Abstract
Mycobacterium tuberculosis is estimated to infect 80 to 100 million people annually, the majority of whom do not develop clinical tuberculosis (TB) but instead maintain the infection in a latent state. These individuals generally become positive in response to a tuberculin skin test and may develop clinical TB at a later date, particularly if their immune systems are compromised. Latently infected individuals are interesting for two reasons. First, they are an important reservoir of M. tuberculosis, which needs to be considered for TB control. Second, if detected prior to recrudescence of the disease, they represent a human population that is making a protective immune response to M. tuberculosis, which is very important for defining correlates of protective immunity. In this study, we show that while responsiveness to early secretory antigenic target 6 is a good marker for M. tuberculosis infection, a strong response to the 16-kDa Rv2031c antigen (HspX or α-crystallin) is largely restricted to latently infected individuals, offering the possibility of differential immunodiagnosis of, or therapeutic vaccination against, TB.
Tuberculosis (TB) is easily transmitted by inhalation of infectious Mycobacterium tuberculosis bacilli from the air and is one of the leading causes of death in adults between the ages of 15 and 45 years (43). The global incidence rate of TB is increasing by approximately 0.4% per year (43), driven by factors such as the human immunodeficiency virus (HIV)/AIDS epidemic, poverty, and increasing population density. Despite the existence of effective treatment regimens, control of TB is complicated by the chronic nature of the disease. Only 5 to 10% of recently exposed individuals develop clinically active TB in the first 2 years after exposure, and this, together with the often transient nature of exposure, makes accurate tracing of recently exposed and potentially infected individuals extremely difficult.
A higher percentage of exposed individuals become infected but are able to limit the growth of the mycobacteria and become latently infected. The exact incidence of latent infection remains unclear, but it is estimated that up to 2 billion people globally may harbor latent TB infections (17, 22, 43). Latency can only be understood as a dynamic process—an active host immune response is essential to keep the disease in the latent stage, as demonstrated by the high rate of TB recrudescence in individuals with HIV infection (8) or the reactivation of the latent infection in individuals given anti-tumor necrosis factor alpha therapy (29). The decline of the immune system due to old age or malnutrition also increases the risk of latent disease becoming clinically significant.
Thus, latently infected individuals provide a highly relevant population to study what human protective immune responses against M. tuberculosis look like. However, identifying these people has been problematic in the past. The classical definition of latent infection (conversion to positivity in the purified protein derivative-based skin test after exposure to M. tuberculosis, without clinical illness) is of limited reliability, especially in TB-endemic countries, where widespread Mycobacterium bovis BCG vaccination or high levels of exposure to environmental mycobacteria often lead to skin test positivity. We have therefore used immune recognition of the M. tuberculosis virulence factor ESAT-6 (early secretory antigenic target 6), encoded by esat6 Rv3875, to define M. tuberculosis infection (12, 16). ESAT-6 is essential for the bacteria to survive and spread in vivo (7, 19), is expressed early in infection, and is recognized by most TB patients (1). Although immune responses to ESAT-6 by themselves cannot separate acute or latent TB (12, 15), recognition by healthy individuals without recent M. tuberculosis exposure is currently the best operational definition of latent M. tuberculosis infection (12, 16, 22).
With this approach, we have recently shown that latently infected healthy individuals produce elevated levels of mRNA for Th1 cytokines such as gamma interferon (IFN-γ) and in particular the antagonistic splice variant of interleukin-4 (IL-4), IL-4δ2 (12, 16). This implicates IL-4 in the clinical form of illness, a hypothesis supported by recent publications showing elevated IL-4 production in individuals with clinical illness or progressing toward clinical illness (28, 32, 33; A. Demissie et al., submitted for publication). However, M. tuberculosis is not a passive participant in this process. The bacteria respond to the host immune response by controlling their own gene expression and may interfere with the host's immune response as well (18, 25, 31, 34).
Typically, in latent infection, bacteria are present only in low numbers. The physical microenvironment where the bacteria survive has not been characterized in detail but is thought to include restricted access to nutrients and to oxygen and a low pH, together with elevated levels of hydrolytic enzymes and reactive nitrogen and oxygen species released by the host's immune response. The abundance of regulatory proteins in the M. tuberculosis genome (10) may explain the ability of the pathogen to adapt to this hostile environment (18, 26) by upregulating so-called latency genes. One of the most prominent of these is the one that encodes Rv2031c (also known as α-crystallin, HspX, or the 16-kDa antigen), whose importance is demonstrated by the reduced ability of bacteria deficient in this gene to grow in macrophages (45). Rv2031c is clearly expressed during infection in humans, as it is recognized by sera from a majority of TB patients (24). Moreover, production of Rv2031c appears to increase as the bacteria go into the metabolically resting stage (44) and to decrease as they revert to exponential growth (20). It therefore serves as the prototypic “latency-associated antigen.”
In this study, we compared immune responses to Rv2031c with responses to ESAT-6 in TB patients, contacts with well-defined recent exposure to someone with TB, and healthy individuals with and without evidence of prior infection with M. tuberculosis. The data, collected at multiple sites in TB-endemic and nonendemic regions, suggest that the ratio of immune responses to ESAT-6 and Rv2031c may be characteristic of different phases of M. tuberculosis infection. These results offer hope that specific diagnostic assays can be developed to distinguish between progressive TB and latent TB, even in individuals who are currently asymptomatic, and suggest that vaccination aimed at boosting immunity to latency phase antigens may be feasible.
MATERIALS AND METHODS
Study sites and subjects.
The study sites included Hossana and Butajira in the southern region of Ethiopia, Fajara in The Gambia, and Leiden in The Netherlands. The African cohorts analyzed are from an ongoing multicenter longitudinal study being carried out in Africa (VACSEL/VACSIS). These cohorts have been previously described in detail (12, 15, 16). Briefly, healthy household contacts (HHC) of TB patients were recruited from TB clinics. In both cases, blood was drawn on the first visit, before treatment of index cases commenced. Community controls (CC) were randomly selected from the same neighborhoods and prior TB disease or contact with TB excluded by questionnaire. Pulmonary TB was confirmed by the presence of acid-fast bacilli in at least two of three consecutive sputum samples or one positive sputum sample and a positive culture. Active TB was excluded in all healthy participants (HHC and CC) on entry into the study by radiological and clinical examination, sputum microscopy, and culture as previously described (15).
In The Netherlands, patients with pulmonary and extrapulmonary TB were recruited at the clinic and blood samples were taken from 9 patients with active TB and from 10 patients 1 to 8 years after successful treatment. Exposed, non-BCG-vaccinated individuals with a tuberculin skin test (TST) of >10 mm induration were recruited based on TB control program records as nonendemic latently infected individuals. Twenty of these had not received any prophylactic antibiotic, whereas three subjects had received isoniazid treatment 1 to 3 years postconversion. At the time this report was written, all TST-positive persons have remained free of clinical TB (12 years mean follow-up). Dutch CC were randomly selected and were TST negative, unvaccinated individuals with no known history of exposure to TB.
Blood samples were obtained from all donors at entry into the study. All participants in Africa were screened for HIV by repeated enzyme-linked immunosorbent assay (ELISA), and samples from HIV-positive individuals were excluded from the study. Pre- and posttest counseling was offered to all of these participants. Only adults (18 years old or older) who had given written consent were included in the study, and this work was performed under a study protocol approved by the institutional and national ethical review boards.
Sample preparation.
Venous blood (15 to 30 ml) was drawn into a Venoject tube containing heparin (Terumo, Leuven, Belgium) with a butterfly needle and gently mixed by inversion. Peripheral blood mononuclear cells (PBMC) were enriched as previously described (15, 30), by centrifugation over Ficoll-Hypaque (Pharmacia Biotech) and washing in complete medium (RPMI 1640 medium supplemented with 5% heat-inactivated pooled human AB serum, 1% l-glutamine, and 1% penicillin-streptomycin) before use for in vitro assays. Samples were then frozen in liquid nitrogen. Frozen cell samples were thawed and washed multiple times in complete medium before use. In all cases, viable cells were counted and diluted to 2 × 106 viable lymphocytes/ml in complete medium and 100 μl per well was used for both ELISA and enzyme-linked immunospot assay (ELISPOT).
Antigen preparations.
All recombinant antigens were produced as previously described (27). Briefly, the full-length genes were PCR amplified from cloned M. tuberculosis genomic DNA and subcloned. His-tagged proteins were expressed in Escherichia coli XL-1 Blue and purified essentially as described previously (37). Purified recombinant antigen was solubilized and stored at −80°C until use.
IFN-γ ELISA.
In vitro restimulation of PBMC with either purified protein derivative (20 μg/ml) or recombinant antigen (2 μg/ml) was carried out as previously described (15). The supernatants were harvested at day 5 after stimulation and stored at −80°C until assayed. The levels of IFN-γ were assayed from duplicate culture supernatants with capture monoclonal antibody 1-D1K and signaling monoclonal antibody 7-B6-1, in accordance with the manufacturer's instructions (Mabtech AB, Nacka Strand, Sweden), and cytokine concentrations were calculated with the standard curve generated from recombinant human IFN-γ or IL-4 (Life Technologies, Paisley, United Kingdom). Results are expressed in picograms per milliliter, and the cutoff for positivity was set at 3 standard deviations above the mean of unstimulated wells (110 pg/ml). The difference between duplicate wells was consistently less than 10% of the mean.
ELISPOT.
The ELISPOT was performed as previously described (16). For this study, we tested ESAT-6 or Rv2031c (10 μg/ml; Statens Serum Institut, Copenhagen, Denmark), phytohemagglutinin (5 μg/ml) as a positive control, or medium as a negative control. Cells were recovered from liquid nitrogen and serially titrated in duplicate wells of anti-human IFN-γ-precoated ELISPOT plates (MAIPS45; Millipore). ELISPOT plates were counted with an AID plate reader (Autoimmun Diagnostika, Strassberg, Germany). The mean number of spot-forming cells (SFC) per well for each antigen was calculated, and the mean number of SFC of the negative control was subtracted and transformed to the number of SFC per 106 cells. A positive response to antigen was taken as twice the background with more than 10 spot-forming units.
Statistics.
Comparisons between groups were assessed by the Kruskal-Wallis and Dunnett multiple-comparison tests. The Mann-Whitney test was used for analyses within groups. In all instances, a P value of <0.05 was considered significant.
RESULTS
Assessment of IFN-γ responses induced by early- and late-stage antigens from M. tuberculosis.
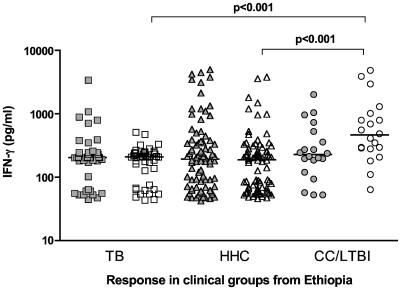
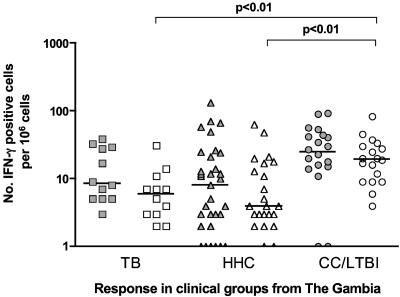
The Ethiopian study cohort was divided into three groups based on their clinical status: index cases (designated TB, n = 44), healthy HHC (n = 82), and CC (n = 20). Cells from the peripheral blood of each group were stimulated in vitro with either ESAT-6 (a so-called early-stage antigen) or RV2031c (the prototypic late-stage antigen), and the IFN-γ responses were assessed by ELISA. As shown in Fig. 1, all three groups contained a wide range of ESAT-6-responsive individuals, but although responses to Rv2031c were also present in all three groups, the median response was significantly higher in the CC group. A similar pattern was observed in the Gambian cohort (TB, n = 12; HHC, n = 32; CC, n = 20), but with the ELISPOT instead of the ELISA. While these assays are not exactly comparable in terms of magnitude, the general trends observed are comparable (14). ESAT-6 responses were not significantly different, but the number of cells responding to Rv2031c in the CC group was significantly elevated compared to the other two clinical groups (Fig. 2).
FIG. 1.
In vitro IFN-γ responses of PBMC from TB (n = 44), HHC (n = 82), and CC (n = 20) clinical groups from Ethiopia to restimulation with ESAT-6 (filled symbols) or Rv2031c (unfilled symbols) as assessed by ELISA. Results are individual responses. The medians of the groups are shown (bars), and levels of cytokine which were significantly different between groups are indicated.
FIG. 2.
In vitro IFN-γ responses of PBMC from TB (n = 12), HHC (n = 32), and CC (n = 20) clinical groups from The Gambia to restimulation with ESAT-6 (filled symbols) or Rv2031c (unfilled symbols) as assessed by ELISPOT. Results are individual responses expressed as numbers of spots per 106 PBMC. The medians of the groups are shown (bars), and groups in which the numbers of IFN-γ-producing cells were significantly different are indicated.
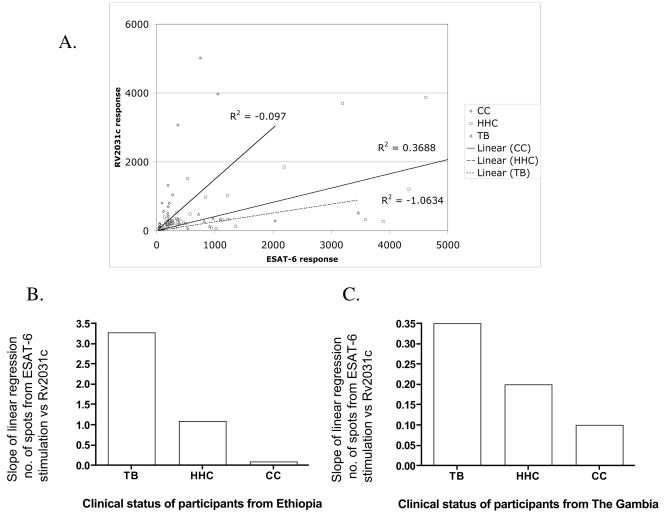
However, responses of the group as a whole tell us little about individual responses. We therefore visualized the results by plotting the IFN-γ response to the two antigens against each other for every antigen-responsive individual. When the responses were compared for each individual, a pattern was discernible, with immune responses to ESAT-6 tending to dominate responses to Rv2031c in subjects from the TB group, while individuals in the CC group were more biased toward Rv2031c, even if responsive to ESAT-6. This yielded a line of regression for each study group, and as shown in Fig. 3A, in the Ethiopian cohort, there was a gradation from the acute TB group to the CC group, with the HHC group in between. This is made plain by comparing the slopes of the lines, as shown in Fig. 3B. The same type of analysis for the Gambian cohort, with the ELISPOT data, yielded the same conclusion (Fig. 3C).
FIG. 3.
Regression analysis of the levels of the IFN-γ produced in response to ESAT-6 or Rv2031c in clinical groups from Ethiopia (A). The slopes of the line of regression for this analysis (B) and an identical analysis performed on ELISPOT data from The Gambia (C) show the bias of individual responses to one or the other antigen.
Elevated Rv2031c responses are associated with latent infection.
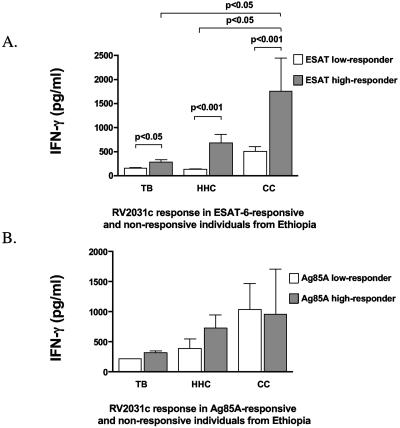
The data presented above suggest that there are substantial numbers of ESAT-6-responsive individuals in each group. For TB patients and HHC, this is hardly surprising and the incidence of positivity in the latter group from The Gambia matches that of a recent, larger study, supporting the representative nature of the data shown here. However, the number of ESAT-6-positive subjects among the CC groups can only be plausibly explained by the assumption that in a TB-endemic community, there will be cases of unsuspected latent TB infection even among those healthy individuals without identified exposure to someone with TB. This finding is consistent with a large body of earlier studies (9, 12, 22, 40), and this group was therefore designated CC/LTBI (for latent TB infection). To evaluate this further, we divided the CC group from the Ethiopian cohort into ESAT-6 high responders (3 or more standard deviations above the mean of unstimulated wells) who were presumptively latently infected (CC-Hi) or low responders (CC-Lo) and reanalyzed the data from the IFN-γ ELISA. As shown in Fig. 4A, the response to Rv2031c was elevated in ESAT-6-positive individuals from all groups (consistent with the hypothesis that the response is due to M. tuberculosis infection. However, the magnitude of the response was greatest in the ESAT-6-responsive (and therefore presumably latently infected) healthy individuals. Analysis of individual responses demonstrated that in the CC group, the strongest Rv2031c responders are also the strongest responders to ESAT-6, as indicated by the relatively low ratio of ESAT-6 to Rv2031c responses shown in Fig. 3. To ensure that this correlation was not simply coincidental, we segregated the CC group by the same criteria, but based on the response to the shared mycobacterial antigen Ag85A instead of ESAT-6. As shown in Fig. 4B, there were no significant differences between the clinical groups when they were separated on the basis of reactivity to Ag85A. These data indicate that the responses to Rv2031c seen in the strongly ESAT-6-responsive individuals from the CC group are consistently associated with responses to ESAT-6 but are particularly elevated in those subjects thought to have latent infections with M. tuberculosis. Subjecting the Gambian cohort to the same analysis led to the same conclusion, with the median response to Rv2031c among the high ESAT-6 responder CC group (45 spots/106 PBMC) being significantly higher than in the low-responder CC group (17 spots/106 PBMC, P < 0.005) and significantly higher than the ESAT-6 high responders from the TB or HHC groups (P < 0.01, data not shown). Thus, this analysis shows that in both African cohorts the strongest Rv2031c responses are found in latently infected healthy individuals (ESAT-6 responders in the CC/LTBI group). Even in those TB patients who strongly respond to ESAT-6, the response to Rv2031c was relatively low compared to the latently infected individuals in the CC group. This indicates that the lower Rv2031c responses observed in the TB patients cannot be entirely attributed to the generalized suppression of immune responses which can be observed during active disease. Since the CC group from both African cohorts was selected as much as possible to avoid recent TB exposure, the responses to ESAT-6 in this group are most easily explained by latent infection arising from a prior, unidentified encounter with someone with infectious TB.
FIG. 4.
In vitro IFN-γ responses of PBMC from the CC groups from Ethiopia to restimulation with Rv2031c after segregation into ESAT-6 high (CC-Hi) and low responders (CC-Lo) (A) or Ag85A high (CC-Hi) and low (CC-Lo) responders (B), as assessed by ELISA. Results are means and standard deviations expressed in pg/ml. Responses that were significantly different between groups are indicated.
Immune responses to ESAT-6 and Rv2031c in clinical cohorts from a non-TB-endemic setting.
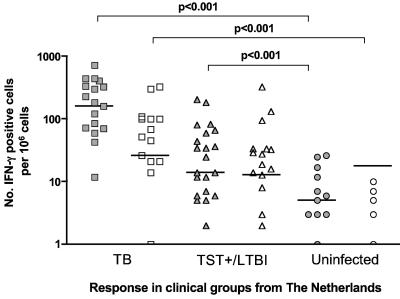
All of the data presented above are from TB-endemic countries, where although it is unlikely that the ESAT-6 responses in the CC group are due to a recent exposure, the possibility cannot be ruled out. Therefore, we carried out a similar analysis in a non-TB-endemic setting. We recruited 19 TB patients, 23 latently infected (TST-positive) individuals, and 13 healthy, non-BCG-vaccinated, TST-negative controls (uninfected). As before, we investigated T-cell recognition of Rv2031c and ESAT6 in the different groups by IFN-γ ELISPOT. Unlike the CC groups from Ethiopia and The Gambia, the unexposed group from The Netherlands would not be expected to contain a latently infected subset. Not unexpectedly, therefore (2), the Dutch uninfected group had no significant response to either ESAT-6 or Rv2031c, suggesting that (at least in a developed-world setting) exposure to environmental mycobacteria was not sufficient to generate any cross-reactivity against M. tuberculosis-derived Rv2031c (Fig. 5). However, the responses of the acute and latent TB groups were also noticeably different from those of the parallel groups derived from the TB-endemic countries. The TB patient group had significantly stronger responses to ESAT-6 than the uninfected group and the TST-positive (latently infected) group. In contrast, the Rv2031c responses in the latently infected, TST-positive group were not significantly different from those of the acutely infected TB group (though both groups were significantly more responsive to both ESAT-6 and Rv2031c than the uninfected group).
FIG. 5.
In vitro IFN-γ responses of PBMC from TB patients (n = 19), healthy TST-positive contacts (n = 23), and uninfected controls (n = 13) from The Netherlands to restimulation with ESAT-6 (filled symbols) or Rv2031c (unfilled symbols) as assessed by ELISPOT. Results are individual responses expressed as numbers of spots per 106 PBMC. The levels of IFN-γ-producing cells that were significantly different between groups are indicated.
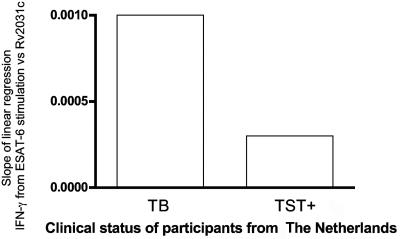
To further analyze the pattern of antigen recognition in TB patients versus latently infected individuals, the number of T cells specific for the early-stage antigen ESAT-6 was plotted against the number of T cells specific to the late-stage antigen RV2031c, as was done for the cohorts from TB-endemic regions as described above. The lines of regression had quite different slopes between the two groups (Fig. 6), with the same bias as seen in the cohorts from TB-endemic countries. These results confirm that individuals with acute infection demonstrate a relatively strong T-cell response toward ESAT-6 compared to Rv2031c, while this is not seen in TST converters, who are assumed to be latently infected.
FIG. 6.
Regression analysis of the levels of IFN-γ produced in response to ESAT-6 or Rv2031c in clinical groups from The Netherlands plotted against one another. The slopes of the line of regression for this analysis show the bias of individual responses to one or the other antigen.
DISCUSSION
The course of an M. tuberculosis infection is very complex, with only 5 to 10% of infections thought to progress to active TB in a short time. In most infected individuals, some bacteria are believed to survive and enter a quiescent phase, resulting in latent infection, which can reactivate later in life. So, infection with M. tuberculosis can give rise to disease at any time, from months to decades, after infection. The estimated one-third of the world's population that harbors latent TB therefore represents an immense infection reservoir and future source of disease transmission (43). Characteristically, M. tuberculosis initially enters the body by infecting alveolar macrophages. The acute phase of infection is characterized by rapid bacterial growth and the development of an immune response dominated by recognition of bacterial antigens actively secreted in the first growth phase, such as ESAT-6 (36). The development of a cell-mediated inflammatory response is clearly involved in arresting bacterial growth and subsequently restricting the infection to the latent stage, as is shown by the significantly increased risk of reactivation attendant on HIV infection (8) or inhibition of tumor necrosis factor alpha (21).
Though an obligatory aerobic organism, M. tuberculosis is able to adapt to and survive in the hypoxic and hostile environment of host macrophages. In this regard, it has been shown that M. tuberculosis undergoes a dramatic change in gene transcription characteristic of nonreplicating persistence (18, 26). When grown in vitro in oxygen-depleted cultures, as well as in cultures exposed to nitric oxide, M. tuberculosis up-regulates overlapping, characteristic sets of genes (34, 41, 44), among which is the gene encoding the small 16-kDa heat shock protein Rv2031c. Expression of this protein is increased by a factor of approximately 4 to 7 during the stationary growth phase (44) and appears to be crucial for survival of the organism (45). Recently, North and colleagues used real-time PCR in a mouse model of TB infection to demonstrate that when the immune response reaches the point where it inhibits the growth of the bacteria, the transcription of a number of genes is down-regulated while that of Rv2031c is up-regulated (35).
Accordingly, in the present study we have compared the recognition of the late-expressed antigen Rv2031c with that of an antigen (ESAT-6) expressed early during bacterial multiplication and also (at a reduced level) later in infection. Immune recognition of ESAT-6 is known to be highly specific for exposure to members of the TB complex (6, 22, 23, 30, 38), so it serves as a marker for prior M. tuberculosis infection. The data presented here demonstrate that Rv2031c, a protein which is known to be induced during stress-restricted growth of M. tuberculosis in vitro, was most strongly recognized by T cells from individuals with evidence of previous M. tuberculosis infection but who had neither symptoms nor any evidence of recent exposure to anyone with infectious TB—a profile most consistent with latent TB. This strongly suggests that Rv2031c is indeed expressed by M. tuberculosis in humans during latent infection. Moreover, in persons with acute TB or recent TB infection, the Th1 immune response, as measured by IFN-γ, while including an Rv2031c-responsive component, is biased toward ESAT-6, while in persons who have become latently infected after exposure to M. tuberculosis, the immune response is predominantly targeted toward Rv2031c. This led to observation of a consistent gradient in the ESAT-6/Rv2031c response ratio in the study groups, with a high ratio in TB patients, a low ratio in CC from endemic regions (due almost entirely to latently infected individuals), and an intermediate ratio in the HHC groups (recently exposed, healthy individuals). Moreover, this bias was detectable in study groups of different ethnicities drawn from three geographically distinct sites with very different rates of TB incidence. The differences in the magnitudes of the responses that were seen may reflect the environment in which the cohorts live, or they may reflect the nature of the patients' infections. In general, TB patients in Ethiopia and The Gambia are self-referred to the clinic and very often present with an advanced state of the disease, compared to TB patients in Europe. This is associated with a generalized suppression of immune responses (13, 15, 40), which may explain why the African TB patients had relatively lower responses to both ESAT-6 and Rv2031c compared to their Dutch counterparts, while the immune responses in the latently infected groups (who were all healthy) were comparable for both sites.
In this analysis, we have focused on cell-mediated immune responses to Rv2031c, whereas most previous work has looked at humoral responses. The most recent study of this kind, which tested serological responses to a panel of antigens, including Rv2031c, reached exactly the same conclusion as we have (11). This strongly suggests that this is a general phenomenon related to M. tuberculosis infection. Other studies are also compatible with this hypothesis—while antibody to Rv2031c increased in TB patients during treatment, it was initially low, suggesting that humoral responses to this antigen are also minimal during active disease (3), while chronically exposed but healthy individuals who may have been latently infected had high titers of antibody against this antigen (4). Interestingly, the strong association of Rv2031c with ESAT-6 reactivity, and therefore with M. tuberculosis infection, implies that exposure to other mycobacteria, whether in the environment or from BCG vaccination, does not induce strong reactivity to Rv2031c, even though genes encoding analogues are present in other mycobacteria. At first glance, this appears to contradict earlier work (42), but since the BCG vaccinees in that study were also exposed to M. tuberculosis, it is impossible to determine whether the immune responses are due to BCG vaccination or latent TB infection. Our work suggests the latter—and this is in agreement with the conclusions of that study, whose authors wrote that, in the case of M. tuberculosis, “…prolonged containment may be partially mediated by IFN-γ-producing CD4+ T cells responding to the 16-kDa antigen that is expressed by nonreplicating bacilli” (42).
There are several implications of these findings. The first is that the differences in antigen recognition described here and the differences in cytokine expression patterns previously described (12, 16) are both consistent with the identification of ESAT-6-responsive, healthy individuals without recent TB contact as latently infected. In addition, these data indicate that a strong IFN-γ response to Rv2031c correlates with a certain level of protection against TB disease, consistent with earlier suggestions (5, 42). If validated, this opens the possibility that generating (or boosting) immune responses against antigens expressed during the latent phase of infection might reduce the reactivation of latent infection. While this is speculative, therapeutic vaccines based on this concept are already being developed by a number of groups. Finally, these data also provide proof of concept for an immunodiagnostic test that could potentially allow clinicians to identify an M. tuberculosis infection by ESAT-6 responsiveness and estimate the risk that it is progressive or latent by determining the ratio of the responses to ESAT-6 and Rv2031c. Such an immunodiagnostic test would make it more feasible to identify and treat active TB at a very early stage, which could finally reduce the transmission of M. tuberculosis. This is of particular importance in TB-endemic countries, where the current emphasis is on treating active TB and where the huge reservoir of latent infection is likely to greatly reduce the specificity of immunodiagnosis based on RD antigens (9, 12, 22, 40). However, longitudinal studies are needed to confirm whether ESAT-6/Rv2031c response ratios are indeed predictive of progression to active TB disease. Further, it is likely that multiple early- and late-stage antigens will be required in order to obtain sufficient sensitivity for such an immunodiagnostic test. In this regard, it is encouraging that immune responses to CFP10, another early antigen from M. tuberculosis (22, 39), show a pattern very similar to those presented here for ESAT-6, albeit that CFP10 was only tested with a subset of the samples tested for ESAT-6 (data not shown) and that other antigens may mimic the pattern seen here with Rv2031c (11). Proteomic and transcriptomic analyses have identified more than 200 genes whose transcription is altered by transition to nonreplicating hypoxic growth conditions, so it will take some time—and much work—to assess the diagnostic potential of these antigens, alone or in combination.
Acknowledgments
The VACSEL Study Group also includes Chifumbe Chintu, Gina Mulundu, and Peter Mwaba (University of Zambia School of Medicine, Lusaka, Zambia); K. P. W. J. McAdam (until 2003), David Warndorff (2001), Christian Lienhardt (until 2001), and Sarah Burl (from 2004) (MRC, Gambia); and Louise Kim (UCL, United Kingdom) (from 2003).
We acknowledge Gebeyehu Haile and Fekede Lemma from the Hossana and Butajira Hospitals for contributions in the selection and screening of patients and Ato Alemayehu Kifle for bleeding and collecting specimens from these sites. We appreciate the Armauer Hansen Research Institute's administration for the support it provided when needed.
This study was funded by EU INCO contracts ICA-CT-1999-10005 and IC4-2001-10050, EU FP6 contract 503367, the Danish Forskningsrådet grant TB-latency, The Netherlands Organization for Scientific Research (NOW/ZonMw), and the institutes' core budgets. The Armauer Hansen Research Institute is supported by the governments of Ethiopia, Norway, and Sweden.
REFERENCES
- 1.Andersen, P., M. E. Munk, J. M. Pollock, and T. M. Doherty. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 356:1099-1104. [DOI] [PubMed] [Google Scholar]
- 2.Arend, S. M., P. Andersen, K. E. van Meijgaarden, R. L. Skjot, Y. W. Subronto, J. T. van Dissel, and T. H. Ottenhoff. 2000. Detection of active tuberculosis infection by T cell responses to early-secreted antigenic target 6-kDa protein and culture filtrate protein 10. J. Infect. Dis. 181:1850-1854. [DOI] [PubMed] [Google Scholar]
- 3.Bothamley, G. H. 2004. Epitope-specific antibody levels demonstrate recognition of new epitopes and changes in titer but not affinity during treatment of tuberculosis. Clin. Diagn. Lab. Immunol. 11:942-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bothamley, G. H., J. S. Beck, R. C. Potts, J. M. Grange, T. Kardjito, and J. Ivanyi. 1992. Specificity of antibodies and tuberculin response after occupational exposure to tuberculosis. J. Infect. Dis. 166:182-186. [DOI] [PubMed] [Google Scholar]
- 5.Bothamley, G. H., R. Rudd, F. Festenstein, and J. Ivanyi. 1992. Clinical value of the measurement of Mycobacterium tuberculosis specific antibody in pulmonary tuberculosis. Thorax 47:270-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brock, I., M. E. Munk, A. Kok-Jensen, and P. Andersen. 2001. Performance of whole blood IFN-γ test for tuberculosis diagnosis based on PPD or the specific antigens ESAT-6 and CFP-10. Int. J. Tuberc. Lung Dis. 5:462-467. [PubMed] [Google Scholar]
- 7.Brodin, P., I. Rosenkrands, P. Andersen, S. T. Cole, and R. Brosch. 2004. ESAT-6 proteins: protective antigens and virulence factors? Trends Microbiol. 12:500-508. [DOI] [PubMed] [Google Scholar]
- 8.Cahn, P., H. Perez, G. Ben, and C. Ochoa. 2003. Tuberculosis and HIV: a partnership against the most vulnerable. J. Int. Assoc. Physicians AIDS Care (Chicago) 2:106-123. [DOI] [PubMed] [Google Scholar]
- 9.Chapman, A. L., M. Munkanta, K. A. Wilkinson, A. A. Pathan, K. Ewer, H. Ayles, W. H. Reece, A. Mwinga, P. Godfrey-Faussett, and A. Lalvani. 2002. Rapid detection of active and latent tuberculosis infection in HIV-positive individuals by enumeration of Mycobacterium tuberculosis-specific T cells. AIDS 16:2285-2293. [DOI] [PubMed] [Google Scholar]
- 10.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. Mclean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M.-A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrel. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 11.Davidow, A., G. V. Kanaujia, L. Shi, J. Kaviar, X. Guo, N. Sung, G. Kaplan, D. Menzies, and M. L. Gennaro. 2005. Antibody profiles characteristic of Mycobacterium tuberculosis infection state. Infect. Immun. 73:6846-6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demissie, A., M. Abebe, A. Aseffa, G. Rook, H. Fletcher, A. Zumla, K. Weldingh, I. Brock, P. Andersen, and T. M. Doherty. 2004. Healthy individuals that control a latent infection with Mycobacterium tuberculosis express high levels of Th1 cytokines and the IL-4 antagonist IL-4δ2. J. Immunol. 172:6938-6943. [DOI] [PubMed] [Google Scholar]
- 13.Demissie, A., P. Ravn, J. Olobo, T. M. Doherty, T. Eguale, M. Geletu, W. Hailu, P. Andersen, and S. Britton. 1999. T-cell recognition of Mycobacterium tuberculosis culture filtrate fractions in tuberculosis patients and their household contacts. Infect. Immun. 67:5967-5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doherty, T. M., A. Demissie, D. Menzies, P. Andersen, G. Rook, and A. Zumla. 2005. Effect of sample handling on analysis of cytokine responses to Mycobacterium tuberculosis in clinical samples using ELISA, ELISPOT and quantitative PCR. J. Immunol. Methods 298:129-141. [DOI] [PubMed] [Google Scholar]
- 15.Doherty, T. M., A. Demissie, J. Olobo, D. Wolday, S. Britton, T. Eguale, P. Ravn, and P. Andersen. 2002. Immune responses to the Mycobacterium tuberculosis-specific antigen ESAT-6 signal subclinical infection among contacts of tuberculosis patients. J. Clin. Microbiol. 40:704-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fletcher, H. A., P. Owiafe, D. Jeffries, P. Hill, G. A. Rook, A. Zumla, T. M. Doherty, and R. H. Brookes. 2004. Increased expression of mRNA encoding interleukin (IL)-4 and its splice variant IL-4δ2 in cells from contacts of Mycobacterium tuberculosis, in the absence of in vitro stimulation. Immunology 112:669-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernandez-Pando, R., M. Jeyanathan, G. Mengistu, D. Aguilar, H. Orozco, M. Harboe, G. A. Rook, and G. Bjune. 2000. Persistence of DNA from Mycobacterium tuberculosis in superficially normal lung tissue during latent infection. Lancet 356:2133-2138. [DOI] [PubMed] [Google Scholar]
- 18.Honer zu Bentrup, K., and D. G. Russell. 2001. Mycobacterial persistence: adaptation to a changing environment. Trends Microbiol. 9:597-605. [DOI] [PubMed] [Google Scholar]
- 19.Hsu, T., S. M. Hingley-Wilson, B. Chen, M. Chen, A. Z. Dai, P. M. Morin, C. B. Marks, J. Padiyar, C. Goulding, M. Gingery, D. Eisenberg, R. G. Russell, S. C. Derrick, F. M. Collins, S. L. Morris, C. H. King, and W. R. Jacobs, Jr. 2003. The primary mechanism of attenuation of bacillus Calmette-Guérin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc. Natl. Acad. Sci. USA 100:12420-12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu, Y., and A. R. Coates. 1999. Transcription of the stationary-phase-associated hspX gene of Mycobacterium tuberculosis is inversely related to synthesis of the 16-kilodalton protein. J. Bacteriol. 181:1380-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keane, J., S. Gershon, R. P. Wise, E. Mirabile-Levens, J. Kasznica, W. D. Schwieterman, J. N. Siegel, and M. M. Braun. 2001. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 345:1098-1104. [DOI] [PubMed] [Google Scholar]
- 22.Lalvani, A., P. Nagvenkar, Z. Udwadia, A. A. Pathan, K. A. Wilkinson, J. S. Shastri, K. Ewer, A. V. Hill, A. Mehta, and C. Rodrigues. 2001. Enumeration of T cells specific for RD1-encoded antigens suggests a high prevalence of latent Mycobacterium tuberculosis infection in healthy urban Indians. J. Infect. Dis. 183:469-477. [DOI] [PubMed] [Google Scholar]
- 23.Lalvani, A., A. A. Pathan, H. Durkan, K. A. Wilkinson, A. Whelan, J. J. Deeks, W. H. Reece, M. Latif, G. Pasvol, and A. V. Hill. 2001. Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet 357:2017-2021. [DOI] [PubMed] [Google Scholar]
- 24.Lee, B. Y., S. A. Hefta, and P. J. Brennan. 1992. Characterization of the major membrane protein of virulent Mycobacterium tuberculosis. Infect. Immun. 60:2066-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manca, C., M. B. Reed, S. Freeman, B. Mathema, B. Kreiswirth, C. E. Barry III, and G. Kaplan. 2004. Differential monocyte activation underlies strain-specific Mycobacterium tuberculosis pathogenesis. Infect. Immun. 72:5511-5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehrotra, J., and W. R. Bishai. 2001. Regulation of virulence genes in Mycobacterium tuberculosis. Int. J. Med. Microbiol. 291:171-182. [DOI] [PubMed] [Google Scholar]
- 27.Olsen, A. W., L. A. van Pinxteren, L. M. Okkels, P. B. Rasmussen, and P. Andersen. 2001. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Infect. Immun. 69:2773-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ordway, D. J., L. Costa, M. Martins, H. Silveira, L. Amaral, M. J. Arroz, F. A. Ventura, and H. M. Dockrell. 2004. Increased interleukin-4 production by CD8 and γδ T cells in health-care workers is associated with the subsequent development of active tuberculosis. J. Infect. Dis. 190:756-766. [DOI] [PubMed] [Google Scholar]
- 29.Ormerod, L. P. 2004. Tuberculosis and anti-TNF-α treatment. Thorax 59:921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravn, P., A. Demissie, T. Eguale, H. Wondwosson, D. Lein, H. A. Amoudy, A. S. Mustafa, A. K. Jensen, A. Holm, I. Rosenkrands, F. Oftung, J. Olobo, F. von Reyn, and P. Andersen. 1999. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J. Infect. Dis. 179:637-645. [DOI] [PubMed] [Google Scholar]
- 31.Reed, M. B., P. Domenech, C. Manca, H. Su, A. K. Barczak, B. N. Kreiswirth, G. Kaplan, and C. E. Barry III. 2004. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature 431:84-87. [DOI] [PubMed] [Google Scholar]
- 32.Seah, G. T., and G. A. Rook. 2001. High levels of mRNA encoding IL-4 in unstimulated peripheral blood mononuclear cells from tuberculosis patients revealed by quantitative nested reverse transcriptase-polymerase chain reaction; correlations with serum IgE levels. Scand. J. Infect. Dis. 33:106-109. [DOI] [PubMed] [Google Scholar]
- 33.Seah, G. T., G. M. Scott, and G. A. Rook. 2000. Type 2 cytokine gene activation and its relationship to extent of disease in patients with tuberculosis. J. Infect. Dis. 181:385-389. [DOI] [PubMed] [Google Scholar]
- 34.Sherman, D. R., M. Voskuil, D. Schnappinger, R. Liao, M. I. Harrell, and G. K. Schoolnik. 2001. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding α-crystallin. Proc. Natl. Acad. Sci. USA 98:7534-7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi, L., Y. J. Jung, S. Tyagi, M. L. Gennaro, and R. J. North. 2003. Expression of Th1-mediated immunity in mouse lungs induces a Mycobacterium tuberculosis transcription pattern characteristic of nonreplicating persistence. Proc. Natl. Acad. Sci. USA 100:241-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sorensen, A. L., S. Nagai, G. Houen, P. Andersen, and A. B. Andersen. 1995. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 63:1710-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theisen, M., J. Vuust, A. Gottschau, S. Jepsen, and B. Hogh. 1995. Antigenicity and immunogenicity of recombinant glutamate-rich protein of Plasmodium falciparum expressed in Escherichia coli. Clin. Diagn. Lab. Immunol. 2:30-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulrichs, T., P. Anding, S. Porcelli, S. H. Kaufmann, and M. E. Munk. 2000. Increased numbers of ESAT-6- and purified protein derivative-specific gamma interferon-producing cells in subclinical and active tuberculosis infection. Infect. Immun. 68:6073-6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Pinxteren, L. A., P. Ravn, E. M. Agger, J. Pollock, and P. Andersen. 2000. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin. Diagn. Lab. Immunol. 7:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vekemans, J., C. Lienhardt, J. S. Sillah, J. G. Wheeler, G. P. Lahai, M. T. Doherty, T. Corrah, P. Andersen, K. P. McAdam, and A. Marchant. 2001. Tuberculosis contacts but not patients have higher gamma interferon responses to ESAT-6 than do community controls in The Gambia. Infect. Immun. 69:6554-6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voskuil, M. I., D. Schnappinger, K. C. Visconti, M. I. Harrell, G. M. Dolganov, D. R. Sherman, and G. K. Schoolnik. 2003. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 198:705-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkinson, R. J., K. A. Wilkinson, K. A. De Smet, K. Haslov, G. Pasvol, M. Singh, I. Svarcova, and J. Ivanyi. 1998. Human T- and B-cell reactivity to the 16kDa α-crystallin protein of Mycobacterium tuberculosis. Scand. J. Immunol. 48:403-409. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization. 2002. Global tuberculosis control. WHO report 2002. WHO/CDS/TB/2001.287. World Health Organization, Geneva, Switzerland.
- 44.Yuan, Y., D. D. Crane, and C. E. Barry III. 1996. Stationary phase-associated protein expression in Mycobacterium tuberculosis: function of the mycobacterial α-crystallin homolog. J. Bacteriol. 178:4484-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuan, Y., D. D. Crane, R. M. Simpson, Y. Q. Zhu, M. J. Hickey, D. R. Sherman, and C. E. Barry III. 1998. The 16-kDa α-crystallin (Acr) protein of Mycobacterium tuberculosis is required for growth in macrophages. Proc. Natl. Acad. Sci. USA 95:9578-9583. [DOI] [PMC free article] [PubMed] [Google Scholar]