Abstract
Oral administration of lactoferrin (LF), an antimicrobial and immunomodulatory protein, shows a protective effect against infectious diseases, possibly via immunomodulation of the host. Initially, we confirmed an immunomodulatory effect of LF by observing changes in the number of cells in the leukocyte subsets in the peripheral blood and spleens of mice 1 day after oral administration of LF. Then we developed a quantitative reverse transcription-PCR method for 20 immunity-related genes of antimicrobial proteins, pattern recognition receptors, cytokines, and lymphocyte mobilization-related proteins, and we assessed the expression of these genes in the small intestines of mice 2 h after administration of water, bovine serum albumin (BSA), or LF. Expression of the LF gene was lower in mice administered LF than in mice administered water or BSA, implying a negative-feedback control. Expression of gamma interferon (IFN-γ) and interleukin-10 (IL-10) was lower in both BSA- and LF-administered mice than in water administered mice, suggesting a nonspecific effect of protein ingestion. Expression of NOD2, IFN-β, and IL-12p40 was higher with LF administration than with water or BSA administration. The expression levels of these three genes were correlated. This study indicated that oral administration of LF modulates the small intestinal expression of genes closely related to the host defense in a specific or a nonspecific manner.
Lactoferrin (LF) is a transferrin-family iron-binding glycoprotein present in milk and other exocrine secretions as well as in neutrophil granules. It is thought to play an important role as an innate-defense protein, because its activities include antimicrobial effects (3) and immunomodulatory effects (1), as shown previously by in vitro studies. The in vitro effects of LF are reported to involve activation of the transcription of genes such as matrix metalloproteinase 1, interleukin-1β (IL-1β), tracheal antimicrobial peptide, and p53 tumor suppressor (14, 15, 22, 32).
Recently, it has been increasingly reported that oral administration of LF or its fragment peptides enhances host protection against infections, cancer, and inflammation in adults as well as in infants (30, 31). Ingestion of LF or its peptides improves the resolution of symptoms or the survival rate, reduces levels of pathogens in the body, and maintains homeostasis in animals or humans infected with bacteria (2, 4), fungi (24, 36, 38), protozoa (7), or viruses (8, 21, 26, 33). Immunomodulatory effects of LF are thought to mediate these effects, because the following effects of orally administered LF have been reported in animal studies (27): enhancement of Th1 cytokine responses in splenocytes and lymph node cells (25, 33), enhancement of effector cell activities in peritoneal macrophages and splenic NK cells (19, 35), and increases in leukocyte numbers in the blood and lymphoid tissues (10, 25, 33, 37). The primary target of orally administered LF may be the intestinal immune system, because intact LF or its functional fragments, which include the antimicrobial peptide lactoferricin B and antibody-reacting peptides, are rarely transferred to the blood after feeding in adult animals without injury in the gut (34). It has also been reported that orally administered LF enhances the production of IL-18 and the activity of its processing enzyme, caspase-1, in the intestinal epithelia of mice (6, 10, 37). However, the effect of orally administered LF on the intestinal immune system has not been fully elucidated, especially at the transcriptional level.
In this study, we analyzed the number of leukocytes in the peripheral blood and spleens of mice to confirm the previously reported effects of orally administered LF. Then we developed a quantitative reverse transcription-PCR (RT-PCR) method for 20 murine immunity-related genes, including genes for antimicrobial proteins, pattern recognition receptors, cytokines, and lymphocyte mobilization-related proteins, and we examined the effects of orally administered LF on the expression of these genes in the small intestine to identify LF-responsive genes in vivo.
MATERIALS AND METHODS
Materials.
Bovine serum albumin (BSA) of protease- and gamma globulin-free grade was purchased from Sigma (Saint Louis, MO). Bovine LF with 8.2% iron saturation was produced by Morinaga Milk Industry Co. (Tokyo, Japan). Although LF can bind endotoxin, the content of endotoxin in the LF used was confirmed to be low (63 ng/g) by the Limulus test (Limulus HS-J Test; Wako Pure Chemical, Osaka, Japan).
Animal experiments.
Specific-pathogen-free female BALB/cByJ Jcl mice bred on diet CE-2 (Clea Japan, Tokyo, Japan) were obtained from Clea Japan and were used for animal experiments at the age of 8 to 9 weeks. Mice were allowed free access to a commercial standard diet (Labo MR stock; Nosan Corporation, Yokohama, Japan) and tap water. Diet CE-2 and Labo MR stock are both free from bovine materials such as milk products, LF, and BSA. Mice were orally administered 200 μl of water, BSA (2.5 g/kg of body weight) solution, or LF (2.5 g/kg of body weight) solution by gavage, because this dose of LF showed host-protective effects in our previous studies using infectious animal models (24, 33, 36). At 24 h after administration, peripheral blood was collected in heparin-coated syringes by cardiac puncture. The spleen was harvested in buffer A (0.3% BSA, 0.01% NaN3 in phosphate-buffered saline), gently disrupted, and filtered through a nylon mesh to obtain a single-cell suspension. The number of leukocytes was measured with a Sysmex F-300 cell counter (Toa, Kobe, Japan). Two, 6, and 24 h after administration of the test compound, a 3-cm length of jejunum was dissected, washed in phosphate-buffered saline with 10 mM EDTA, frozen with liquid nitrogen, and stored at −80°C until RNA extraction. All animal studies were approved by the Morinaga Milk Industry Animal Research Committee, and mice were maintained according to the guidelines for the care and use of laboratory animals of Morinaga Milk Industry.
Flow cytometry.
Cells were preincubated with 0.2 mg/ml of mouse immunoglobulin G (IgG; Chemicon, Temecula, CA) in buffer A for 30 min at 4°C to block FcγR and were subsequently treated with the indicated antibodies at 1.3 μg/106 cells in buffer A at 4°C for 45 min. The following panel of anti-mouse antibodies (BD Biosciences, San Jose, CA) was used: anti-CD3 molecular complex (R-phycoerythrin [PE]-conjugated rat IgG2b), anti-CD4 (fluorescein isothiocyanate [FITC]-conjugated rat IgG2a), anti-CD8α (FITC-conjugated rat IgG2a), anti-CD11b (FITC-conjugated rat IgG2b), anti-Gr-1 (Ly-6G, PE-conjugated rat IgG2b), and anti-γδ T-cell receptor (anti-TCRγδ) (FITC-conjugated hamster IgG). We also used the following isotype control antibodies (BD Biosciences): FITC-conjugated rat IgG2a and IgG2b, PE-conjugated rat IgG2b, and FITC-conjugated hamster IgG. Red blood cells were lysed, and leukocytes were fixed with fluorescence-activated cell sorter lysing solution (BD Biosciences) for 10 min at room temperature. Cells were analyzed using a FACSCalibur cytometer and CellQuest software (Becton Dickinson, Franklin Lakes, NJ). Positive populations were gated on the basis of staining profiles with an isotype-matched control antibody.
Quantitative RT-PCR.
RNA was extracted from the intestinal tissue by homogenization in RNA-Bee (TEL-TEST, Friendswood, TX). The amounts of RNA in the samples were quantified by spectrophotometry, and the quality of the RNA was verified by agarose gel electrophoresis. cDNA synthesis was performed with an Advantage RT-for-PCR kit (BD Biosiences Clontech, Palo Alto, CA). One microgram of total RNA and 20 pmol of oligo(dT)18 primer were incubated at 70°C for 2 min to denature the RNA template and then quench-cooled for 1 min. Moloney murine leukemia virus reverse transcriptase (200 U), 0.5 mM deoxynucleoside triphosphates, RNase inhibitor (20 U), and 1× reaction buffer were added to yield a final volume of 20 μl, and the reaction mixtures were incubated at 42°C for 60 min and at 94°C for 5 min.
cDNA reaction mixtures were diluted 1/10, and 5 μl of the diluted cDNA reaction mixture was added to 20 μl of Ex Taq R-PCR, version 1.0 (Takara Bio, Otsu, Japan) containing each primer pair at 0.3 μM (Table 1), 1/30,000-diluted SYBR Green I (Cambrex, Rockland, ME), and 3 mM MgCl2. Reactions were conducted in reaction tubes (Takara Bio) in the Cepheid Smart Cycler II System (Takara Bio), beginning with a 30-s hot-start activation of the Taq polymerase at 95°C, followed by 45 cycles of amplification in two steps (denaturation at 95°C for 10 s and annealing/extension/fluorescence detection) or three steps (denaturation at 95°C for 10 s, annealing/extension, and fluorescence detection) as indicated in Table 1. In cases in which nonspecific products were amplified aside from the specific product, only the fluorescence of the specific product was detected at the third step in the three-step amplification method. The purity and correct size of the amplified PCR products were confirmed by agarose gel electrophoresis and the melting curve. For real-time analysis, samples were quantified by comparison with a standard curve generated by amplifying serial fivefold dilutions of a nondiluted cDNA template with the respective primers. Relative gene expression units were calculated by normalization: the value for the target gene was divided by the value for the glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) gene.
TABLE 1.
Primer sequences and reaction parameters of genes tested by quantitative RT-PCR
| Gene type | Sequenceb | Size (bp) | Annealing, extension, and detection conditions |
|---|---|---|---|
| GAPDH | F GGGTGGAGCCAAACGGGTC | 532 | 69°C, 60 s |
| R GGAGTTGCTGTTGAAGTCGCA | |||
| Lysozyme P | F GGTCTACAATCGTTGTGAGTTGG | 386 | 65°C, 40 s |
| R CTCCGCAGTTCCGAATATACT | |||
| Cryptdin 4 | F AAGAGACTAAAACTGAGGAGCAGC | 196 | 69°C, 30 s |
| R CGGCGGGGGCAGCAGTA | |||
| Intelectin | F AGGGAATTTACTGCAGGATATGTTCAG | 331 | 63°C, 40 s |
| R CCAGTTACTAAGTCAGTGTTTCTCATG | |||
| LF | F AGGAAAGCCCCCCTACAAAC | 276 | 64°C, 20 s |
| R GGAACACAGCTCTTTGAGAAGAAC | |||
| TLR2 | F AAGAGGAAGCCCAAGAAAGC | 199 | 68°C, 30 s |
| R CGATGGAATCGATGATGTTG | |||
| TLR3 | F CACAGGCTGAGCAGTTTGAA | 190 | 68°C, 30 s |
| R TTTCGGCTTCTTTTGATGCT | |||
| TLR4 | F ACCTGGCTGGTTTACACGTC | 201 | 68°C, 30 s |
| R CTGCCAGAGACATTGCAGAA | |||
| NOD2 | F GGAGGAGCTTCCAGGAGTTT | 195 | 67°C, 30 s |
| R ACTCGTCCAAGCCATCAAAG | |||
| IFN-β | F TTCCTGCTGTGCTTCTCCAC | 606 | 69°C, 60 s |
| R GATTCACTACCAGTCCCAGAGTC | |||
| IFN-γ | F CCTGCAGAGCCAGATTATCTCTTTCTACC | 284 | 62°C, 30 s |
| R CCACCCCGAATCAGCAGCGA | |||
| IL-10 | F GGACAACATACTGCTAACCGAC | 256 | 70°C, 30 s |
| R AAAATCACTCTTCACCTGCTCC | |||
| IL-12p35 | F CATCGATGAGCTGATGCAGT | 163 | 70°C, 30 s |
| R CAGATAGCCCATCACCCTGT | |||
| IL-12p40 | F TGGAAGCACGGCAGCAGAATAAAT | 201 | 68°C, 30 s; 87°C, 6 sa |
| R TGCGCTGGATTCGAACAAAGAACT | |||
| IL-15 | F TTAACTGAGGCTGGCATTCATGTCTTC | 194 | 66°C, 20 s |
| R CAGTTCATTGCAGTAACTTTGCAACTG | |||
| IL-18 | F ACTGTACAACCGCAGTAATAC | 433 | 56°C, 50 s |
| R AGTGAACATTACAGATTTATCCC | |||
| IL-23p19 | F TGCTGGATTGCAGAGCAGTAA | 410 | 70°C, 20 s; 89.5°C, 6 sa |
| R CTGGAGGAGTTGGCTGAGTC | |||
| TECK/CCL25 | F TGAGGAGTGCCACCCTAGGTCATC | 234 | 70°C, 30 s |
| R GAGGGCCTTTAGCGCAGTAGTTCC | |||
| CCR9 | F GCCGTCTCCAACCTCTTGTTTGTAG | 212 | 69°C, 30 s |
| R AACTGGGCCTTCGGTCTGTGGAG | |||
| α4 integrin | F CCCAGGCTACATCGTTTTGT | 203 | 68°C, 30 s |
| R CATGAATGGGGGTAAGGATG | |||
| S1P receptor1 | F AACTTTGCGAGTGAGCTGGT | 227 | 68°C, 30 s; 91°C, 6 sa |
| R CTAGAGGGCGAGGTTGAGTG |
For three step amplification, the annealing/extension step was followed by the fluorescence detection step.
F, forward; R, reverse.
Statistical analysis.
Data are expressed as means ± standard errors of the means (SEM). Statistical analysis was done by a two-tailed Student t test. P values of <0.05 and <0.10 were considered to indicate significant differences and a tendency to be different, respectively. The Pearson correlation test was also done.
RESULTS
In the initial experiment, the effect of LF administration on the number of leukocytes in the peripheral blood and spleen was tested. Water, BSA, or LF was administered orally to mice, and after 1 day, the numbers of leukocytes and of various leukocyte subsets in the peripheral blood and spleen were determined by flow cytometry (Table 2). The number of peripheral blood leukocytes (PBL) tended to be higher in LF-administered mice than in water-administered mice (P = 0.0507). The numbers of CD4+ T cells (CD3+ CD4+) and granulocytes (CD11blo-hi Gr-1hi), but not those of CD8+ T cells (CD3+ CD8α+) or CD3− CD8α+ cells, in the peripheral blood were significantly higher in LF-administered mice than in water-administered mice. Mice given BSA showed similar numbers of those cells as mice given water. The number of γδ T cells (CD3+ TCRγδ+) in the blood was significantly higher in LF-administered mice than in BSA-administered mice. On the other hand, the numbers of total splenocytes and CD3− CD8α+ splenocytes tended to be lower in LF-administered mice than in BSA-administered mice (P = 0.0651 and 0.0999, respectively). The number of splenic CD4+ T cells, but not those of CD8+ T cells and granulocytes, was significantly lower in LF-administered mice than in BSA-administered mice.
TABLE 2.
Effects of LF administration on numbers of PBL and splenocytes
| Subset | No. of cells (106)a after administration of the indicated agent
|
||||
|---|---|---|---|---|---|
| PBL in 1 ml of blood
|
Splenocytes
|
||||
| Water | BSA | LF | BSA | LF | |
| Total | 7.62 ± 0.88 | 7.78 ± 0.67 | 10.48 ± 0.88# | 117.2 ± 9.1 | 96.2 ± 3.6# |
| CD3+ CD4+ | 1.78 ± 0.22 | 1.72 ± 0.40 | 2.34 ± 0.08* | 26.4 ± 1.2 | 22.4 ± 1.0* |
| CD3+ CD8α+ | 0.67 ± 0.10 | 0.60 ± 0.13 | 0.85 ± 0.08 | 13.0 ± 0.9 | 11.0 ± 0.7 |
| CD3− CD8α+ | 0.049 ± 0.025 | 0.026 ± 0.010 | 0.113 ± 0.047 | 0.76 ± 0.11 | 0.53 ± 0.05# |
| CD3+ TCRγδ+ | ND | 0.056 ± 0.007 | 0.142 ± 0.025* | ND | ND |
| CD11blo-hi Gr-1hi | 1.12 ± 0.12 | 0.94 ± 0.21 | 1.72 ± 0.12* | 3.84 ± 0.11 | 3.72 ± 1.18 |
The numbers of PBL, splenocytes, and their subsets were estimated 1 day after administration of water, BSA, or LF. Data are means ± SEM for five animals from one experiment representative of two independent experiments with similar results. *, P < 0.05; #, P < 0.10. P values refer to comparison with the BSA group for splenocytes and CD3+ TCRγδ+ PBL, or with the water group for other PBL. ND, not determined.
Next, the effect of LF administration on the expression of immunity-related genes in the small intestine was investigated. A method for quantitative RT-PCR for 20 immunity-related genes of mice was developed (Table 1), and the expression of these genes in the small intestine was compared 2 h after administration of water, BSA, or LF. Because in our preliminary experiment a change in gene expression was most apparent 2 h after LF treatment, we chose this interval until tissue collection.
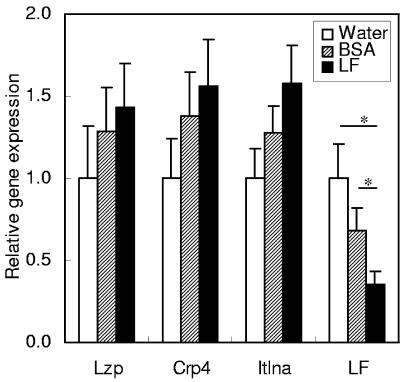
Among the four tested genes of antimicrobial proteins, lysozyme P, cryptdin 4, and intelectin (whose human homologue is also known to be a lactoferrin receptor in the intestine [23]) showed similar expression patterns (Fig. 1). The expression levels of these genes tended to increase in the order of: water, BSA, and LF administration, but there was no significant difference between these groups. On the other hand, the expression of the mouse LF gene was downregulated by LF administration.
FIG. 1.
Gene expression of antimicrobial proteins in the small intestines of mice. The gene expression levels of lysozyme P (Lzp), cryptdin 4 (Crp4), intelectin (Itlna), and LF were estimated by quantitative RT-PCR 2 h after administration of water, BSA, or LF. Data are means ± SEM for 5 to 10 animals from two independent experiments. *, P < 0.05.
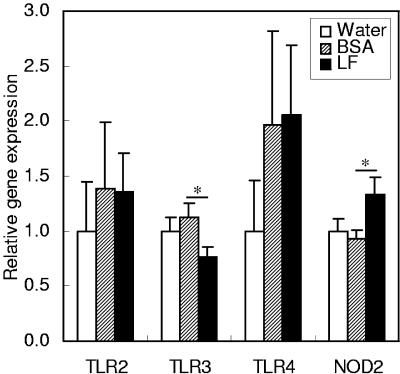
As pattern recognition receptors, TLR2, TLR3, TLR4, and NOD2 were examined (Fig. 2). Expression of the TLR3 gene was lower with LF administration than with BSA administration. Although the expression of the TLR4 gene appeared to be higher with BSA or LF administration than with water administration, the expression levels were highly variable between individual animals and there was no significant difference between the groups. Expression of the NOD2 gene was higher with LF administration than with BSA administration.
FIG. 2.
Gene expression of pattern recognition receptors in the small intestines of mice. The gene expression levels of TLR2, TLR3, TLR4, and NOD2 were estimated by quantitative RT-PCR 2 h after administration of water, BSA, or LF. Data are means ± SEM for 5 to 10 animals from two independent experiments. Expression of TLR4 is shown on a logarithmic scale. *, P < 0.05.
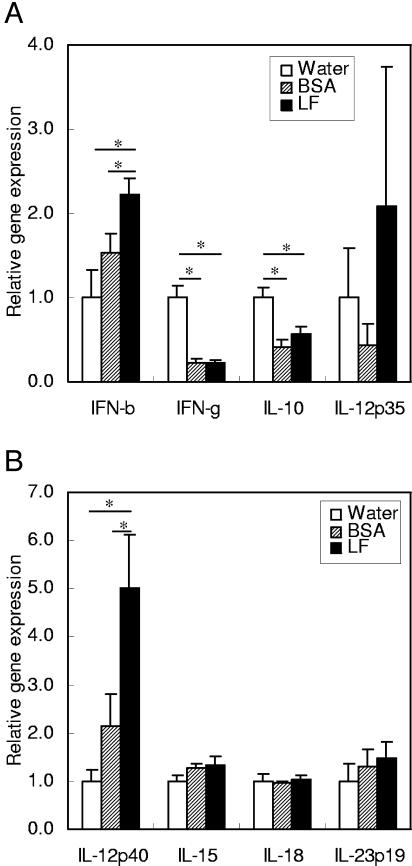
As cytokines, beta interferon (IFN-β), IFN-γ, IL-10, IL-12p35, IL-12p40, IL-15, IL-18, and IL-23p19 were examined (Fig. 3). Expression of IFN-β and IL-12p40 was higher with LF administration than with water or BSA administration. Expression of IFN-γ and IL-10 was lower with either BSA or LF administration than with water administration. Expression of other cytokines did not show a significant difference among the treatment groups.
FIG. 3.
Gene expression of cytokines in the small intestines of mice. The gene expression levels of IFN-β, IFN-γ, IL-10, and IL-12p35 (A) and of IL-12p40, IL-15, IL-18, and IL-23p19 (B) were estimated by quantitative RT-PCR 2 h after administration of water, BSA, or LF. Data are means ± SEM for 5 to 10 animals from two independent experiments. *, P < 0.05.
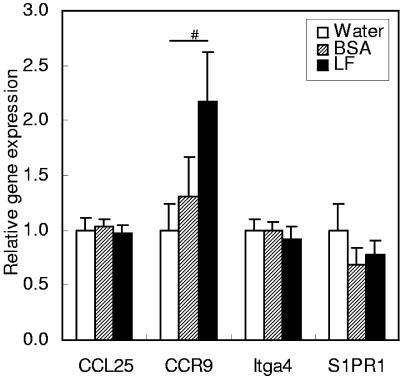
As lymphocyte mobilization-related proteins, TECK/CCL25 (a chemokine that chemoattracts CCR9-expressing intestine-directed lymphocytes), CCR9 and α4 integrin (both of which are expressed in intestine-directed lymphocytes), and S1P receptor 1 (a mediator of lymphocyte egress from lymphoid organs) were examined (Fig. 4). Among these four genes, CCR9 showed a tendency to be expressed at a higher level with LF administration than with water administration (P = 0.0768). Other genes did not show significantly different expression among the treatment groups.
FIG. 4.
Gene expression of lymphocyte mobilization-related proteins in the small intestines of mice. The gene expression levels of TECK/CCL25, CCR9, α4 integrin (Itga4), and S1P receptor 1 (S1PR1) were estimated by quantitative RT-PCR 2 h after administration of water, BSA, or LF. Data are means ± SEM for 5 to 10 animals from two independent experiments. #, P < 0.10.
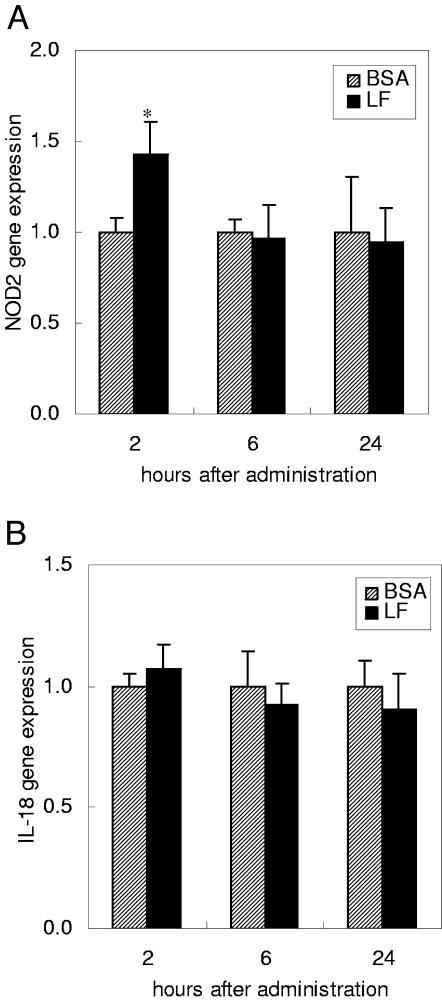
The time course of the gene expression of NOD2 and IL-18 was examined 2, 6, and 24 h after the administration of BSA or LF (Fig. 5). IL-18 was chosen for the time course experiment because it did not induce any change at 2 h (Fig. 3B), in contrast to previously reported findings (10, 37). LF administration enhanced the expression of NOD2 at 2 h but not at 6 or 24 h. IL-18 expression was not changed from 2 to 24 h by LF administration.
FIG. 5.
Time course of gene expression of NOD2 (A) and IL-18 (B) in the small intestines of mice. The gene expression levels were estimated by quantitative RT-PCR 2, 6, and 24 h after administration of BSA or LF. Data are means ± SEM for 4 to 10 animals from two independent experiments. *, P < 0.05.
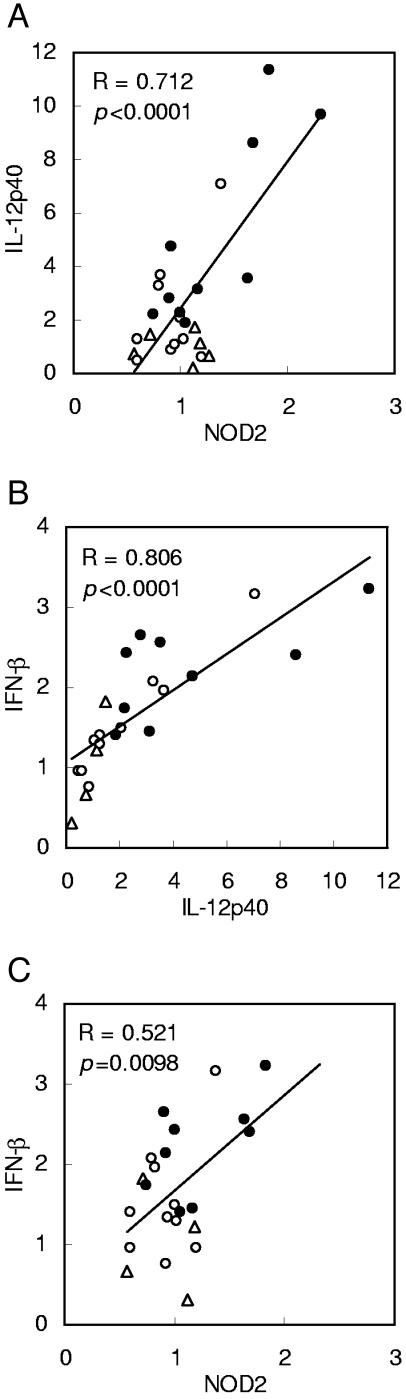
Finally, correlation among the expression levels of NOD2, IFN-β, and IL-12p40, which were upregulated 2 h after LF administration, was analyzed (Fig. 6). The expression level of IL-12p40 was significantly correlated with that of NOD2 and IFN-β with a high regression value. The expression levels of IFN-β and NOD2 were also significantly correlated.
FIG. 6.
(A to C) Correlation between gene expression levels of NOD2, IFN-β, and IL-12p40 in the small intestines of mice. The expression levels of the genes were upregulated 2 h after LF administration, as shown in Fig. 2 and 3, and they were further analyzed by Pearson correlation analysis. The regression curves for the expression of the genes after administration of water (triangles), BSA (open circles), and LF (closed circles), and the regression values (R) and P values, are shown.
DISCUSSION
Because a previous report demonstrated that orally administered LF increases some leukocyte subsets in the peripheral blood (10), we examined the effect of oral LF on the numbers of PBL, splenocytes, and their subsets in the initial experiment here. We observed that LF administration increases the number of CD4+ T cells in the peripheral blood, as previously reported (10). In addition, we found that LF administration also increases the numbers of blood granulocytes and γδ T cells but decreases the number of CD4+ T cells in the spleen. These findings may reflect mobilization of leukocytes from the spleen to peripheral blood. Since we previously observed that LF administration prevents the decrease in the number of splenocytes induced by herpes simplex type 1 infection of mice (33), the effect of LF on the behavior of leukocytes may change depending on the host immune status. In any case, we confirmed the immunomodulatory effect of orally administered LF in our experimental system using healthy mice, and we further examined the immunomodulation, focusing on the small intestine.
In the next step, we developed a real-time RT-PCR method for quantification of 20 immunity-related genes. We examined gene expression in the small intestines of mice administered water, BSA, or LF. Among the genes of four antimicrobial proteins examined, lysozyme P, cryptdin 4, and intelectin showed similar expression patterns, namely, increasing expression in the order of water, BSA, and LF administration, although the effect was not significant. These genes are known to be expressed in the paneth cells of the crypt (9, 16, 18), and their expression levels were correlated in our experiments (R = 0.809 to 0.864 [details not shown]). Human intelectin is reported to be an intestinal receptor of human LF (23). In our study, LF administration had no obvious effect on intelectin expression. Expression of the LF gene was detected in the small intestine and was downregulated by administration of bovine LF. This result implies that there is negative-feedback control of LF gene expression.
Regarding the four pattern recognition receptors studied here, expression of TLR3 was downregulated by LF administration, whereas expression of NOD2 was upregulated. NOD2 expression was transiently enhanced by LF at 2 h. The NOD2 gene is expressed in paneth cells and mononuclear cells in the intestine (12) and is associated with susceptibility to Crohn's disease (5, 13). It has been reported that oral administration of LF reduces trinitrobenzenesulfonic acid- or dextran sulfate-induced colitis in animals (28, 29). Although NOD2 expression was not investigated in those studies, the possible involvement of NOD2 in the effect of LF on intestinal bowel diseases is intriguing.
The expression of eight cytokine genes was investigated. Since administration of both BSA and LF decreased the expression of IFN-γ and IL-10, this effect is considered to be a nonspecific protein-induced effect. These cytokines exhibit opposite effects: IFN-γ is proinflammatory and IL-10 is antiinflammatory. We administered a relatively high dose (2.5 g/kg of body weight) of proteins to mice, and this may have caused nonspecific transcriptional downregulation of these cytokines. Expression of IFN-β and IL-12p40 was upregulated solely by LF administration. These cytokines are well characterized as important mediators of host defense against infections and cancer. The expression levels of IFN-β, IL-12p40, and NOD2 were correlated. Therefore, expression of these genes may be regulated by a common mechanism. It would be rational to examine these genes as LF-responsive markers in the small intestine for investigation of LF effects. Expression of IL-18 was not altered by LF administration from 2 to 24 h, although previous studies found that oral LF induces IL-18 production (10, 37). IL-18 production is probably upregulated at the step of processing of the protein by caspase-1 (6, 37) rather than being regulated at the transcriptional level.
Gene expression of lymphocyte mobilization-related proteins was not significantly changed by LF administration. CCR9, which is expressed in intestine-directed lymphocytes (17), showed only a nonsignificant tendency to higher expression in LF-administered mice. In view of the fact that previous investigations showed that LF administration increases the numbers of some subsets of lymphocytes in the intestine (10, 37), enhancement of CCR9 expression may have been due to an increase in the number of CCR9-expressing lymphocytes.
It has been reported that after 1 week of oral administration of bovine LF, antigen-specific antibody and cytokine responses are induced (20). However, the effect of bovine LF observed here shortly after administration would not have been due to action as a foreign antigen. Because the mice were bred with diets free from bovine materials, they were not sensitized to bovine LF or BSA. We used LF with 8.2% iron saturation. After oral administration, a large part of LF is degraded into peptides in the gastrointestinal tracts of mice (11). Therefore, the iron saturation level of the ingested LF may not influence the immunomodulatory effects.
In conclusion, oral administration of LF modulated the expression of immunity-related genes specifically or nonspecifically in the small intestines of mice. The specific actions may be related to the systemic immunomodulatory and host-protective effects of LF administration. We speculate that systemic circulation of immune cells would transmit the immunomodulation by LF in the intestinal compartment to other systemic compartments. We observed that LF increased the number of PBL but decreased the number of splenic leukocytes. This action may be a part of the process of transmission of immunomodulation.
REFERENCES
- 1.Baveye, S., E. Elass, J. Mazurier, G. Spik, and D. Legrand. 1999. Lactoferrin: a multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin. Chem. Lab. Med. 37:281-286. [DOI] [PubMed] [Google Scholar]
- 2.Bhimani, R. S., Y. Vendrov, and P. Furmanski. 1999. Influence of lactoferrin feeding and injection against systemic staphylococcal infections in mice. J. Appl. Microbiol. 86:135-144. [DOI] [PubMed] [Google Scholar]
- 3.Chierici, R. 2001. Antimicrobial actions of lactoferrin. Adv. Nutr. Res. 10:247-269. [DOI] [PubMed] [Google Scholar]
- 4.Haversen, L. A., I. Engberg, L. Baltzer, G. Dolphin, L. A. Hanson, and I. Mattsby-Baltzer. 2000. Human lactoferrin and peptides derived from surface-exposed helical region reduce experimental Escherichia coli urinary tract infection in mice. Infect. Immun. 68:5816-5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hugot, J. P., M. Chamaillard, H. Zouali, S. Lesage, J. P. Cezard, J. Belaiche, S. Almer, C. Tysk, C. A. O'Morain, M. Gassull, V. Binder, Y. Finkel, A. Cortot, R. Modigliani, P. Laurent-Puig, C. Gower-Rousseau, J. Macry, J. F. Colombel, M. Sahbatou, and G. Thomas. 2001. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411:599-603. [DOI] [PubMed] [Google Scholar]
- 6.Iigo, M., M. Shimamura, E. Matsuda, K. Fujita, H. Nomoto, J. Satoh, S. Kojima, D. B. Alexander, M. A. Moore, and H. Tsuda. 2004. Orally administered bovine lactoferrin induces caspase-1 and interleukin-18 in the mouse intestinal mucosa: a possible explanation for inhibition of carcinogenesis and metastasis. Cytokine 25:36-44. [DOI] [PubMed] [Google Scholar]
- 7.Isamida, T., T. Tanaka, Y. Omata, K. Yamauchi, K. Shimazaki, and A. Saito. 1998. Protective effects of lactoferricin against Toxoplasma gondii infection in mice. J. Vet. Med. Sci. 60:241-244. [DOI] [PubMed] [Google Scholar]
- 8.Iwasa, M., M. Kaito, J. Ikoma, M. Takeo, I. Imoto, Y. Adachi, K. Yamauchi, R. Koizumi, and S. Teraguchi. 2002. Lactoferrin inhibits hepatitis C virus viremia in chronic hepatitis C patients with high viral loads and HGV genotype 1b. Am. J. Gastroenterol. 97:766-767. [DOI] [PubMed] [Google Scholar]
- 9.Komiya, T., Y. Tanigawa, and S. Hirohashi. 1998. Cloning of the novel gene intelectin, which is expressed in intestinal paneth cells in mice. Biochem. Biophys. Res. Commun. 251:759-762. [DOI] [PubMed] [Google Scholar]
- 10.Kuhara, T., M. Iigo, T. Itoh, Y. Ushida, K. Sekine, N. Terada, H. Okamura, and H. Tsuda. 2000. Orally administered lactoferrin exerts an antimetastatic effect and enhances production of IL-18 in the intestinal epithelium. Nutr. Cancer 38:192-199. [DOI] [PubMed] [Google Scholar]
- 11.Kuwata, H., T.-T. Yip, K. Yamauchi, S. Teraguchi, H. Hayasawa, M. Tomita, and T. W. Hutchens. 1998. The survival of ingested lactoferrin in the gastrointestinal tract of adult mice. Biochem. J. 334:321-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lala, S., Y. Ogura, C. Osborne, S. Y. Hor, A. Bromfield, S. Davies, O. Ogunbiyi, G. Nuñez, and S. Keshav. 2003. Crohn's disease and the NOD2 gene: a role for paneth cells. Gastroenterology 125:47-57. [DOI] [PubMed] [Google Scholar]
- 13.Ogura, Y., D. K. Bonen, N. Inohara, D. L. Nicolae, F. F. Chen, R. Ramos, H. Britton, T. Moran, R. Karaliuskas, R. H. Duerr, J. P. Achkar, S. R. Brant, T. M. Bayless, B. S. Kirschner, S. B. Hanauer, G. Nuñez, and J. H. Cho. 2001. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411:603-606. [DOI] [PubMed] [Google Scholar]
- 14.Oh, S. M., D. H. Hahm, I. H. Kim, and S. Y. Choi. 2001. Human neutrophil lactoferrin trans-activates the matrix metalloproteinase 1 gene through stress-activated MAPK signaling modules. J. Biol. Chem. 276:42575-42579. [DOI] [PubMed] [Google Scholar]
- 15.Oh, S. M., C. W. Pyo, Y. Kim, and S. Y. Choi. 2004. Neutrophil lactoferrin upregulates the human p53 gene through induction of NF-κB activation cascade. Oncogene 23:8282-8291. [DOI] [PubMed] [Google Scholar]
- 16.Ouellette, A. J., D. Darmoul, D. Tran, K. M. Huttner, J. Yuan, and M. E. Selsted. 1999. Peptide localization and gene structure of cryptdin 4, a differentially expressed mouse paneth cell α-defensin. Infect. Immun. 67:6643-6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pabst, O., L. Ohl, M. Wendland, M. A. Wurbel, E. Kremmer, B. Malissen, and R. Forster. 2004. Chemokine receptor CCR9 contributes to the localization of plasma cells to the small intestine. J. Exp. Med. 199:411-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salzman, N. H., M. M. Chou, H. de Jong, L. Liu, E. M. Porter, and Y. Paterson. 2003. Enteric Salmonella infection inhibits paneth cell antimicrobial peptide expression. Infect. Immun. 71:1109-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sekine, K., Y. Ushida, T. Kuhara, M. Iigo, H. Baba-Toriyama, M. A. Moore, M. Murakoshi, Y. Satomi, H. Nishino, T. Kakizoe, and H. Tsuda. 1997. Inhibition of initiation and early stage development of aberrant crypt foci and enhanced natural killer activity in male rats administered bovine lactoferrin concomitantly with azoxymethane. Cancer Lett. 121:211-216. [DOI] [PubMed] [Google Scholar]
- 20.Sfeir, R. M., M. Dubarry, P. N. Boyaka, M. Rautureau, and D. Tomé. 2004. The mode of oral bovine lactoferrin administration influences mucosal and systemic immune responses in mice. J. Nutr. 134:403-409. [DOI] [PubMed] [Google Scholar]
- 21.Shin, K., H. Wakabayashi, K. Yamauchi, S. Teraguchi, Y. Tamura, M. Kurokawa, and K. Shiraki. 2005. Effects of orally administered bovine lactoferrin and lactoperoxidase on influenza virus infection in mice. J. Med. Microbiol. 54:717-723. [DOI] [PubMed] [Google Scholar]
- 22.Son, K. N., J. Park, C. K. Chung, D. K. Chung, D. Y. Yu, K. K. Lee, and J. Kim. 2002. Human lactoferrin activates transcription of IL-1β gene in mammalian cells. Biochem. Biophys. Res. Commun. 290:236-241. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki, Y., K. Shin, and B. Lönnerdal. 2001. Molecular cloning and functional expression of a human intestinal lactoferrin receptor. Biochemistry 40:15771-15779. [DOI] [PubMed] [Google Scholar]
- 24.Takakura, N., H. Wakabayashi, H. Ishibashi, S. Teraguchi, Y. Tamura, H. Yamaguchi, and S. Abe. 2003. Oral lactoferrin treatment of experimental oral candidiasis in mice. Antimicrob. Agents Chemother. 47:2619-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takakura, N., H. Wakabayashi, H. Ishibashi, K. Yamauchi, S. Teraguchi, Y. Tamura, H. Yamaguchi, and S. Abe. 2004. Effect of orally administered bovine lactoferrin on the immune response in the oral candidiasis murine model. J. Med. Microbiol. 54:495-500. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka, K., M. Ikeda, A. Nozaki, N. Kato, H. Tsuda, S. Saito, and H. Sekihara. 1999. Lactoferrin inhibits hepatitis C virus viremia in patients with chronic hepatitis C: a pilot study. Jpn. J. Cancer Res. 90:367-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teraguchi, S., H. Wakabayashi, H. Kuwata, K. Yamauchi, and Y. Tamura. 2004. Protection against infections by oral lactoferrin: evaluation in animal models. Biometals 17:231-234. [DOI] [PubMed] [Google Scholar]
- 28.Togawa, J., H. Nagase, K. Tanaka, M. Inamori, A. Nakajima, N. Ueno, T. Saito, and H. Sekihara. 2002. Oral administration of lactoferrin reduces colitis in rats via modulation of the immune system and correction of cytokine imbalance. J. Gastroenterol. Hepatol. 17:1291-1298. [DOI] [PubMed] [Google Scholar]
- 29.Togawa, J., H. Nagase, K. Tanaka, M. Inamori, T. Umezawa, A. Nakajima, M. Naito, S. Sato, T. Saito, and H. Sekihara. 2002. Lactoferrin reduces colitis in rats via modulation of the immune system and correction of cytokine imbalance. Am. J. Physiol. Gastrointest. Liver Physiol. 283:G187-G195. [DOI] [PubMed] [Google Scholar]
- 30.Tomita, M., H. Wakabayashi, K. Yamauchi, S. Teraguchi, and H. Hayasawa. 2002. Bovine lactoferrin and lactoferricin derived from milk: production and applications. Biochem. Cell Biol. 80:109-112. [DOI] [PubMed] [Google Scholar]
- 31.Tsuda, H., K. Sekine, K. Fujita, and M. Iigo. 2002. Cancer prevention by bovine lactoferrin and underlying mechanisms—a review of experimental and clinical studies. Biochem. Cell Biol. 80:131-136. [DOI] [PubMed] [Google Scholar]
- 32.Velliyagounder, K., J. B. Kaplan, D. Furgang, D. Legarda, G. Diamond, R. E. Parkin, and D. H. Fine. 2003. One of two human lactoferrin variants exhibits increased antibacterial and transcriptional activation activities and is associated with localized juvenile periodontitis. Infect. Immun. 71:6141-6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakabayashi, H., M. Kurokawa, K. Shin, S. Teraguchi, Y. Tamura, and K. Shiraki. 2004. Oral lactoferrin prevents body weight loss and increases cytokine responses during herpes simplex virus type 1 infection of mice. Biosci. Biotechnol. Biochem. 68:537-544. [DOI] [PubMed] [Google Scholar]
- 34.Wakabayashi, H., H. Kuwata, K. Yamauchi, S. Teraguchi, and Y. Tamura. 2004. No detectable transfer of dietary lactoferrin or its functional fragments to portal blood in healthy adult rats. Biosci. Biotechnol. Biochem. 68:853-860. [DOI] [PubMed] [Google Scholar]
- 35.Wakabayashi, H., N. Takakura, S. Teraguchi, and Y. Tamura. 2003. Lactoferrin feeding augments peritoneal macrophage activities in mice intraperitoneally injected with inactivated Candida albicans. Microbiol. Immunol. 47:37-43. [DOI] [PubMed] [Google Scholar]
- 36.Wakabayashi, H., K. Uchida, K. Yamauchi, S. Teraguchi, H. Hayasawa, and H. Yamaguchi. 2000. Lactoferrin given in food facilitates dermatophytosis cure in guinea pig models. J. Antimicrob. Chemother. 46:595-601. [DOI] [PubMed] [Google Scholar]
- 37.Wang, W. P., M. Iigo, J. Sato, K. Sekine, I. Adachi, and H. Tsuda. 2000. Activation of intestinal mucosal immunity in tumor-bearing mice by lactoferrin. Jpn. J. Cancer Res. 91:1022-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamauchi, K., M. Hiruma, N. Yamazaki, H. Wakabayashi, H. Kuwata, S. Teraguchi, H. Hayasawa, N. Suegara, and H. Yamaguchi. 2000. Oral administration of bovine lactoferrin for treatment of tinea pedis. A placebo-controlled, double-blind study. Mycoses 43:197-202. [DOI] [PubMed] [Google Scholar]