performed in the control liver to confirm that ABCG8 colocalizes with a known apical protein, MRP2 (Figure 2A). When the sitosterolemic liver was stained with the same anti-ABCG8 antibody, a similar apical distribution is seen as compared with control “healthy” liver (Figure 2B). It must be noted that the quality of the sitosterolemic liver was very poor secondary to it being cirrhotic. Additionally, the anti-ABCG5 antibody was not used because the genetic defect was found to be in ABCG8; therefore, it is presumed that ABCG5 is normal.
Figure 2.
Immunohistochemistry of normal human liver and the proband’s liver. (A) Colocalization of ABCG8 and MRP2 indicating an apical expression of ABCG8 in the normal human liver. (B) The proband’s liver shows the same apical distribution, despite having a mutation in the gene.
Most of the hematologic, immunologic, and liver function abnormalities seen pretransplantation normalized after liver transplantation. Posttransplantation, the patient experienced 2 episodes of acute rejection treated with large dose methylprednisolone, followed by a change in his cyclosporine-based immunosuppression to tacrolimus. Furthermore, the patient had bouts of diarrhea leading to colonoscopy that revealed findings consistent with features of ulcerative colitis.
Discussion
The defect in sitosterolemia is expressed at 2 levels.19,20 In the intestine, there is a failure to discriminate between luminal/dietary cholesterol and noncholesterol sterols, allowing both to enter. In the liver, there is an inability to excrete sterols, either cholesterol or noncholesterol sterols into the bile. We report a clinical case that has several novel findings. First, no case with hepatitis progressing to liver failure has ever been reported to be associated with (or possibly caused by) sitosterolemia. Cholestatic liver disease has been reported to develop sometimes during therapy with parenteral nutrition using different fat preparations containing plant sterols, almost all of these cases in children.21 Increased cholestanol and plant sterol values have been reported in patients with primary biliary cirrhosis,22 but the values, especially of plant sterols, are markedly lower than those seen in even the mildest cases of sitosterolemia. To date, disorders of the liver or intestine do not seem to cause an elevation in plasma plant sterols. Cholestatic liver damage has also not been reported previously in known cases of sitosterolemia.4 Given the rarity of sitosterolemia and its relative unawareness by most clinicians, perhaps this report may help in increasing this awareness. Because, by definition, idiopathic cirrhosis has no known causes, and the diagnosis of sitosterolemia (essentially a biochemical disorder) can only be made by GLC or high-performance liquid chromatography (HPLC) analyses of plasma, it is possible that cases of chronic active hepatitis and/or cirrhosis may contain a subset of patients who may have sitosterolemia. The drug ezetimibe has been approved for the therapy of sitosterolemia, and thus screening for this treatable disease has merit.23
Second, it is not routine for patients with a typical history of chronic active hepatitis to be screened for elevated serum plant sterol levels. Although nonimmune hemolysis is unusual with most cases of hepatitis, the presence of this may be a good indicator for a clinician to perform plasma sterol analyses by GLC in these select patients. Because this is the first reported association of liver disease and sitosterolemia, we cannot exclude the possibility of association by chance. However, there is a possible mechanism(s) whereby mutations in ABCG8 could lead to liver disease. Misfolded proteins in the liver can cause liver disease, and a classical example of this is the accumulation of misfolded proteins in the endoplasmic reticulum (ER) seen with α-1 antitrypsin deficiency.24 In vitro evidence suggests that coexpression of both normal ABCG5 and ABCG8 proteins is required for apical expression, and each of these proteins acts as a “mutual chaperone.” 11 In the absence of one, the other is retained in the ER and degraded. However, the immunohistochemistry data shown here go against this hypothesis. Alternatively, there may be direct damage to the liver by the accumulating plant sterols, although this feature has not been reported in other cases of sitosterolemia. On the other hand, sitosterolemia is a very rare disease, and it is likely underdiagnosed.
Third, this patient affords a unique window into the relative roles of the liver and intestine on the roles of these organs in regulating noncholesterol sterol levels. Although we did not perform genotype analyses of the donor liver, we assume that it came from a nonsitosterolemic individual (because this disease is very rare). Thus, normal biliary sterol clearance and a normal ability to excrete noncholesterol sterols were restored in the liver, in the face of a continuing genetic defect in his intestine. The latter presumably continues to allow un-regulated dietary sterols to enter, yet the transplantation led to a near normalization of plasma plant sterol levels. Although tissue levels of accumulated plant sterols are not easily assessable, one assumes that these have also been steadily declining. Thus, the liver seems to have the capacity to prevent the accumulation of noncholesterol sterols, despite an on-going intestinal defect. One explanation for this may be that the input from the diet is intermittent, dictated by feeding patterns, whereas the hepatobiliary excretion is continuous. During the last 4 preoperative years, the plant sterol pattern in serum remained unchanged, despite continuous cholestyramine treatment. The postoperative serum plant sterol values were clearly improved but remained mildly increased at levels roughly similar to values seen occasionally in obligatory heterozygous subjects.
In conclusion, we report the first case of sitosterolemia, presenting with progressive liver disease, initially categorized as chronic active hepatitis leading to cirrhosis, who was found to have sitosterolemia as a chance finding. Mutational analyses confirmed that the patient carried 2 mutant alleles for ABCG8. Following liver transplantation, his sitosterolemia improved dramatically and allows us to surmise that restoration of normal ABCG8 function in this organ alone is sufficient to correct the biochemical abnormality. If or when gene therapy to the liver becomes feasible and safe, this disease may be a suitable one for such therapies.
Footnotes
- GLC
- gas-liquid chromatography.
Supported by grants from the Helsinki University Central Hospital (to T.A.M.) and the National Institutes of Health PHS HL060613 (to S.B.P.).
References
- Schoenheimer R. Uber die Bedeutung der Pflanzensterine fur den tierischen Organismus. Hoppe-Seyler’s Z für Physiol Chem. 1929;180:1–5. [Google Scholar]
- Bhattacharyya AK, Connor WE. β-Sitosterolemia and xanthomatosis. A newly described lipid storage disease in two sisters. J Clin Invest. 1974;53:1033–1043. doi: 10.1172/JCI107640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty TH, Kwiterovich P, Jr, Khoury MJ, et al. Genetic analysis of plasma sitosterol, apoprotein B, and lipoproteins in a large Amish pedigree with sitosterolemia. Am J Hum Genet. 1986;38:492–504. [PMC free article] [PubMed] [Google Scholar]
- Bjorkhem I, Boberg KM. Inborn errors in bile acid biosynthesis and storage of sterols other than cholesterol. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic basis of inherited disease. 7.th ed. McGraw-Hill Inc.; New York: 1995. pp. 2073–2102. [Google Scholar]
- Salen G, Shefer S, Nguyen L, Ness GC, Tint GS, Shore V. Sitosterolemia. J Lipid Res. 1992;33:945–955. [PubMed] [Google Scholar]
- Salen G, Patel SB, Batta AK. Sitosterolemia. Cardiovasc Drug Rev. 2002;20:255–270. doi: 10.1111/j.1527-3466.2002.tb00096.x. [DOI] [PubMed] [Google Scholar]
- Miettinen TA. Phytosterolaemia, xanthomatosis and premature atherosclerotic arterial disease: a case with high plant sterol absorption, impaired sterol elimination and low cholesterol synthesis. Eur J Clin Invest. 1980;10:27–35. doi: 10.1111/j.1365-2362.1980.tb00006.x. [DOI] [PubMed] [Google Scholar]
- Patel SB, Salen G, Hidaka H, et al. Mapping a gene involved in regulating dietary cholesterol absorption. The sitosterolemia locus is found at chromosome 2p21. J Clin Invest. 1998;102:1041–1044. doi: 10.1172/JCI3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M-H, Lu K, Hazard S, et al. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat Genet. 2001;27:79–83. doi: 10.1038/83799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berge KE, Tian H, Graf GA, et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- Graf GA, Cohen J, Hobbs HH. Missense mutations in ABCG5 and ABCG8 disrupt heterodimerization and trafficking. J Biol Chem. 2004;279:24881–24888. doi: 10.1074/jbc.M402634200. [DOI] [PubMed] [Google Scholar]
- Graf GA, Li WP, Gerard RD, et al. Coexpression of ATP-binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface. J Clin Invest. 2002;110:659–669. doi: 10.1172/JCI16000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf GA, Yu L, Li WP, et al. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J Biol Chem. 2003;278:48275–48282. doi: 10.1074/jbc.M310223200. [DOI] [PubMed] [Google Scholar]
- Klett EL, Lee MH, Adams DB, Chavin KD, Patel SB. Localization of ABCG5 and ABCG8 proteins in human liver, gall bladder and intestine. BMC Gastroenterol. 2004;4:21. doi: 10.1186/1471-230X-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klett EL, Lu K, Kosters A, et al. A mouse model of sitosterolemia: absence of Abcg8/sterolin-2 results in failure to secrete biliary cholesterol. BMC Med. 2004;2:5. doi: 10.1186/1741-7015-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plosch T, Bloks VW, Terasawa Y, et al. Sitosterolemia in ABC-transporter G5-deficient mice is aggravated on activation of the liver-X receptor. Gastroenterology. 2004;126:290–300. doi: 10.1053/j.gastro.2003.10.074. [DOI] [PubMed] [Google Scholar]
- Miettinen TA. Cholesterol metabolism during ketoconazole treatment in man. J Lipid Res. 1988;29:43–51. [PubMed] [Google Scholar]
- Lu K, Lee M-H, Hazard S, et al. Two genes that map to the STSL locus cause sitosterolemia: genomic structure and spectrum of mutations involving sterolin-1 and sterolin-2, encoded by ABCG5 and ABCG8, respectively. Am J Hum Genet. 2001;69:278–290. [Google Scholar]
- Lu K, Lee M, Patel SB. Dietary cholesterol absorption: more than just bile. Trends Endocrinol Metab. 2001;12:314–320. doi: 10.1016/s1043-2760(01)00433-7. [DOI] [PubMed] [Google Scholar]
- Klett EL, Patel S. Genetic defenses against noncholesterol sterols. Curr Opin Lipidol. 2003;14:341–345. doi: 10.1097/01.mol.0000083763.66245.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton PT, Bowron A, Mills KA, Massoud A, Casteels M, Milla PJ. Phytosterolemia in children with parenteral nutrition-associated cholestatic liver disease. Gastroenterology. 1993;105:1806–1813. doi: 10.1016/0016-5085(93)91079-w. [DOI] [PubMed] [Google Scholar]
- Nikkila K, Hockerstedt K, Miettinen TA. Serum and hepatic cholestanol, squalene and noncholesterol sterols in man: a study on liver transplantation. Hepatology. 1992;15:863–870. doi: 10.1002/hep.1840150519. [DOI] [PubMed] [Google Scholar]
- Salen G, von Bergmann K, Lutjohann D, et al. Ezetimibe effectively reduces plasma plant sterols in patients with sitosterolemia. Circulation. 2004;109:966–971. doi: 10.1161/01.CIR.0000116766.31036.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch WJ. Role of quality control pathways in human diseases involving protein misfolding. Semin Cell Dev Biol. 2004;15:31–38. doi: 10.1016/j.semcdb.2003.12.011. [DOI] [PubMed] [Google Scholar]