Abstract
Idiosyncratic drug-induced hepatitis (IDDIH) is the third most common cause for acute liver failure in the United States. Previous studies have attempted to identify susceptible patients or early stages of disease with various degrees of success. To determine if total serum immunoglobulin subclasses, CYP2E1-specific subclass autoantibodies, complement components, or immune complexes could distinguish persons with IDDIH from others exposed to drugs, we studied persons exposed to halogenated volatile anesthetics, which have been associated with IDDIH and CYP2E1 autoantibodies. We found that patients with anesthetic-induced IDDIH had significantly elevated levels of CYP2E1-specific immunoglobulin G4 (IgG4) autoantibodies, while anesthetic-exposed healthy persons had significantly elevated levels of CYP2E1-specific IgG1 autoantibodies. Anesthetic IDDIH patients had significantly lower levels of C4a, C3a, and C5a compared to anesthetic-exposed healthy persons. C1q- and C3d-containing immune complexes were significantly elevated in anesthetic-exposed persons. In conclusion, our data suggest that anesthetic-exposed persons develop CYP2E1-specific IgG1 autoantibodies which may form detectable circulating immune complexes subsequently cleared by classical pathway activation of the complement system. Persons susceptible to anesthetic-induced IDDIH develop CYP2E1-specific IgG4 autoantibodies which form small, nonprecipitating immune complexes that escape clearance because of their size or by direct inhibition of complement activation.
Idiosyncratic drug-induced hepatitis (IDDIH), a form of drug-induced hypersensitivity reaction, accounts for approximately 13% of acute liver failure cases in the United States and is the third most common cause of acute liver failure (19, 25, 33, 39). IDDIH is the primary reason for recalling drugs from the commercial market (42). Unfortunately, no widely used laboratory tests can identify susceptible individuals or the early stagesof drug-induced hypersensitivity reactions. Hence, IDDIH is usually diagnosed when the patient becomes significantly symptomatic.
Recent studies suggest that individuals susceptible to drug reactions may be identifiable using pharmacogenetics (27, 36). Other studies have attempted to identify susceptible individuals by skin-testing, serum histamine, tryptase, C3a, C4a, drug hapten- or native protein-specific immunoglobulin E (IgE) levels or IgG antibodies. Sadly, skin testing may potentially identify patients after drug-exposure, but may also induce life-threatening reactions in highly sensitized patients (18). Serum histamine and tryptase levels can identify persons who have experienced severe hypersensitivity reactions and mast cell degranulation; however, these tests are not specific for hepatitis. Additionally, C3a and C4a levels have been inconsistent (18). Serum antibodies to drug haptens and native proteins have been more widely used to identify IDDIH patients (4, 24).
A form of IDDIH, anesthetic-induced IDDIH (AH), is believed to be triggered by native hepatic proteins, such as cytochrome P450 2E1 (CYP2E1) (4, 21), which have been covalently modified by trifluoroacetyl chloride (TFA), formed during CYP2E1-mediated anesthetic oxidative metabolism (28, 29, 37). Elevated serum levels of CYP2E1-specific IgG autoantibodies have been found in 45 to 70% of patients diagnosed with halothane-induced IDDIH (4). However, antigen-specific autoantibodies are not unique to AH. In fact, CYP2E1-specific IgG autoantibodies are found in patients with alcoholic liver disease (46) and toxic liver injury from commercial refrigerant hydroclorofluorocarbons (12). Moreover, previous studies have suggested that IDDIH caused by antiseizure medications, antidepressants, antibiotics or nonsteroidal anti-inflammatory agents results from immune-mediated hepatocellular injury involving drug hapten-altered cytochrome P450 enzymes (1, 6). Thus, CYP2E1 and other CYP autoantibodies are not specific for IDDIH. Additionally, serum CYP autoantibodies are not diagnostic for IDDIH since high levels of CYP2E1 IgG autoantibodies have been demonstrated in persons merely exposed to halogenated volatile anesthetics (4, 30).
Alternative serum markers are needed to identify persons susceptible to IDDIH. Immunoglobulin subclasses are believed to have a determining role in several autoimmune diseases. For example, antinuclear (ANA) and anti-double-stranded DNA (anti-dsDNA) autoantibodies are predominantly IgG1 and IgG3 subclasses (23). Additionally, elevated IgG1 and IgG3 autoantibodies to type II collagen in rheumatoid arthritis (8), to glutamic acid decarboxylase in IDDM (9), and to ganglioside in Guillain-Barre have been demonstrated (14), while elevated IgG4 autoantibodies have been seen in idiopathic membranous nephropathy (17), to desmoglein-1 in pemphigus foliaceus (47), to collagen in systemic lupus erythematosus (SLE) (8), to thyroid peroxidase in subclinical hypothyroidism (40) and to thyroglobulin in Graves' disease (7). So, it is reasonable to hypothesize that IgG subclass-restricted CYP2E1 autoantibodies may predict the development of IDDIH.
Demonstrating immune complexes or complement activation may also identify patients with IDDIH. Previous studies have described transient complement activation and depletion by immune complexes during drug reactions (48). Recent studies suggest that the complement system can also regulate B cell (5) and granulocyte activation (11) following C3a and C5a formation. Moreover, current investigations show that the anaphylatoxins C3a and C5a have divergent roles in the generation of immune responses where C3a promotes T helper 2 (Th2) responses and C5a promotes T helper 1 responses. Whether IDDIH in humans is a Th2-generated disease is unknown; however, a recent report of an animal model of IDDIH suggested critical roles for Th2 mechanisms (31). Yet, there are no studies suggesting a role for immune complexes, C3a or C5a in IDDIH patients.
To extend our previous findings where we detected elevated serum CYP2E1 IgG autoantibodies in AH patients and persons exposed to high levels of halogenated volatile anesthetics in their working environment, our present study was designed to differentiate the role of autoantibodies in these groups. We compared AH patients with control persons with no history of anesthetic exposure and those with high or low environmental exposure to halogenated volatile anesthetics. We analyzed sera for Ig subclass levels, CYP2E1-specific Ig subclass autoantibodies, components of complement pathways as well as immune complexes. Our findings suggest that persons environmentally exposed to halogenated volatile anesthetics develop CYP2E1-specific IgG1 autoantibodies which may form immune complexes that are probably cleared by classical activation of the complement system. In contrast, AH patients develop CYP2E1-specific IgG4 autoantibodies which may form small, nonprecipitating immune complexes that escape clearance because of their size or by direct inhibition of complement activation.
MATERIALS AND METHODS
Chemicals and reagents.
Alkaline phosphatase-conjugated mouse anti-human IgG1, clone 4E3, isotype mouse IgG1, mouse anti-human IgG2, clone 31-74, isotype mouse IgG1(κ), mouse anti-human IgG3, clone HP6050, isotype mouse IgG1(κ) and mouse anti-human IgG4, clone HP6025, isotype mouse IgG1(κ) were from Southern Biotech (Birmingham, AL); alkaline phosphatase substrate kit, from Bio-Rad (Hercules, CA); antiactin enzyme-linked immunosorbent assay (ELISA), anti-liver/kidney microsome 1 (LKM-1) ELISA, and soluble liver antigen (SLA) ELISA, from QUANTA Lite INOVA Diagnostics, Inc. (San Diego, CA); anti-dsDNA enzyme immunoassay (EIA), from Scimedx Corporation (Denville, NJ); ANA/HEp-2 cell culture immunofluorescence test system, from Zeus Scientific, Inc. (Raritan, NJ); C1q and C3d circulating immune complexes EIA kits, from ALPCO Diagnostics (Windham, NH); fetal calf serum, from Gibco-BRL, Life Technologies (Grand Island, NY); human C3a, C4a, and C5a ELISA kits from BD OptEIA, BD Biosciences (San Diego, CA); human CYP2E1 plus P450 reductase plus cytochrome b5 Supersomes, from GenTest, BD Biosciences (Woburn, MA); human IgG subclass profile ELISA kit, from Zymed Laboratories, Inc. (San Francisco, CA); Immulon 2HB 96-well microtiter plates from ISC BioExpress (Kaysville, UT); phosphate-buffered saline (PBS), from Biofluids (Rockville, MD); and polyoxyethylene-sorbitan monolaurate (Tween 20), from Sigma (St. Louis, MO). All kits were used according to the manufacturer's instructions.
Human sera.
Studies were approved by the Johns Hopkins School of Medicine Institutional Review Board. Four experimental groups were studied: control persons (control, n = 54), persons with anesthetic-induced IDDIH (AH, n = 24), pediatric anesthesiologists (pediatric, n = 53) and general anesthesiologists (general, n = 53). Control sera were preemployment samples from healthy persons. AH patients were self-referred patients with hepatitis following exposure to the halogenated volatile anesthetics halothane, isoflurane, and desflurane. AH was diagnosed using the following criteria: clinical hepatitis following exposure to halothane, isoflurane or desflurane, serologically negative for infectious causes of hepatitis, including hepatitis virus types A, B, and C, cytomegalovirus, and Epstein-Barr virus, negative history of exposure to toxic levels of acetaminophen, nonsteroidal anti-inflammatory agents or antiseizure medications; and serum TFA hapten, 58-kDa endoplasmic reticulum protein, or CYP2E1 antibodies. Sera from pediatric and general anesthesiologists were from a previous study where pediatric anesthesiologists had elevated CYP2E1 autoantibodies (30), which were attributed to higher levels of exposure to halogenated volatile anesthetics during mask induction of anesthesia and from the practice of using uncuffed endotracheal tubes in pediatric patients. Blood was collected in serum separator tubes, allowed to clot and then centrifuged (1,000 × g, 4°C). To avoid repeat freeze-thaw cycles, sera aliquots were removed and stored at −80°C.
Serum assays for antibodies to soluble liver antigen, nuclear, actin, liver/kidney microsome, and double-stranded DNA.
To detect patients who may have autoimmune hepatitis, anti-SLA antibodies were measured using the QUANTA Lite SLA ELISA (26). Positive values were valued at >25 units. ANA and antiactin antibodies were measured to detect autoimmune hepatitis type 1 (AIH-1) (3). ANA antibodies were measured using the ANA/HEp-2 cell culture IFA test system, antiactin IgG antibodies, using the QUANTA Lite Actin ELISA. Positive ANA values were set at 1:40 dilution while weakly positive antiactin values were set at >20 units with strongly positive antiactin values at >30 units. To detect AIH-2 (22), anti-LKM-1 (CYP2D6) antibodies were measured using the QUANTA Lite LKM-1 ELISA. Positive anti-LKM-1 values were set at >25 units. To detect systemic lupus erythematosus (49), anti-dsDNA antibodies were measured using the SCIMEDX anti-dsDNA EIA kit (20). Positive values for anti-dsDNA autoantibodies were as follows: borderline 25 to 30 IU, low positive 30 to 60 IU, positive 60 to 200 IU, and strongly positive >200 IU.
Serum assays for total immunoglobulin G subclasses.
IgG subclasses were measured by ELISA in randomly selected samples using a human IgG subclass profile ELISA kit: control (n = 41), AH (n = 23), pediatric anesthesiologists (n= 32), and general anesthesiologists (n= 32). Normal values ± standard deviations (SD) were as follows: IgG1, 6,641 ± 824 μg/ml; IgG2, 2,867 ± 490 μg/ml; IgG3, 785 ± 118 μg/ml; and IgG4, 324 ± 93 μg/ml.
Serum assays for CYP2E1-specific IgG subclass antibodies.
Human serum samples (1:100) were tested for CYP2E1-specific subclasses using a previously described ELISA (30), human CYP2E1 (0.5 μg/100 ul) as the test antigen and alkaline phosphatase-conjugated second antibodies (1:1,000) as follows: mouse anti-human IgG1, IgG2, IgG3, and IgG4. Plates were read after 60 min for CYP2E1 IgG1, IgG2, and IgG3 and 90 min for CYP2E1 IgG4.
Serum analysis for activation of the classical and common complement pathways.
To determine if halogenated volatile anesthetics activate complement pathways, sera were analyzed for C4a, C3a, and C5a using a BD OptEIA ELISA kits. Control values were C3a-desArg, 8,707.2 ± 1,797.3 ng/ml; C4a-desArg, 732.2 ng/ml; and C5a-desArg, 168.6 ± 70.6 ng/ml.
Serum analysis for circulating immune complexes.
Using circulating immune complexes—C1q- and C3d-circulating immune complex EIA kits, respectively— sera were analyzed for immune complexes containing C1q, implicating the classical, or C3d, implicating the common, complement pathways. Elevated C1q and C3d immune complex values for these EIA kits were 5 μEq/ml and 40 μg/ml, respectively.
Statistical analysis.
Statistical analyses were performed using GraphPad Prism version 4.02 for Windows (GraphPad Software, Incorporated, San Diego, CA [www.graphpad.com]). Demographic data and anti-SLA, -ANA, -actin, -LKM-1 and-dsDNA antibodies, as well as C1q and C3d circulating immune complexes were analyzed using Fisher's exact test. Total Ig levels, CYP2E1 Ig subclass autoantibodies, C3a, C4a, and C5a were analyzed by analysis of variance with Tukey's posttest. A P value of <0.05 was considered significant.
RESULTS
Demographic analysis.
Experimental groups were questioned for baseline characteristics including age, sex, history of asthma, drug or seasonal allergies and history of autoimmune diseases (Table 1). Pediatric and general anesthesiologists were also previously demographically analyzed (30). General anesthesiologists were younger than AH patients and pediatric anesthesiologists (P < 0.05); however, other baseline characteristics were balanced.
TABLE 1.
Study designa
| Group | No. of subjects (M/F) | Age (yr) ± SE | No. (%) with history of drug allergies | No. (%) with history of allergic rhinitis or asthma | No. (%) with history of autoimmune disease |
|---|---|---|---|---|---|
| Control | 54 (28/26) | 37.5 ± 0.6 | 0 | 0 | 0 |
| Anesthetic IDDIH | 23 (15/8) | 46.0 ± 3.9 | 2 (8.7) | 2 (8.7) | 1 (4.3) |
| Pediatric anesthesiologists | 53 (30/23) | 43.5 ± 1.1 | 4 (7.5) | 2 (3.8) | 6 (11.3) |
| General anesthesiologists | 53 (30/23) | 37.8 ± 1.3* | 6 (11.3) | 3 (5.7) | 2 (3.8) |
*, P < 0.05.
Sera from experimental groups do not have markers consistent with autoimmune hepatitis or SLE.
Previous studies have detected CYP2E1 IgG autoantibodies in AH patients (4, 10, 30); however, these studies did not test for autoimmune hepatitis or SLE. We tested sera for anti-SLA antibodies, ANAand antiactin antibodies, anti-LKM antibodies, and anti-dsDNA antibodies.
Experimental groups do not contain patients with undiagnosed autoimmune hepatitis.
Anti-SLA autoantibodies were not detected in pediatric and general anesthesiologists. However, one AH sample had anti-SLA antibodies, but also had high levels of autoantibodies to other liver proteins, namely ERp58 and CYP2E1.
To screen for persons with undiagnosed AIH-1 or AIH-2, randomly selected samples from each study group were tested. Three of 23 of AH patients (13.0%), 14 of 54 pediatric anesthesiologists (25.9%), and 7 of 43 general anesthesiologists (21%) had ANAantibodies. The majority of positive antiactin values were weakly positive. Nonetheless, 1 of 23 AH patients (4.3%), 4 of 54 pediatric anesthesiologists (7.4%) and 2 of 43 general anesthesiologists (4.7%) had antiactin antibodies. Only three samples were positive for both ANA and antiactin antibodies (one AH patient and three pediatric anesthesiologists). Medical histories from these persons did not support the diagnosis of AIH. Moreover, a two-sided Fisher's exact test revealed that there were no statistical differences in the numbers of persons with positive ANA or antiactin between the experimental groups (not shown). Additionally, no sera from AH patients and pediatric and general anesthesiologists had LKM-1 antibodies. Therefore, these findings suggest that differences in our experimental groups could not be explained by undiagnosed AIH-1 or AIH-2.
Experimental groups do not contain patients with undiagnosed SLE.
To test for undiagnosed SLE or other connective tissue diseases, samples were analyzed for anti-dsDNA antibodies. Few samples were positive for anti-dsDNA antibodies: 1 of 23 AH persons (4.3%), 5 of 54 pediatric anesthesiologists (9.3%), and 1 of 33 general anesthesiologists (3.0%). Fewer were positive for both ANA and anti-dsDNA antibodies: 1 of 23 AH persons (4.3%), 1 of 54 pediatric anesthesiologists (1.9%) and 1 of 43 general anesthesiologists (2.3%). Further clinical analysis did not support the diagnosis of SLE. Moreover, a two-sided Fisher's exact test revealed that there were no statistical differences in the numbers of positive anti-dsDNA or combined ANA and anti-dsDNA antibodies between experimental groups. Therefore, these findings and those above suggest that our findings could not be explained by undiagnosed SLE or autoimmune hepatitis.
Total sera IgG3 levels are significantly higher in patients with anesthetic-induced IDDIH.
Total IgG subclass levels were measured. Since our IgG subclass levels were higher than normal values reported for the kit, we used the mean value of our control group as the normal levels in each subclass. Data are presented as means ± SD in Fig. 1.
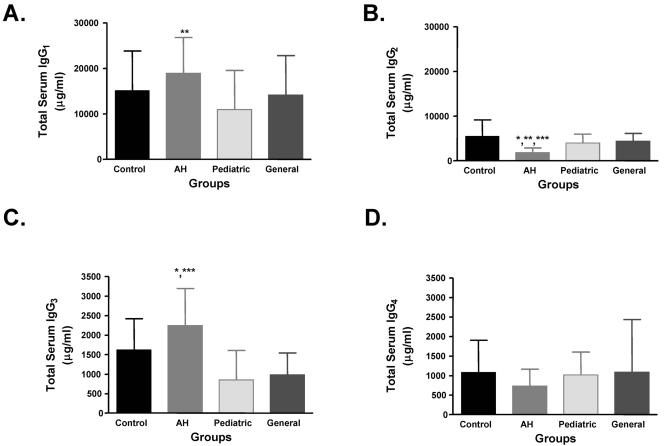
FIG. 1.
IgG3 levels are significantly higher while IgG2 levels are significantly lower in anesthetic-induced IDDIH. IgG subclasses were measured by ELISA. Data are expressed in μg/ml (mean ± SD). IgG3 levels were significantly higher in AH persons than controls (P < 0.05 [*]), pediatric anesthesiologists (P < 0.001 [***]), or general anesthesiologists (P < 0.001) (C), while IgG2 levels were significantly lower in AH persons than controls (P < 0.001), pediatric anesthesiologists (P < 0.05) or general anesthesiologists (P < 0.01 [**]) (B). IgG1 levels were significantly higher in AH persons compared to the pediatric group (P < 0.01) (A) while IgG4 levels were not significantly different between groups (D).
Analysis of complement-fixing Ig subclasses showed that although persons in AH and pediatric anesthesiologist categories had similar levels of CYP2E1 IgG autoantibodies (30), IgG1 levels were significantly higher in AH patients than in pediatric anesthesiologists (Fig. 1A), but not significantly different from control or general anesthesiologist groups. Conversely, IgG3 levels were significantly higher in AH patients than all other groups (Fig. 1C). Finding elevated IgG3 levels was surprising since AH patients did not have increased ANA or anti-dsDNA autoantibodies, also of the IgG3 subclass, and could suggest another previously unidentified IgG3 autoantibody.
Non-complement-fixing subclass analyses revealed that IgG2 levels were significantly lower in AH than all other groups (Fig. 1B). Conversely, there were no statistical differences in the levels of IgG4 between all of the study groups (Fig. 1D). These results suggest that AH may increase IgG3 levels representing a previously unidentified autoantibody or circulating immune complexes, but may decrease IgG2 levels.
CYP2E1 IgG4 levels are significantly higher in patients with anesthetic-induced IDDIH.
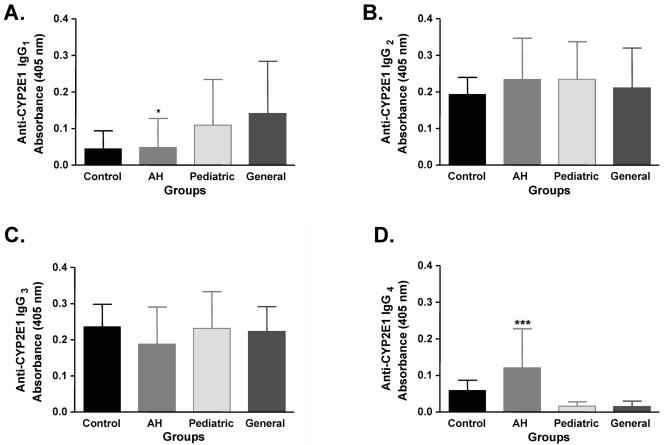
We did not directly quantify CYP2E1-specific IgG subclass levels; rather, we compared absorbance differences between groups. The total IgG subclass distribution did not parallel CYP2E1-specific IgG subclasses. Specifically, while IgG1 levels were significantly higher in AH patients than in pediatric anesthesiologists, CYP2E1-specific IgG1 autoantibody levels were significantly lower than general anesthesiologists (Fig. 2A). Notably, differences in CYP2E1-specific IgG1 levels between AH patients and pediatric anesthesiologists approached significance. IgG2 levels were significantly lower in AH than all other groups while CYP2E1-specific IgG2 levels were not significantly different (Fig. 2B). Conversely, IgG3 levels were significantly higher in AH than all other groups while CYP2E1-specific IgG3 levels were also not significantly different (Fig. 2C). Lastly, IgG4 levels were not different between groups while CYP2E1-specific IgG4 levels were significantly higher than all other groups (Fig. 2D).
FIG. 2.
CYP2E1-specific IgG4 autoantibody levels are significantly higher in anesthetic IDDIH. CYP2E1 IgG1, IgG2, IgG3, and IgG4 subclass antibodies were detected by ELISA and expressed as optical density at 405 nm ± SD. CYP2E1-specific IgG1 autoantibody levels were significantly lower in AH persons compared to general anesthesiologists (P < 0.05 [*]) (A). However, CYP2E1-specific IgG4 autoantibody levels were significantly higher in AH persons than controls (P < 0.001 [***]), pediatric anesthesiologists (P < 0.001), or general anesthesiologists (P < 0.001) (D). CYP2E1-specific IgG2 and IgG3 autoantibody levels were not significantly different between groups.
Patients with anesthetic-induced IDDIH have reduced serum components of classical and common complement pathways.
Reduced complement components have been documented in patients with hepatitis following halothane (50), cromolyn sodium (38), hydralazine (15), trimethoprim-sulfamethoxazole (2), and carbamazepine (13). We measured C3a, C4a, and C5a levels and used the mean value in our control group for normal levels.
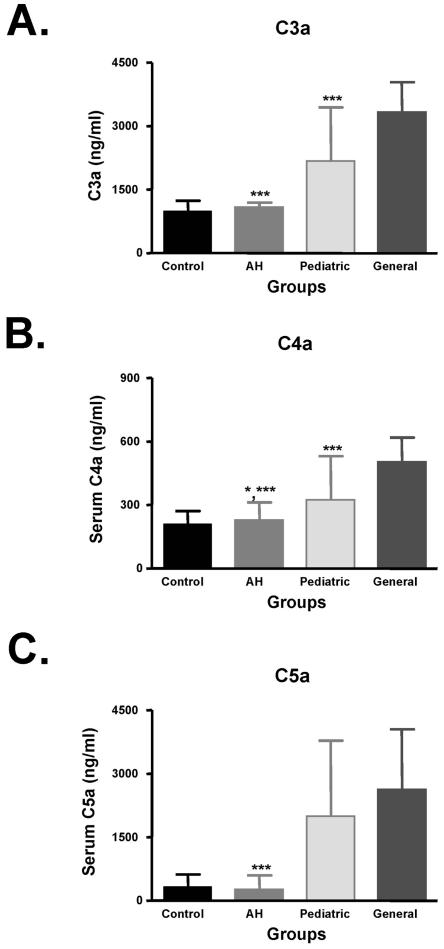
We found that patients with AH had significantly lower levels of complement components when compared to anesthetic-exposed persons but these levels were not statistically different from controls. Specifically, C4a, C3a, and C5a levels were significantly lower in AH patients when compared to pediatric and general anesthesiologists, but not significantly different from controls (Fig. 3), suggesting the complement system may not be activated or may be inhibited in IDDIH.
FIG. 3.
Classical and common complement components are significantly lower in anesthetic-induced IDDIH. C3a, C4a, and C5a levels were measured by ELISA. Data are expressed in ng/ml (mean ± SD). C3a (A) and C5a (C) levels were significantly lower in AH patients than pediatric and general anesthesiologists (P < 0.001 [***]). C4a (B) levels were also significantly lower in AH patients than pediatric anesthesiologists (P < 0.05 [*]) and general (P < 0.001) anesthesiologists.
Lastly, C4a and C3a levels in pediatric anesthesiologists were significantly lower than general anesthesiologists (Fig. 3A and B), while C5a levels were not statistically different. These findings may suggest that classical complement activation may occur at different rates in these groups, may be exposure dependent or may be triggered by distinct immune complexes.
Occupationally exposure to halogenated volatile anesthetics induces detectable immune complexes.
Earlier studies found autoantibodies in persons occupationally exposed to halogenated volatile anesthetics (4, 30), but these studies did not test for immune complexes. Sera were tested to determine if C1q- and C3d-binding immune complexes were detectable following anesthetic exposure.
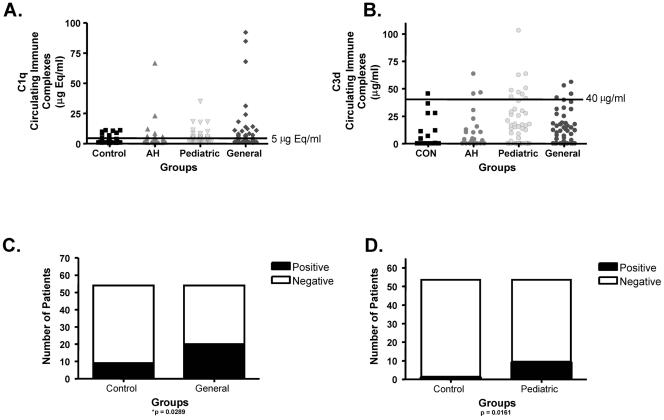
Although immune complexes were detectable at low levels in each group, only occupationally exposed persons had significantly higher levels of immune complexes when compared to controls. Specifically, general anesthesiologists had significantly higher levels of C1q-containing immune complexes (Fig. 4A and C), and pediatric anesthesiologists had significantly higher levels of C3d-containing immune complexes (Fig. 4B and D). Hence, serum immune complexes which could activate complement were detectable in occupationally exposed persons, while not detectable at significant levels in AH patients and controls.
FIG. 4.
Immune complexes are significantly elevated in persons occupationally exposed to halogenated volatile anesthetics. C1q- and C3d-containing immune complexes were determined by ELISA and are presented as μEq/ml (C1q) and μg/ml (C3d); 5 μEq/ml and 40 μg/ml represent elevated C1q and C3d values, respectively, for these kits. General and pediatric anesthesiologists had significantly elevated levels of C1q- (A and C) (P= 0.0289) and C3d- (B and D) (P = 0.0161) containing immune complexes, respectively.
DISCUSSION
In the present study, we showed that AH patients have significantly elevated CYP2E1-specific IgG4 antibodies while environmentally exposed persons have significantly elevated CYP2E1 IgG1 antibodies. Additionally, AH patients have significantly increased total IgG3, but decreased IgG2. Serum analyses for complement activation shows significantly lower C3a, C4a, and C5a levels in AH patients than in the environmental exposure groups. Significant levels of immune complexes were detected only in environmentally exposed but not in persons with AH. To our knowledge there is no prior study that demonstrates a role for antigen-specific IgG4 autoantibodies or immune complexes in the pathogenesis of IDDIH.
Prior investigations have demonstrated that autoimmune diseases were associated with distinct distributions of Ig subclasses (34). We found increased IgG3 levels in AH patients. Increased IgG3 subclasses have been associated with primary biliary cirrhosis (34), anti-nuclear and anti-double-stranded DNA antibodies and circulating immune complexes. We were not able to demonstrate anti-mitochondrial antibodies which would suggest primary biliary cirrhosis (results not shown), nor significantly elevated ANA autoantibodies, anti-dsDNA autoantibodies or immune complexes. Interestingly, we also showed that IDDIH patients had decreased total IgG2, which has been associated with SLE (35), autoimmune connective tissue disease (16) and Sjogrens syndrome (34). Whether this finding in IDDIH is pathogenic or compensatory will be the subject of further investigations in our laboratory.
Although IgG subclass analysis did not expose a clear mechanism that may clarify the development of IDDIH, analysis for CYP2E1-specific IgG subclasses showed elevated CYP2E1-specific IgG4 autoantibodies in AH persons, while anesthetic-exposed persons had elevated levels of CYP2E1-specific IgG1 autoantibodies. Previous studies have demonstrated that both IgG1 and IgG4 antigen-specific subclasses are key players in inhalant allergies and asthma (43). However, only CYP2E1-specific IgG4 autoantibodies, the least of the IgG subclasses, were present in patients who developed hepatitis, which may suggests distinct roles for these subclasses in IDDIH.
IgG1 and IgG4 subclasses have distinct roles in complement activation where IgG1 subclasses activate and IgG4 subclasses inhibit complement activation (44). We found that pediatric and general anesthesiologists, persons exposed to high and low levels of halogenated volatile anesthetics, had circulating C4a, as well as C3a and C5a, respectively, suggesting classical and common complement activation, respectively. Complement activation in exposed groups is a novel finding and could be indicative of immune complexes containing IgG1 subclass antibodies. We were able to demonstrate significantly elevated C1q-containing immune complexes in general anesthesiologists with significantly elevated CYP2E1 IgG1 subclass autoantibodies. IgG1-containing immune complexes are usually cleared by classical activation of complement pathways. Interestingly, we were also able to demonstrate significantly elevated C3d-containing immune complexes in pediatric anesthesiologists with high levels of CYP2E1 IgG1 autoantibodies. Demonstrating C3d-containing immune complexes strongly suggests that halogenated volatile anesthetic induction of IgG1-containing immune complexes activate common complement pathways. Moreover, finding distinct immune complexes in low (general)-exposure groups may explain significantly elevated levels of C3a and C4a in general compared to pediatric anesthesiologists. Thus, demonstration of IgG1 autoantibodies and immune complexes in exposed groups without hepatitis suggests that complement activation has a crucial role in clearing potentially damaging anesthetic-induced immune complexes.
Further evidence suggesting a vital role for complement activation in the pathogenesis of AH, and possibly IDDIH, is the detection of CYP2E1-specific IgG4 autoantibodies in AH patients. IgG4 autoantibodies can form immune complexes that are smaller and may escape clearance (45). More importantly, previous studies have shown that IgG4 autoantibodies can inhibit complement activation and thus prevent clearance of immune complexes (44). In fact, we found that C4a, C3a, and C5a in AH patients were similar to the levels in controls. Additionally, in spite of elevated levels of CYP2E1-specific IgG4 autoantibodies, significantly elevated levels of immune complexes were not demonstrated. Low levels of complement components have been previously reported in persons with drug-induced liver injury (13, 15, 38, 50). Finding elevated levels of IgG4 autoantibodies in AH or any other form of IDDIH to our knowledge has not previously been demonstrated. Our data suggest that hypocomplementemia in drug-induced IDDIH may occur because of inhibition of complement activation. In addition liver injury in IDDIH may occur through IgG4-containing immune complexes by mechanisms similar to those suggested in idiopathic membranous nephropathy (32). We are in the process of verifying these mechanisms in our animal model of drug-induced IDDIH (31).
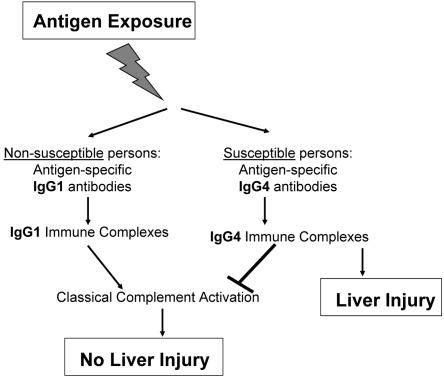
We have demonstrated that IgG3 levels are significantly higher and IgG2 levels are significantly lower in AH patients than all other groups studied. Significantly elevated levels of CYP2E1-specific IgG4 autoantibodies were present in AH patients, while persons environmentally exposed to halogenated volatile anesthetics had predominantly CYP2E1-specific IgG1 autoantibodies. Moreover, classical complement activation and immune complexes were only present in environmentally exposed groups. Thus, our findings suggest that that persons environmentally exposed to halogenated volatile anesthetics develop CYP2E1-specific IgG1 autoantibodies which may form detectable immune complexes that are normally cleared by complement system possibly through the classical pathway. AH persons develop CYP2E1-specific IgG4 autoantibodies which form small, nonprecipitating immune complexes that may escape clearance because of their size or by direct inhibition of complement activation (Fig. 5). Since C3a and C5a are also essential for liver regeneration (41), all of these mechanisms may have a role in the development of AH or other forms of IDDIH.
FIG. 5.
Proposed role of complement and subclass antibodies in IDDIH. We propose that following exposure to drugs associated with IDDIH, nonsusceptible persons develop antigen-specific IgG1 antibodies, from which complement-activating IgG1-containing immune complexes are subsequently cleared. Susceptible persons develop antigen-specific IgG4 antibodies directly or through subclass switching, which form complement-inhibiting IgG4-containing immune complexes that escape clearance and may induce liver injury.
Acknowledgments
This work was supported by a grant from the American Autoimmune Related Diseases Association, and the Joseph F. Scoby and Gail I. Zimmerman Foundation.
REFERENCES
- 1.Adkinson, N. F., Jr., D. Essayan, R. Gruchalla, H. Haggerty, T. Kawabata, J. D. Sandler, L. Updyke, N. H. Shear, and D. Wierda. 2002. Task force report: future research needs for the prevention and management of immune-mediated drug hypersensitivity reactions. J. Allergy Clin. Immunol. 109:S461-S478. [DOI] [PubMed] [Google Scholar]
- 2.Alberti-Flor, J. J., M. E. Hernandez, J. P. Ferrer, S. Howell, and L. Jeffers. 1989. Fulminant liver failure and pancreatitis associated with the use of sulfamethoxazole-trimethoprim. Am. J. Gastroenterol. 84:1577-1579. [PubMed] [Google Scholar]
- 3.Alvarez, F., P. A. Berg, F. B. Bianchi, L. Bianchi, A. K. Burroughs, E. L. Cancado, R. W. Chapman, W. G. Cooksley, A. J. Czaja, V. J. Desmet, P. T. Donaldson, A. L. Eddleston, L. Fainboim, J. Heathcote, J. C. Homberg, J. H. Hoofnagle, S. Kakumu, E. L. Krawitt, I. R. Mackay, R. N. MacSween, W. C. Maddrey, M. P. Manns, I. G. McFarlane, K. H. Meyer zum Buschenfelde, and M. Zeniya. 1999. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J. Hepatol. 31:929-938. [DOI] [PubMed] [Google Scholar]
- 4.Bourdi, M., W. Chen, R. M. Peter, J. L. Martin, J. T. Buters, S. D. Nelson, and L. R. Pohl. 1996. Human cytochrome P450 2E1 is a major autoantigen associated with halothane hepatitis. Chem. Res. Toxicol. 9:1159-1166. [DOI] [PubMed] [Google Scholar]
- 5.Carroll, M. C. 2000. The role of complement in B cell activation and tolerance. Adv. Immunol. 74:61-88. [DOI] [PubMed] [Google Scholar]
- 6.Castell, J. V. 1998. Allergic hepatitis: a drug-mediated organ-specific immune reaction. Clin. Exp. Allergy 28(Suppl. 4):13-19. [PubMed] [Google Scholar]
- 7.Caturegli, P., R. C. Kuppers, S. Mariotti, C. L. Burek, A. Pinchera, P. W. Ladenson, and N. R. Rose. 1994. IgG subclass distribution of thyroglobulin antibodies in patients with thyroid disease. Clin. Exp. Immunol. 98:464-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook, A. D., I. R. Mackay, F. M. Cicuttini, and M. J. Rowley. 1997. IgG subclasses of antibodies to type II collagen in rheumatoid arthritis differ from those in systemic lupus erythematosus and other connective tissue diseases. J. Rheumatol. 24:2090-2096. [PubMed] [Google Scholar]
- 9.Couper, J. J., L. C. Harrison, J. J. Aldis, P. G. Colman, M. C. Honeyman, and A. Ferrante. 1998. IgG subclass antibodies to glutamic acid decarboxylase and risk for progression to clinical insulin-dependent diabetes. Hum. Immunol. 59:493-499. [DOI] [PubMed] [Google Scholar]
- 10.Eliasson, E., and J. G. Kenna. 1996. Cytochrome P450 2E1 is a cell surface autoantigen in halothane hepatitis. Mol. Pharmacol. 50:573-582. [PubMed] [Google Scholar]
- 11.Hawlisch, H., M. Wills-Karp, C. L. Karp, and J. Kohl. 2004. The anaphylatoxins bridge innate and adaptive immune responses in allergic asthma. Mol. Immunol. 41:123-131. [DOI] [PubMed] [Google Scholar]
- 12.Hoet, P., M. L. Graf, M. Bourdi, L. R. Pohl, P. H. Duray, W. Chen, R. M. Peter, S. D. Nelson, N. Verlinden, and D. Lison. 1997. Epidemic of liver disease caused by hydrochlorofluorocarbons used as ozone-sparing substitutes of chlorofluorocarbons. Lancet 350:556-559. [DOI] [PubMed] [Google Scholar]
- 13.Horneff, G., H. G. Lenard, and V. Wahn. 1992. Severe adverse reaction to carbamazepine: significance of humoral and cellular reactions to the drug. Neuropediatrics 23:272-275. [DOI] [PubMed] [Google Scholar]
- 14.Ilyas, A. A., Z. W. Chen, S. D. Cook, F. A. Mithen, and B. S. Singhal. 2001. Immunoglobulin G subclass distribution of autoantibodies to gangliosides in patients with Guillain-Barre syndrome. Res. Commun. Mol. Pathol. Pharmacol. 109:115-123. [PubMed] [Google Scholar]
- 15.Itoh, S., A. Ichinoe, Y. Tsukada, and Y. Itoh. 1981. Hydralazine-induced hepatitis. Hepatogastroenterology 28:13-16. [PubMed] [Google Scholar]
- 16.Kay, R. A., K. J. Wood, R. M. Bernstein, P. J. Holt, and R. S. Pumphrey. 1988. An IgG subclass imbalance in connective tissue disease. Ann. Rheum. Dis. 47:536-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuroki, A., T. Shibata, H. Honda, D. Totsuka, K. Kobayashi, and T. Sugisaki. 2002. Glomerular and serum IgG subclasses in diffuse proliferative lupus nephritis, membranous lupus nephritis, and idiopathic membranous nephropathy. Intern. Med. 41:936-942. [DOI] [PubMed] [Google Scholar]
- 18.Laroche, D., I. Aimone-Gastin, F. Dubois, H. Huet, P. Gerard, M. C. Vergnaud, C. Mouton-Faivre, J. L. Gueant, M. C. Laxenaire, and H. Bricard. 1998. Mechanisms of severe, immediate reactions to iodinated contrast material. Radiology 209:183-190. [DOI] [PubMed] [Google Scholar]
- 19.Lee, W. M., and J. R. Senior. 2005. Recognizing drug-induced liver injury: current problems, possible solutions. Toxicol. Pathol. 33:155-164. [DOI] [PubMed] [Google Scholar]
- 20.Locker, J. D., M. E. Medof, R. M. Bennett, and S. Sukhupunyaraksa. 1977. Characterization of DNA used to assay sera for anti-DNA antibodies; determination of the specificities of anti-DNA antibodies in SLE and non-SLE rheumatic disease states. J. Immunol. 118:694-701. [PubMed] [Google Scholar]
- 21.Mackay, I. R., and B. H. Toh. 2002. Autoimmune hepatitis: the way we were, the way we are today and the way we hope to be. Autoimmunity 35:293-305. [DOI] [PubMed] [Google Scholar]
- 22.Manns, M. P., E. F. Johnson, K. J. Griffin, E. M. Tan, and K. F. Sullivan. 1989. Major antigen of liver kidney microsomal autoantibodies in idiopathic autoimmune hepatitis is cytochrome P450db1. J. Clin. Investig. 83:1066-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manolova, I., M. Dancheva, and K. Halacheva. 2002. Predominance of IgG1 and IgG3 subclasses of autoantibodies to neutrophil cytoplasmic antigens in patients with systemic lupus erythematosus. Rheumatol. Int. 21:227-233. [DOI] [PubMed] [Google Scholar]
- 24.Martin, J. L., G. F. Reed, and L. R. Pohl. 1993. Association of anti-58 kDa endoplasmic reticulum antibodies with halothane hepatitis. Biochem. Pharmacol. 46:1247-1250. [DOI] [PubMed] [Google Scholar]
- 25.Masubuchi, Y., M. Bourdi, T. P. Reilly, M. L. Graf, J. W. George, and L. R. Pohl. 2003. Role of interleukin-6 in hepatic heat shock protein expression and protection against acetaminophen-induced liver disease. Biochem. Biophys. Res. Commun. 304:207-212. [DOI] [PubMed] [Google Scholar]
- 26.McFarlane, I. G. 1998. The relationship between autoimmune markers and different clinical syndromes in autoimmune hepatitis. Gut 42:599-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer, U. A. 2000. Pharmacogenetics and adverse drug reactions. Lancet 356:1667-1671. [DOI] [PubMed] [Google Scholar]
- 28.Neuberger, J., G. Mieli-Vergani, J. M. Tredger, M. Davis, and R. Williams. 1981. Oxidative metabolism of halothane in the production of altered hepatocyte membrane antigens in acute halothane-induced hepatic necrosis. Gut 22:669-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Njoku, D., M. J. Laster, D. H. Gong, E. I. Eger, G. F. Reed, and J. L. Martin. 1997. Biotransformation of halothane, enflurane, isoflurane, and desflurane to trifluoroacetylated liver proteins: association between protein acylation and hepatic injury. Anesth. Analg. 84:173-178. [DOI] [PubMed] [Google Scholar]
- 30.Njoku, D. B., R. S. Greenberg, M. Bourdi, C. B. Borkowf, E. M. Dake, J. L. Martin, and L. R. Pohl. 2002. Autoantibodies associated with volatile anesthetic hepatitis found in the sera of a large cohort of pediatric anesthesiologists. Anesth. Analg. 94:243-249. [DOI] [PubMed] [Google Scholar]
- 31.Njoku, D. B., M. V. Talor, D. Fairweather, S. Frisancho-Kiss, O. A. Odumade, and N. R. Rose. 2005. A novel model of drug hapten-induced hepatitis with increased mast cells in the BALB/c mouse. Exp. Mol. Pathol. 78:87-100. [DOI] [PubMed] [Google Scholar]
- 32.Oliveira, D. B. 1998. Membranous nephropathy: an IgG4-mediated disease. Lancet 351:670-671. [DOI] [PubMed] [Google Scholar]
- 33.Ostapowicz, G., R. J. Fontana, F. V. Schiodt, A. Larson, T. J. Davern, S. H. Han, T. M. McCashland, A. O. Shakil, J. E. Hay, L. Hynan, J. S. Crippin, A. T. Blei, G. Samuel, J. Reisch, and W. M. Lee. 2002. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann. Intern. Med. 137:947-954. [DOI] [PubMed] [Google Scholar]
- 34.Outschoorn, I., M. J. Rowley, A. D. Cook, and I. R. Mackay. 1993. Subclasses of immunoglobulins and autoantibodies in autoimmune diseases. Clin. Immunol. Immunopathol. 66:59-66. [DOI] [PubMed] [Google Scholar]
- 35.Oxelius, V. A. 1984. Immunoglobulin G (IgG) subclasses and human disease. Am. J. Med. 76:7-18. [DOI] [PubMed] [Google Scholar]
- 36.Phillips, K. A., D. L. Veenstra, E. Oren, J. K. Lee, and W. Sadee. 2001. Potential role of pharmacogenomics in reducing adverse drug reactions: a systematic review. JAMA 286:2270-2279. [DOI] [PubMed] [Google Scholar]
- 37.Pohl, L. R., D. Thomassen, N. R. Pumford, L. E. Butler, H. Satoh, V. J. Ferrans, A. Perrone, B. M. Martin, and J. L. Martin. 1991. Hapten carrier conjugates associated with halothane hepatitis. Adv. Exp. Med. Biol. 283:111-120. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg, J. L., D. Edlow, and R. Sneider. 1978. Liver disease and vasculitis in a patient taking cromolyn. Arch. Intern. Med. 138:989-991. [PubMed] [Google Scholar]
- 39.Schiodt, F. V., E. Atillasoy, A. O. Shakil, E. R. Schiff, C. Caldwell, K. V. Kowdley, R. Stribling, J. S. Crippin, S. Flamm, K. A. Somberg, H. Rosen, T. M. McCashland, J. E. Hay, and W. M. Lee. 1999. Etiology and outcome for 295 patients with acute liver failure in the United States. Liver Transpl. Surg. 5:29-34. [DOI] [PubMed] [Google Scholar]
- 40.Silva, L. M., J. Chavez, M. H. Canalli, and C. R. Zanetti. 2003. Determination of IgG subclasses and avidity of antithyroid peroxidase antibodies in patients with subclinical hypothyroidism—a comparison with patients with overt hypothyroidism. Horm. Res. 59:118-124. [DOI] [PubMed] [Google Scholar]
- 41.Strey, C. W., M. Markiewski, D. Mastellos, R. Tudoran, L. A. Spruce, L. E. Greenbaum, and J. D. Lambris. 2003. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J. Exp. Med. 198:913-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Temple, R. J., and M. H. Himmel. 2002. Safety of newly approved drugs: implications for prescribing. JAMA 287:2273-2275. [DOI] [PubMed] [Google Scholar]
- 43.Vance, G. H., C. A. Thornton, T. N. Bryant, J. A. Warner, and J. O. Warner. 2004. Ovalbumin-specific immunoglobulin G and subclass responses through the first 5 years of life in relation to duration of egg sensitization and the development of asthma. Clin. Exp. Allergy 34:1542-1549. [DOI] [PubMed] [Google Scholar]
- 44.van der Zee, J. S., P. van Swieten, and R. C. Aalberse. 1986. Inhibition of complement activation by IgG4 antibodies. Clin. Exp. Immunol. 64:415-422. [PMC free article] [PubMed] [Google Scholar]
- 45.van der Zee, J. S., P. van Swieten, and R. C. Aalberse. 1986. Serologic aspects of IgG4 antibodies. II. IgG4 antibodies form small, nonprecipitating immune complexes due to functional monovalency. J. Immunol. 137:3566-3571. [PubMed] [Google Scholar]
- 46.Vidali, M., S. F. Stewart, R. Rolla, A. K. Daly, Y. Chen, E. Mottaran, D. E. Jones, J. B. Leathart, C. P. Day, and E. Albano. 2003. Genetic and epigenetic factors in autoimmune reactions toward cytochrome P4502E1 in alcoholic liver disease. Hepatology 37:410-419. [DOI] [PubMed] [Google Scholar]
- 47.Warren, S. J., L. A. Arteaga, E. A. Rivitti, V. Aoki, G. Hans-Filho, B. F. Qaqish, M. S. Lin, G. J. Giudice, and L. A. Diaz. 2003. The role of subclass switching in the pathogenesis of endemic pemphigus foliaceus. J. Investig. Dermatol. 120:104-108. [DOI] [PubMed] [Google Scholar]
- 48.Wen, L., J. P. Atkinson, and P. C. Giclas. 2004. Clinical and laboratory evaluation of complement deficiency. J. Allergy Clin. Immunol. 113:585-593. [DOI] [PubMed] [Google Scholar]
- 49.White, R. H., and D. L. Robbins. 1987. Clinical significance and interpretation of antinuclear antibodies. West. J. Med. 147:210-213. [PMC free article] [PubMed] [Google Scholar]
- 50.Williams, B. D., N. White, P. L. Amlot, J. Slaney, and P. A. Toseland. 1977. Circulating immune complexes after repeated halothane anaesthesia. Br. Med. J. 2:159-162. [DOI] [PMC free article] [PubMed] [Google Scholar]