Abstract
Ferric uptake regulation protein (Fur) is a bacterial global regulator that uses iron as a cofactor to bind to specific DNA sequences. The function of Fur is not limited to iron homeostasis. A wide variety of genes involved in various mechanisms such as oxidative and acid stresses are under Fur control. Flavohemoglobin (Hmp) is an NO-detoxifying enzyme induced by NO and nitrosothiol compounds. Fur recently was found to regulate hmp in Salmonella typhimurium, and in Escherichia coli, the iron-chelating agent 2,2′-dipyridyl induces hmp expression. We now establish direct inhibition of E. coli Fur activity by NO. By using chromosomal Fur-regulated lacZ reporter fusion in E. coli, Fur activity is switched off by NO at micromolar concentration. In vitro Fur DNA-binding activity, as measured by protection of restriction site in aerobactin promoter, is directly sensitive to NO. NO reacts with FeII in purified FeFur protein to form a S = 1/2 low-spin FeFur–NO complex with a g = 2.03 EPR signal. Appearance of the same EPR signal in NO-treated cells links nitrosylation of the iron with Fur inhibition. The nitrosylated Fur protein is still a dimer and is stable in anaerobiosis but slowly decays in air. This inhibition probably arises from a conformational switch, leading to an inactive dimeric protein. These data establish a link between control of iron metabolism and the response to NO effects.
Microorganisms have developed several mechanisms to survive in their hosts' environments. These include competition with their hosts for metal acquisition (1) and resistance to host defenses such as nitric oxide (NO), a cytotoxic weapon generated by macrophages (2). In eukaryotic cells, NO is metabolically produced by NO synthase from arginine, O2, and NADPH (3). In macrophages, an inducible NO synthase is produced after activation by endotoxins or cytokines and generates copious amounts of NO to poison pathogens (2). A few examples of bacterial NO synthases have been described (4, 5). An endogenous source of NO also may be found in some denitrifying bacteria (6). Because they use nitrate in place of oxygen for energy production, NO is a metabolic intermediate in the denitrification pathway. Denitrifiers possess an improved NO reductase that catalyzes conversion to N2O, keeping NO at a nontoxic level inside the cell (1–50 nM). NO, which is uncharged and nonpolar, can cross membranes to trigger its target responses (7). NO can injure cells by attacking the iron centers (8) in various key proteins such as nitrogenase (9) and ribonucleotide reductase (10). NO also induces modification of thiol-containing proteins, yielding nitrosothiol groups (11). Specific responses to NO are found in bacteria to prevent NO or NO-mediated damages. NO induces the expression of specific NO-detoxifying enzymes, the flavohemoglobin (Hmp) (12) and the flavorubredoxin (13). Hmp is an O2-nitroxylase in aerobic condition that catalyzes the reaction of NO− with O2 to give NO (14, 15). In anaerobic conditions, the flavorubredoxin has an O2-sensitive NO reductase activity in Escherichia coli (13). NO also induces the expression of enzymes involved in the oxidative stress response such as the manganese-containing superoxide dismutase (SodA). At the level of gene expression, the regulation occurs through SoxR activation (7). Indeed, evidence has been provided that SoxR may be an NO sensor (7). NO activates the SoxR protein by direct formation of a dinitrosyliron species, leading to the induction of the oxidative stress response (16). Concerning the Hmp, NO has been shown to induce 19-fold the hmp gene expression, in aerobic conditions, independently of the SoxRS regulon (12). Moreover, the same level of activation of hmp expression was observed after treatment with the iron-chelating agent 2,2′-dipyridyl in aerobic and anaerobic conditions, suggesting the involvement of an iron-dependent regulatory protein (12). Furthermore, in E. coli, this activation cannot be explained by the other described regulators of hmp: fumarate nitrate reductase, an anaerobic repressor, and the MetR protein (methionine biosynthetic pathway regulation). Indeed, a mutation in fumarate nitrate reductase stimulates only 4-fold hmp gene expression (12), and MetR activates hmp gene expression in response to nitrosothiol but not to NO (17). Thus, in E. coli, there is a missing link between NO stress and Hmp expression. Results obtained in other bacteria suggest that the ferric uptake regulation protein (Fur) may be the link. Indeed, in Salmonella typhimurium, expression of hmp also is induced after NO treatment (18), and its control is independent of the SoxS and OxyR transcription factors but relies on the iron-dependent Fur repressor (18). The control of hmp expression by Fur in S. typhimurium suggests a link between the control of iron metabolism and NO detoxification.
(14, 15). In anaerobic conditions, the flavorubredoxin has an O2-sensitive NO reductase activity in Escherichia coli (13). NO also induces the expression of enzymes involved in the oxidative stress response such as the manganese-containing superoxide dismutase (SodA). At the level of gene expression, the regulation occurs through SoxR activation (7). Indeed, evidence has been provided that SoxR may be an NO sensor (7). NO activates the SoxR protein by direct formation of a dinitrosyliron species, leading to the induction of the oxidative stress response (16). Concerning the Hmp, NO has been shown to induce 19-fold the hmp gene expression, in aerobic conditions, independently of the SoxRS regulon (12). Moreover, the same level of activation of hmp expression was observed after treatment with the iron-chelating agent 2,2′-dipyridyl in aerobic and anaerobic conditions, suggesting the involvement of an iron-dependent regulatory protein (12). Furthermore, in E. coli, this activation cannot be explained by the other described regulators of hmp: fumarate nitrate reductase, an anaerobic repressor, and the MetR protein (methionine biosynthetic pathway regulation). Indeed, a mutation in fumarate nitrate reductase stimulates only 4-fold hmp gene expression (12), and MetR activates hmp gene expression in response to nitrosothiol but not to NO (17). Thus, in E. coli, there is a missing link between NO stress and Hmp expression. Results obtained in other bacteria suggest that the ferric uptake regulation protein (Fur) may be the link. Indeed, in Salmonella typhimurium, expression of hmp also is induced after NO treatment (18), and its control is independent of the SoxS and OxyR transcription factors but relies on the iron-dependent Fur repressor (18). The control of hmp expression by Fur in S. typhimurium suggests a link between the control of iron metabolism and NO detoxification.
Fur is a global regulator ubiquitous in Gram-negative bacteria that controls the expression of >90 genes in E. coli (19). This dimeric protein (2 × 17 kDa) first was described as being involved in iron-uptake regulation. Fur is the key protein for the control of the intracellular iron concentration. The active form of the Fur protein, FeFur, contains a nonheme ferrous iron site with oxygen and nitrogen donor ligands (20, 21). When the cellular iron level becomes too low, the active Fur repressor looses Fe2+, its corepressor, and is no longer able to bind to specific DNA sequences. Fur links iron metabolism and the regulation of oxidative stress defenses. It regulates the expression of the superoxide dismutases (repression of the manganese superoxide dismutase and activation of the iron one) (22, 23), and fur expression is under control of OxyR and SoxRS (24).
In this paper, we present investigations of NO action on the Fur protein from E. coli by using in vivo and in vitro assays of Fur activity as well as spectroscopic characterization of nitrosyl adduct of pure, reconstituted FeFur. We find that the FeFur protein reacts with NO to form an inactive and stable iron nitrosyl adduct with a g = 2.03 EPR signal. Nitrosylation of Fur also occurs in vivo because the same EPR signal is observed in intact Fur-overproducing cells treated with NO. We show that NO inhibits Fur repressor activity in vivo and Fur DNA-binding activity in vitro. These data establish a link between the control of iron metabolism and the response to NO effects.
Materials and Methods
Chemicals.
Mohr's salt Fe(SO4)2(NH4)2⋅6 H2O, diethylamine, 1,3-bis[tris(hydroxymethyl)methylamino]propane, EDTA, 14NO gas (98.5% purity), and 15NO gas (98% 15N) were obtained from Sigma, and DEANO [diethylamine 2,2′-(hydroxynitrosohydrazino) (NONOate)] was obtained from Cayman Chemicals, Ann Arbor, MI. DEANO solutions were prepared in 10 mM NaOH; they decompose to 1.5 NO molecules in acidic solution (25).
Construction of the Mutant Strains.
Bacterial strains used in the study were all Δlac E. coli K-12 [QC 2461 (23)] derivatives of MG1655 (wild type, E. coli Genetic Stock Center). (fhuF:lacZ) and (fiu:lacZ) fusions (26) and Δfur:cat mutation (23) were introduced by P1 transduction as described (23), giving QC2949 (Δlac fhuF:lacZ), QC2950 (Δlac fiu:lacZ), QC6009 (Δlac Δfur:cat fhuF:lacZ), and QC6008 (Δlac Δfur:cat fiu:lacZ).
Media, Growth Conditions, and β-Galactosidase (β-Gal) Assays.
Cells were grown in anaerobiosis (Forma Scientific anaerobic chamber) in LB adjusted at pH 7.0 containing 1% glucose and 40 μg/ml kanamycin.
For cultures in the presence of NONOate, 0.8 ml was inoculated in 20 ml of medium at 37°C with an anaerobic culture to OD600 of 0.05–0.1. After exponential growth recovery, NONOate at 50 mM was added. Various times after inoculation, concentrations (10–100 μΜ) of DEANO (see Fig. 1) were assayed. Samples for measuring optical density and β-gal activity were taken at intervals. β-Gal assays were performed as described according to Miller and others (23, 27).
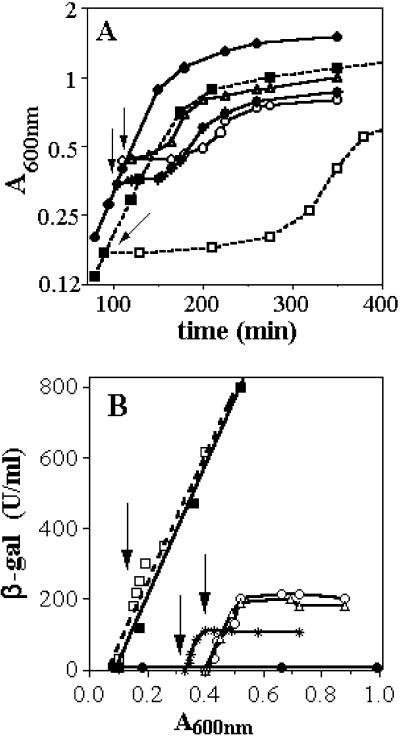
Fig 1.
Effect of NO on growth and β-gal expression in strains carrying fhuF:lacZ fusion. Strains fhuF:lacZ and Δ-Fur fhuF:lacZ were grown in the presence or absence of DEANO in anaerobiosis at 37°C (A) and assayed for β-gal (B). The differential rate of β-gal synthesis is represented as the total β-gal synthesized by milliliter of culture: β-gal activity expressed in Miller units × A600 (β-gal units/ml) in function of the absorbance at 600 nm (A600). Arrows indicate time of addition of DEANO. ○, Strains fhuF:lacZ with 25 μM DEANO; ▵ and *, with 10 μM DEANO; and •, no DEANO; □, Δ-Fur fhuF:lacZ with 200 μM DEANO; ▪, no DEANO.
Overproduction and Purification of Fur.
The T7 RNA polymerase/promoter system was used to overproduce Fur. The corresponding coding sequence was cloned into the NdeI/XhoI sites of a pET-30c expression vector (Novagen). The resulting plasmid, called pFur1, was transformed into BL21 (DE3) E. coli strain. Freshly transformed bacteria were plated on LB (0.1% agar) containing 50 μg/ml kanamycin and grown at 37°C. Cells were grown at 37°C from an overnight culture in 300 ml of LB containing 50 μg/ml kanamycin. Fur expression was induced at OD600 = 0.7–0.8 by isopropyl β-d-thiogalactoside (200 mM, 600 μl) during 2 h and 30 min. Dimeric Fur protein was purified as described (20). The buffer was exchanged for 100 mM 1,3-bis[tris(hydroxymethyl)methylamino]propane/100 mM KCl, pH 7.5. The purity was checked from SDS/PAGE. Protein concentrations were calculated by using an absorption coefficient at 275 nm of 0.4 mg−1⋅ml⋅cm−1 for the monomer of pure apo-Fur (20).
NO Treatment on Intact Cells.
Fur expression was induced in 200 ml of culture, as described in the previous section, during 2 h and 30 min. DEANO (200 mM) then was added to a final concentration of 10 μM. Aliquots (200 μl) were taken after 10, 60, and 180 min and transferred to an EPR tube. To increase signal intensity, 50 ml of bacterial culture immediately was concentrated 100-fold (by centrifugation at 900 × g and suspension of the pellet in 300 μl of LB) and 200 μl was transferred to an EPR tube. The supernatant after centrifugation also was checked by EPR.
Preparation of FeFur.
Fur apoprotein samples (1 mM, 500 μl) have been reconstituted anaerobically with 0.95 equivalent of Fe2+ as described (21). In the case of isotopically enriched 57Fe samples, the following modifications were used. Solid 57Fe Mohr's salt from Chemgas (100 mg, 255 μmol; Boulogne, France) was dissolved in water (5 ml), and the iron concentration was titrated by ferrozine (28). Fe2+ is incorporated efficiently in Fur as shown by the absence of the iron in the filtrate (checked with the ferrozine assay) of a 2-fold concentrated sample by using 10-kDa Ultrafree 0.5 (Millipore).
NO Complex Preparation.
For the FeFur EPR sample without isotopic labeling, FeFur (500 μl, 1 mM) was exposed for 1 h and 30 min at 20°C to 1 equivalent of NO using DEANO solution (240 mM, 1.3 μl), under gentle stirring in a container with limited free volume above the sample to minimize NO equilibration with the gas phase. The sample finally was concentrated to 2.5 mM by using 10-kDa Ultrafree 0.5. For the EPR quantification sample, 250 μl of a solution of 57FeFur at 2.6 mM was exposed to three successive additions of DEANO (7 μl at 240 mM) followed each time by 1 h and 30 min of incubation at 20°C. To remove unbound material from NO-treated FeFur, the sample was loaded on NAP-5 column (Amersham Pharmacia) followed by concentration to 200 μl at 3.25 mM on 10-kDa Ultrafree 0.5.
For the addition of 14NO and 15NO via NO gas, NO gas first was purified by passage through 5 M KOH to remove NO2. Then, an NO-saturated solution (1–2 mM, 250 μl) was prepared by bubbling purified NO gas for 30 min through a buffer solution deaerated by equilibration with argon. The solution was frozen in liquid nitrogen before it was added to concentrated FeFur solution (6 mM, 50 μl) in anaerobic conditions. A change from colorless to yellow-green was observed immediately. Samples were concentrated to 200 μl (1.5 mM). Controls by UV-visible and EPR spectroscopies of the filtrate as well as ferrozine assay showed the absence of Fe2+ and Fe–NO complex.
Activity Assay.
The capacity of metal-substituted Fur to bind DNA was investigated by using the method developed by Bagg and Neilands (29), which is based on the protection by the activated Fur protein of a hinfI site located in a Fur box inserted in a plasmid. pDT10 is a pUC19 derivative carrying the aerobactin promoter region: a 165-bp DNA fragment from the iuc (aerobactin gene) promoter region (−143 to +32), encompassing the Fur-box, was amplified by PCR by using primers carrying BamHI and HindIII restriction sites at their 5′ extremities. The resulting fragment was purified, digested with BamHI and HindIII, and inserted between the corresponding sites of pUC19, giving a 2,834-bp plasmid. pDT10 was transformed into a DH5α E. coli strain (Novagen). Plasmid DNA purification was performed according to the protocol of the Flexiprep kit (Amersham Pharmacia) and yielded 1.8 mg/ml plasmid DNA. pDT10 concentrations were obtained by using an absorption coefficient at 260 nm of 20 mg−1⋅ml⋅cm−1 and a molecular weight of 1.87 × 106 g⋅mol−1.
All buffers were treated on chelex resin (Bio-Rad) (30) to get reliable assays. pDT10 and apoFur (Fur protein without activating metal) samples were dialyzed for 2 h against metal-free buffer (100 mM 1,3-bis[tris(hydroxymethyl)methylamino]propane/100 mM KCl, pH 7.5). Protein (1 μl at various concentrations) and pDT10 (500 nM, 1 μl) were mixed under anaerobic conditions to a final volume of 10 μl in the metal-free buffer containing MgSO4 at 1 mM and incubated for 30 min at room temperature. Several conditions were assayed varying the final FeFur concentration from 200 nM to 200 μM. For assays in the presence of NO, the FeFur–pDT10 complex first was prepared with FeFur (2 and 20 μM) before the addition of two equivalents of DEANO (1 μl of solution at 4 and 40 μM, respectively) and incubation for 1 h. Digestion was carried out at 37°C by adding 1 unit of HinfI (1 μl). The reaction was stopped after 1 h by the addition of EDTA (0.25 M, 1 μl).
Gel-Exclusion Chromatography.
Samples containing 200 μM Apo-Fur, FeFur, and FeFur–NO complex in 100 mM 1,3-bis[tris(hydroxymethyl)methylamino]propane/100 mM KCl, pH 7.5, were loaded on analytical Superdex 75 10/30 (Amersham Pharmacia FPLC system) equilibrated with the same buffer and eluted at a flow rate of 1 ml/min at 4°C.
UV-Visible and EPR Spectroscopies.
UV-visible spectra were recorded on a Hewlett–Packard 8453 diode array spectrophotometer. X-band EPR spectra were recorded on a Varian E109 spectrometer equipped with an ESR-9 continuous-flow liquid helium cryostat (Oxford). Spin concentrations were measured by double-integration of the first-derivative EPR spectra. The resulting areas were compared with the signal from 1 mM aqueous Cu(H2O)6 recorded with identical instrument settings. A standard was prepared from 10 mM stock solution as follows: solid CuSO4 (100 mg) and NaClO4 (11.24 g) were dissolved in HCl (10 mM, 40 ml). EPR simulations were obtained with Frank Neese's program named EPR V.1.0 (University of Konstanz, Konstanz, Germany). The axis systems of the [g] and [A] tensors of Fur-57Fe-NO were assumed to be colinear, in the absence of any further information.
Results
In Vivo, the Activity of Fur Repressor Is Sensitive to NO.
To probe the influence of NO on Fur activity in vivo, we used an E. coli strain harboring a single copy of a fhuF:lacZ operon fusion. The fhuF gene (ferrioxiamin B utilization), a Fur-regulated gene, was chosen as reporter because its expression is very sensitive to small changes in Fur activity (31). To generate NO in the medium, DEANO, an NO donor, has been used. Each DEANO molecule liberates 1.5 NO, and t1/2 = 2 min at 37°C (pH 7.5). These experiments were done in strict anaerobic conditions to avoid the reaction of NO with O2. In the absence of NO, only a basic level of β-gal activity was observed as a result of Fur repressor activity. After adding DEANO at 10 μM, a growth delay of ≈50 min was observed. This delay increased with DEANO concentration and approached 1 h and 30 min at 25 μM (Fig. 1A). The growth delay is caused by the cytostatic action of NO. As cells recovered growth ability, β-gal was induced (Fig. 1B). The induction was not persistent, and the maximum of β-gal expressed was not proportional to the concentration of DEANO but rather to the amount of bacteria initially present (OD600) before DEANO decomposition. These effects mirrored irreversible modifications after the short burst of NO. Moreover, the slope of the curves were almost identical (1,700 Miller units) to that obtained with the Δfur mutant (Fig. 1B). This indicates that 15 μM NO (10 μM DEANO) was sufficient to lead to a complete derepression of the fhuF:lacZ fusion. In the same experiment, the Δfur mutant treated with 200 μM DEANO showed a large growth delay but no alteration in the β-gal induction (Fig. 1B).
In contrast, by using a fiu:lacZ fusion that needs a severe iron deficiency to be derepressed (32), no induction was observed by NO treatment (data not shown) even at 200 μM DEANO. This suggests that this NO concentration was not sufficient to avoid Fur binding at the fiu promoter.
Although these experiments showed inhibition of Fur activity, indirect Fur inactivation mediated by NO could not be ruled out; thus, in vitro assays were performed.
FeFur Is Sensitive to NO in Vitro.
The Fur repressor acts by binding to a specific sequence located upstream of the regulated genes, thereby inhibiting RNA polymerase binding. In the restriction site protection assay, Fur protects a hinfI site from digestion, located between the −10 and −35 regions of the aerobactin promoter. According to Bindereif and Neilands (33), a 152-bp fragment of the aerobactin promoter is sufficient to provide regulation of a downstream iucA:lacZ fusion. Therefore, the protection of the hinfI site in pDT10 containing a 166-bp fragment of this promoter would indicate that the active Fur is bound to the promoter as shown in Fig. 2. Total HinfI digestion of pDT10 gave six fragments, including the 1,530- and 251-bp fragments, which came from the cleaved aerobactin promoter. A 1,781-bp fragment was expected when active Fur had protected the hinfI site. The Fur apoprotein did not bind the aerobactin promoter, but required a metal, Fe2+ in our case, to protect the hinfI site within the aerobactin promoter. FeFur was able to bind the aerobactin promoter in the whole range of molar ratios [FeFur dimer]/[pDT10] superior to 20 (not shown). When EDTA, a strong iron chelator, was added, protection was not observed anymore.
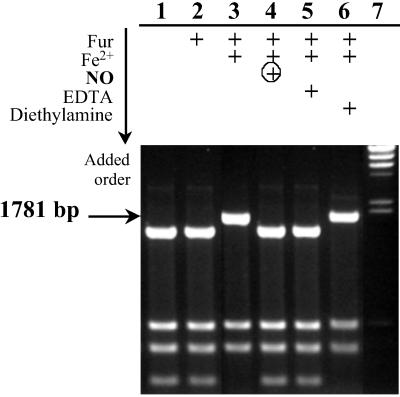
Fig 2.
In vitro assay of NO effect on Fur DNA binding. Fifty nanomolar plasmid pDT10 was cleaved by HinfI in the absence or presence of active Fur and after NO treatment. Reaction mixtures were analyzed on 1.5% agarose gel electrophoresis. Lanes: 1, no addition; 2, 20 μM apoFur; 3, 20 μM FeFur; 4, 20 μM FeFur + 40 μM DEANO after 1 h at 20°C; 5, 20 μM FeFur + 50 μM EDTA; 6, 20 μM FeFur + 40 μM diethylamine after 1 h at 20°C; 7, ladder λ DNA HindIII digest (arrow indicates the restriction fragment (1,781 bp) carrying the Fur box that was not cleaved (into 1,530-bp + 251-bp fragments) in the presence of active Fur.
When FeFur, first incubated with pDT10, was exposed to three equivalents of NO via DEANO, the 1,781-bp fragment was totally cleaved and generated the 1,530- and 251-bp fragments, suggesting direct inactivation of FeFur by NO. A control experiment showed that the aerobactin promoter remained fully protected in the presence of diethylamine, the other product of DEANO decomposition.
NO Binds to FeII in FeFur to Form a S = 1/2 Dimeric Species.
Spectroscopic studies on NO-modified FeFur were performed to characterize the interaction between NO and FeFur. The addition of NO gas to an anaerobic solution of FeFur resulted in immediate change from colorless to yellow-green, associated to the appearance of absorption bands in the UV-visible spectra as well as a g = 2.03 EPR signal. Both features are characteristics of NO–iron complexes (34–38). The rate of NO complex formation was controlled by the decomposition of DEANO (t1/2 = 15 min at 20°C, pH 7.5). The NO-complex formation developed >1 h and 30 min after exposure to one equivalent of NO. There was no difference in the EPR spectra when using NO gas instead of DEANO.
The X-band EPR spectrum of the FeFur–NO complex was characterized by an anisotropic signal around g = 2, with an isotropic g factor of giso = 2.03. This signal arose from a species with a S = 1/2 ground state (Fig. 3A). When the apoprotein was treated with NO in the same conditions, no EPR-detectable species appeared.
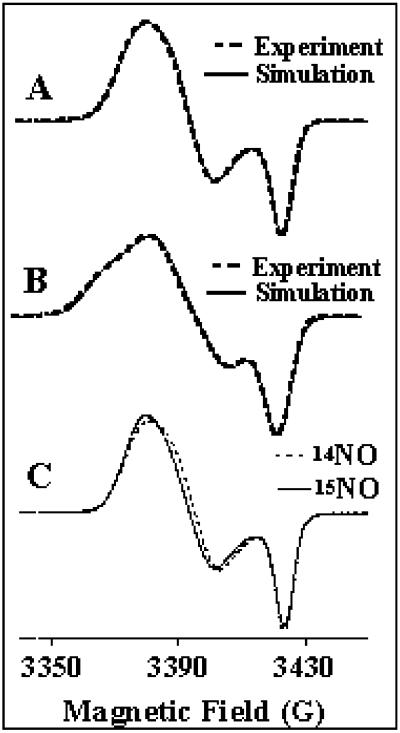
Fig 3.
EPR spectrum of isotopically labeled FurFe–NO complex. (A) FurFe–NO at 2.5 mM. The simulation is obtained with the g values (2.042; 2.032; 2.015) by using the linewidths 7.9, 7.0, and 4.0 G. (B) Fur57Fe-NO at 3.25 mM. The simulation is achieved by using the [g] values obtained previously with FurFe-NO and the hfs constants A/h (45.6, 35.9, and 3.8 MHz) with the respective linewidths (8.3, 7.5, and 4.6 G). (C) Comparison of Fe(II)-Fur–NO complex at 1.5 mM generated with unlabeled NO and 15NO. The simulations are achieved with the same set of g values (2.042; 2.032; 2.015) and the same linewidths as in A for unlabeled species, but with the respective linewidths (6.7, 7.2, and 3.5 G) for 15NO complex. Nonsaturating EPR conditions: microwave frequency, 9.655 GHz; power, 5 μW; modulation amplitude, 4 G; modulation frequency, 100 kHz; temperature, 30 K.
Furthermore, the 57Fe-labeled FeFur–NO complex displayed a distinct X-band EPR spectrum, especially when a shoulder was visible in the low-field region (Fig. 3B). This feature was due to the hyperfine contribution arising from the interaction between the electronic spin S = 1/2 and the nuclear spin of 57Fe (I = 1/2). This interaction demonstrates that iron is directly involved in the S = 1/2 species.
The double integration of this signal indicated that the corresponding species represented 85% of the iron in the sample, which was in agreement with Mössbauer quantification analysis (not shown). This proportion remained unchanged after new additions of NO as well as after gel-exclusion chromatography to remove any adventitious element bound to the protein.
The use of 15NO in place of the unlabeled NO gas to generate the FeFur–NO complex yielded a narrower EPR spectrum, especially in the low-field region (Fig. 3C). These changes arose from the distinct contribution of the 15N nucleus of NO to the hyperfine interactions. As expected for the contribution of the 15N nuclear spin I = 1/2, compared with the 14N nuclear spin I = 1, the linewidths were smaller than the linewidths associated to the unlabeled NO. The 15N labeling of NO demonstrates the binding of NO to the iron.
UV-visible spectra (not shown) revealed three bands at 310, 365, and 400 nm, characteristic of charge-transfer bands observed with small NO–iron complexes (35–38). The appearance of these bands grew in parallel with the increase of the EPR signal intensity. This links the S = 1/2 species and the UV-visible spectrum. The spectrum remained unchanged during 24 h in anaerobic conditions at 20°C. In air, the NO complex was stable for 1 h. Nevertheless, the UV-visible spectrum disappeared over several hours.
NO-Treated FeFur Is Still a Dimer.
We used analytical gel filtration to study the oligomeric state of the protein after the treatment by NO. FeFur treated with NO was eluted at the same volume as apo-Fur and FeFur species, which were both dimers (not shown), showing that NO-modified FeFur was still a dimer.
Fur Is Nitrosylated by NO Inside the Cell.
To analyze EPR species resulting from the interaction between NO and Fur inside cells, bacteria overexpressing the Fur protein were used. The cells treated by NO via DEANO exhibited a strong EPR signal in the g = 2.03 region (Fig. 4A). The signal was absent in untreated cells (Fig. 4B) or in treated cells in which Fur was not overexpressed (Fig. 4C). The signal also was observed when cultures were done in anaerobic conditions (data not shown). This S = 1/2 species revealed [g] values, linewidths, and saturation properties identical to those of the FeFur–NO complex. This EPR signal is associated to the presence of the FeFur–NO complex inside the cells.
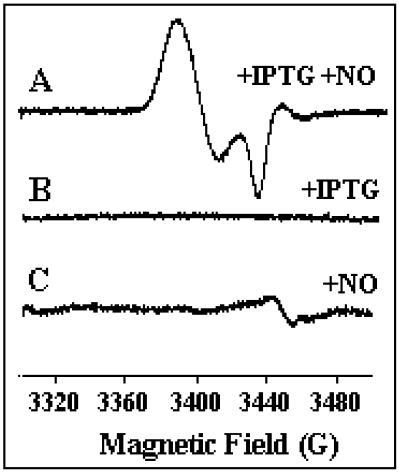
Fig 4.
EPR spectrum of intact cells treated with NO. Bacterial strains containing pFur1 were grown at 37°C in aerobiosis from the same preculture and sampled at the same time. (A) Fur synthesis was induced by isopropyl β-d-thiogalactoside during 2 h and 30 min and a sample was taken 60 min after the addition of DEANO (10 μM). (B) A sample was taken after Fur synthesis was induced during 3 h and 30 min without any addition of DEANO. (C) A sample was taken 60 min after the addition of DEANO (10 μM) in noninduced culture. EPR conditions: microwave frequency, 9.655 GHz; power, 5 μW; modulation amplitude, 10 G; modulation frequency, 100 kHz; temperature, 30 K.
The concentration of this NO complex estimated by EPR quantification was ≈0.3 μM in the bacterial culture in aerobic conditions. Because 50 mg of Fur/liter of culture usually was expressed, we expected a maximum Fur concentration of 3 μM in the cell suspension. Nevertheless, no iron has been added to the medium, so the concentration of active Fur protein, FeFur, is probably <3 μM. Moreover, these experiments have been done in aerobic conditions, which reduce the NO concentration because of its oxidation. Thus, the quantification of the EPR signal shows the possibility to complex large amounts of FeFur with NO inside cells. The intensity of the EPR signal was the same when bacterial culture was sampled 2 h after the addition of NO.
Discussion
The mechanism by which bacteria sense NO and protect themselves against NO is still poorly understood. Several studies established that Hmp, inducible by NO and nitrosothiol compounds, protects against nitrosative stress. In contrast to several NO-inducible genes involved in oxidative stress protection (7), induction of hmp expression by NO was not soxRS-dependent (12). Membrillo-Hernandez et al. (17) showed that induction by nitrosothiol depended on the MetR regulatory protein and proceeded via the nitrosation of its homocysteine cofactor. The mechanism of hmp induction by NO seems different. In E. coli, treatment with the iron chelator 2,2′-dipyridyl greatly induced hmp expression, but the underlying mechanism was unclear (12). In S. typhimurium, hmp expression was induced in a fur mutant and induction by NO was Fur-dependent, but it remained unclear whether this was a direct or an indirect effect (18).
Here, we establish, both in vitro and in vivo, that the active iron-containing form of Fur from E. coli, FeFur, is sensitive to NO at micromolar concentrations. The induction of the fur regulon thus appears as a new pathway for the cell response to NO stress in bacteria.
Fur-regulated lacZ fusions were used to probe Fur activity against NO in vivo. Fusions with different Fur-regulated genes were chosen because of their different inductions in response to iron chelators that presumably reflect specific Fur affinity for promoter regions. The fiu:lacZ fusion requires seven times more ferrozine to yield half of the derepression compared with fhuF:lacZ (32). Consistently, four overlapping Fur boxes were found in the σ promoter region of the fiu operon (39), and only two were found in the fhuF promoter (40). In our normal growing conditions, Fur was active. To assay Fur activity against NO, a range of NO concentrations close to the estimated intracellular Fur concentration was used. A value of ≈5,000 Fur copies per cell in exponentially growing E. coli cultures has been measured (24). Considering a cell volume 2 × 10−15 liters in the exponential growth phase (41), the intracellular Fur concentration would be ≈5 μM. Addition of 15 μM of NO, using DEANO, to anaerobic cell cultures led to complete derepression of the fhuF:lacZ fusion. Comparatively, hmp:lacZ fusions were derepressed in aerobic or anaerobic cultures by 20 μM NO gas in E. coli (12) and by 1 mM SperNO, another NO donor (smaller concentrations were not tested), in S. typhimurium (18).
In contrast, the fiu:lacZ fusion still was repressed at 300 μM NO. The promoter-dependent expression reflected the specific affinity of Fur for each promoter and then clearly involved inhibition of Fur activity. These data suggested that Fur was able to modulate the genes' expression in response to NO, depending on Fur affinity for the promoter.
The interaction between NO and Fur suggested by in vivo assays was investigated on the purified Fur protein. The DNA-binding ability of FeFur was assayed in vitro by using the aerobactin promoter region. In agreement with in vivo assays, we observed that a 3-fold excess of NO was sufficient to switch off Fur binding to the aerobactin promoter. According to the in vitro assays, Fur was inactivated directly by a small excess of NO. To specify the interaction between NO and FeFur, we recorded EPR and UV-visible spectra.
The addition of NO by using NO gas resulted in the immediate complexation of NO with FeFur, yielding a species stable in anaerobic conditions that stood during >1 h in aerobiosis. EPR spectroscopy showed that NO interacts directly with FeII in FeFur, inducing a low spin configuration characterized by an S = 1/2 ground state. The same characteristic EPR signal was recorded from Fur-overexpressing cells treated with NO in aerobic and anaerobic conditions. This result indicated that NO was able to cross the cell membrane and to target the active Fur protein. Furthermore, the stability of the S = 1/2 EPR signal in the cell was compatible with the timing of the Fur inhibition observed in the in vivo activity assay. It showed the link between the formation of the S = 1/2 FeFur–NO complex and Fur inhibition reflected by the derepression of gene expression under Fur control.
EPR investigations on 57Fe- and 15N-labeled FurFe–NO complex established that iron and NO were involved in a nitrosyliron unit. The EPR signal had an isotropic g value of g = 2.03, commonly associated with bis-cysteine dinitrosyl iron complexes of the type Fe(NO)2(RS)2 (34, 37, 42). They are referred to as the “g = 2.03” complexes because of their characteristic isotropic g factor. These kinds of complexes have been proposed in iron–sulfur proteins such as soxR and aconitase after reaction with NO only by means of their EPR properties (16, 43). However, the first, direct structural insight concerning the g = 2.03 family has been obtained with a bis-imidazole-coordinated complex mimicking histidine residues (34). In addition, some mononitrosyl–iron complexes containing nitrogen donor ligands also exhibit these types of EPR features (35, 36). Recent data concerning the nature of the ligands coordinated to iron in FeFur are much more in agreement with the environments in these compounds. Indeed, spectroscopic data on cobalt- and iron-substituted Fur indicated that the metal is hexacoordinated with one ligand at a longer distance and only nitrogen and oxygen donor ligands including two (or three) histidines and one (or two) aspartate or glutamate (20, 21). In conclusion, the nature of the EPR signal of the nitrosyl iron species in the FeFur–NO complex cannot be assigned without further spectroscopic characterizations. On this purpose, the studies by Mössbauer, x-ray absorption, electron nuclear double resonance, and Fourier transform–IR spectroscopies currently are in progress.
To fully explain in vivo observations, we wondered how the interaction of NO with FeFur could lead to the inactivation of Fur. It has been proposed that coordination of Fe2+ induces a conformational change of Fur dimer. Recent work has established that this conformational change enhances solvent accessibility of the DNA-binding region of Fur (44). Coordination of a divalent metal and a dimeric structure both are essential for DNA-binding activity. Gel filtration showed that the dimeric structure was not broken in the FurFe–NO complex. As NO binds to the iron, it may remove one of the six ligands. This change in the metal environment could alter the conformation of the protein and, therefore, modify the ability of Fur to bind to DNA. On this purpose, site-directed mutagenesis experiments have shown that a single mutation of residue likely involved in Fe2+ coordination yields an inactive Fur protein (45, 46). Mutation especially of His-90, a highly conserved amino acid in Fur sequences, leads to an inactive protein still able to dimerize (45).
Taken together, in vivo as well as in vitro assays and spectroscopic studies suggested that a fast and irreversible inhibition of Fur occurs by nitrosylation of FeII. It is the first time that reactivity of Fur iron site with an exogenous molecule is observed. We demonstrate that Fur is not only a sensor of iron, but through reactivity with an exogenous molecule at the iron site, provides a fine tuning of transcriptional control.
The modulation of transcription control via Fur in response to NO stress may provide protection against NO by several ways. The coupled protection against oxidative stress via regulation of superoxide dismutases by Fur minimizes the formation of extremely deleterious peroxynitrite. The activation of hmp expression through Fur inhibition in S. typhimurium directly detoxifies NO. E. coli (148 aa) and S. typhimurium (150 aa) proteins share 99% identity (or 96% identity and 2% similarity if the comparison is done the other way) with a strict conservation of the iron putative ligands. This suggests strongly that the mechanism of induction by NO of hmp expression in S. typhimurium is done by inactivation of Fur, via nitrosylation of the iron of the Fur protein, as demonstrated in this paper for Fur of E. coli. In E. coli, the mechanism of hmp regulation appears more subtle and depends on many regulators. However, a slight effect of Fur (2-fold induction in fur mutant; unpublished data) also is seen, coupling iron metabolism and defense against NO. Stimulation of iron metabolism as shown by the derepression of fhuF and iuc expression in E. coli (this work) and derepression of ircA and ircC in Salmonella (18) presumably favors the reconstitution of the iron proteins damaged by NO.
Acknowledgments
We are indebted to Sarah Dubrac for her assistance with the in vivo experiments, and Jacques Gaillard and Laurent Le Pape for their help with the EPR experiments.
Abbreviations
Fur, ferric uptake regulation protein
FeFur, Fe(II)–Fur complex
Hmp, flavohemoglobin
hmp, flavohemoglobin gene
NONOate, 2,2′-(hydroxynitrosohydrazino)
DEANO, diethylamine NONOate
β-gal, β-galactosidase
References
- 1.Neilands J. B. (1972) Struct. Bonding (Berlin) 11, 145-170. [Google Scholar]
- 2.Hibbs J. B., Taintor, R. R., Vavrin, Z. & Rachlin, E. M. (1988) Biochem. Biophys. Res. Commun. 157, 87-94. [DOI] [PubMed] [Google Scholar]
- 3.Marletta M. A., Yoon, P. S., Iyengar, R., Leaf, C. D. & Wishnok, J. S. (1988) Biochemistry 27, 8706-8711. [DOI] [PubMed] [Google Scholar]
- 4.Sari M.-A., Moali, C., Boucher, J. L., Jaouen, M. & Mansuy, D. (1998) Biochem. Biophys. Res. Commun. 250, 364-368. [DOI] [PubMed] [Google Scholar]
- 5.Adak S., Aulak, K. S. & Stuehr, D. J. (2002) J. Biol. Chem. 277, 16167-16171. [DOI] [PubMed] [Google Scholar]
- 6.Goretski J., Zafiriou, O. C. & Hollocher, T. C. (1990) J. Biol. Chem. 265, 11535-11538. [PubMed] [Google Scholar]
- 7.Nunoshiba T., DeRojas-Walker, T., Wishnok, J. S., Tannenbaum, S. R. & Demple, B. (1993) Proc. Natl. Acad. Sci. USA 90, 9993-9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lancaster J. R. & Hibbs, J. B., Jr. (1990) Proc. Natl. Acad. Sci. USA 87, 1223-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michalski W. P. & Nicholas, D. J. D. (1987) Arch. Microbiol. 147, 304-308. [Google Scholar]
- 10.Lepoivre M., Flaman, J. M. & Henry, Y. (1992) J. Biol. Chem. 267, 22994-23000. [PubMed] [Google Scholar]
- 11.Hausladen A., Privalle, C. T., Keng, T., DeAngelo, J. & Stamler, J. S. (1996) Cell 86, 719-729. [DOI] [PubMed] [Google Scholar]
- 12.Poole R. K., Anjum, M. F., Membrillo-Hernandez, J., Kim, S. O., Hughes, M. N. & Stewart, V. (1996) J. Bacteriol. 178, 5487-5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardner A. M., Helmick, R. A. & Gardner, P. R. (2002) J. Biol. Chem. 277, 8172-8177. [DOI] [PubMed] [Google Scholar]
- 14.Gardner A. M. & Gardner, P. R. (2002) J. Biol. Chem. 277, 8166-8171. [DOI] [PubMed] [Google Scholar]
- 15.Hausladen A., Gow, A. & Stamler, J. S. (2001) Proc. Natl. Acad. Sci. USA 98, 10108-10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding H. & Demple, B. (2000) Proc. Natl. Acad. Sci. USA 97, 5146-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Membrillo-Hernandez J., Coopamah, M. D., Channa, A., Hughes, M. N. & Poole, R. K. (1998) Mol. Microbiol. 29, 1101-1112. [DOI] [PubMed] [Google Scholar]
- 18.Crawford M. J. & Goldberg, D. E. (1998) J. Biol. Chem. 273, 34028-34032. [DOI] [PubMed] [Google Scholar]
- 19.Hantke K. (2001) Curr. Opin. Microbiol. 4, 172-177. [DOI] [PubMed] [Google Scholar]
- 20.Adrait A., Jacquamet, L., Le Pape, L., Gonzalez de Peredo, A., Aberdam, D., Hazemann, J. L., Latour, J. M. & Michaud-Soret, I. (1999) Biochemistry 38, 6248-6260. [DOI] [PubMed] [Google Scholar]
- 21.Jacquamet L., Dole, F., Jeandey, C., Oddou, J. L., Perret, E., Le Pape, L., Aberdam, D., Hazemann, J. L., Michaud-Soret, I. & Latour, J. M. (2000) J. Am. Chem. Soc. 122, 394-395. [Google Scholar]
- 22.Tardat B. & Touati, D. (1991) Mol. Microbiol. 5, 455-465. [DOI] [PubMed] [Google Scholar]
- 23.Dubrac S. & Touati, D. (2000) J. Bacteriol. 182, 3802-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng M., Doan, B., Schneider, T. D. & Storz, G. (1999) J. Bacteriol. 181, 4639-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maragos C. M., Morley, D., Wink, D. A., Dunams, T. M., Saavedra, J. E., Hoffman, A., Bove, A. A., Isaac, L., Hrabie, J. A. & Keefer, L. K. (1991) J. Med. Chem. 34, 3242-3247. [DOI] [PubMed] [Google Scholar]
- 26.Hantke K. (1984) Mol. Gen. Genet. 197, 337-341. [DOI] [PubMed] [Google Scholar]
- 27.Miller J. H., (1992) A Short Course in Bacterial Genetics (Cold Spring Harbor Lab. Press, Plainview, NY), pp. 352–355.
- 28.Stookey L. L. (1970) Anal. Chem. 42, 779-781. [Google Scholar]
- 29.Bagg A. & Neilands, J. B. (1987) Biochemistry 26, 5471-5477. [DOI] [PubMed] [Google Scholar]
- 30.Holmquist B. (1988) Methods Enzymol. 158, 6-12. [DOI] [PubMed] [Google Scholar]
- 31.Stojiljkovic I., Bäumler, A. J. & Hantke, K. (1994) J. Mol. Biol. 236, 531-545. [DOI] [PubMed] [Google Scholar]
- 32.Niehaus F., Hantke, K. & Unden, G. (1991) FEMS Microbiol. Lett. 68, 319-323. [DOI] [PubMed] [Google Scholar]
- 33.Bindereif A. & Neilands, J. B. (1985) J. Bacteriol. 162, 1039-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reginato N., McCrory, C. T. C., Pervitsky, D. & Li, L. (1999) J. Am. Chem. Soc. 121, 10217-10218. [Google Scholar]
- 35.Franz K. J. & Lippard, S. J. (1999) J. Am. Chem. Soc. 121, 10504-10512. [Google Scholar]
- 36.Hauser C., Glaser, T., Bill, E., Weyhermüller, T. & Wieghardt, K. (2000) J. Am. Chem. Soc. 122, 4352-4365. [Google Scholar]
- 37.Costanzo S., Ménage, S., Purrello, R., Bonomo, R. P. & Fontecave, M. (2001) Inorg. Chim. Acta 318, 1-7. [Google Scholar]
- 38.Jo D.-H., Chiou, Y.-M. & Que, L., Jr. (2001) Inorg. Chem. 40, 3181-3190. [DOI] [PubMed] [Google Scholar]
- 39.Newman D. L. & Shapiro, J. A. (1999) Mol. Microbiol. 33, 18-32. [DOI] [PubMed] [Google Scholar]
- 40.Zheng M., Wang, X., Doan, B., Lewis, K. A., Schneider, T. D. & Storz, G. (2001) J. Bacteriol. 183, 4571-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ali Azam T., Iwata, A., Nishimura, A., Ueda, S. & Ishihama, A. (1999) J. Bacteriol. 181, 6361-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boese M., Mordvintcev, P. I., Vanin, A. F., Busse, R. & Mülsch, A. (1995) J. Biol. Chem. 270, 29244-29249. [DOI] [PubMed] [Google Scholar]
- 43.Kennedy M. C., Antholine, W. E. & Beinert, H. (1997) J. Biol. Chem. 272, 20340-20347. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez de Peredo A., Saint-Pierre, C., Latour, J. M., Michaud-Soret, I. & Forest, E. (2001) J. Mol. Biol. 310, 83-91. [DOI] [PubMed] [Google Scholar]
- 45.Braun V., Schäffer, S., Hantke, K. & Tröger, W., (1990) 41. Colloquium Mosbach 1990 (Springer, Berlin), pp. 164–179.
- 46.Coy M., Doyle, C., Besser, J. & Neilands, J. B. (1994) Biol. Metals 7, 292-298. [DOI] [PubMed] [Google Scholar]