Abstract
Highly sensitive and specific, quantitative assays are needed to detect varicella-zoster virus (VZV) immunoglobulin G in human sera, particularly for determining immune status and response following vaccination. A time-resolved fluorescence immunoassay (TRFIA) has been developed, and its performance was compared to that of two commercial enzyme immunoassays (EIAs) and Merck glycoprotein EIA (gpEIA). The TRFIA had equivalent sensitivity (97.8%) and high specificity (93.5%) in relation to gpEIA. A commercial (Behring) EIA compared favorably with TRFIA in terms of sensitivity (98.4%) but had lower specificity (80.7%). Another commercial EIA (Diamedix) had high specificity (97.1%) but low sensitivity (76.4%) compared to TRFIA if equivocal test results were treated as negative for VZV antibody. A novel feature of the TRFIA was that the cutoff was generated using population mixture modeling and was expressed in mIU/ml, as the assay was calibrated using the British standard VZV antibody.
Determination of varicella-zoster virus (VZV) susceptibility is established practice (1) in assessing the need for administration of hyperimmune globulin following exposure to chicken pox in selected patient groups (neonates, immunosuppressed, cyesis). Recently (4), a live attenuated VZV vaccine (Oka strain) has become available, and this has been used in universal childhood vaccination programs in a number of countries. There is now the possibility that immunosuppressed individuals, susceptible women of childbearing age, and susceptible healthcare workers can be vaccinated. In the United Kingdom, immunization with the live attenuated vaccine is currently recommended (8) for susceptible individuals over the age of 12 years and children aged less than 12 years if deemed at risk (e.g., immunosuppressed). There is concern that widespread vaccination of children could lead to a shift in the average age at infection from children to adults, in whom the risk of complications is greater (17). Furthermore, the impact of vaccination on the incidence of zoster needs to be assessed together with determination of the most appropriate vaccination schedules to be adopted (5). For these reasons, it is important to have validated laboratory tests available which are sensitive and specific (16) but which also can be used to quantitatively monitor changes in VZV antibody levels in populations.
The fluorescent antibody to membrane antigen (FAMA) assay, which measures antibodies to viral glycoproteins (22), is generally considered to be the “reference” standard assay for measuring antibodies to VZV. This assay is semiquantitative, not suited for testing large numbers of sera, and cannot be automated, and its interpretation can be subjective. We needed a highly sensitive method to determine VZV immunoglobulin G (IgG) levels in a large number of sera, and from previous experience (14), we considered time-resolved fluorescence immunoassay (TRFIA) to be ideal for this purpose. There are very few data available on the population distribution of VZV IgG concentrations determined using highly sensitive, quantitative immunoassays and standardized reference antibody preparations. Such data are important as a baseline for monitoring population changes following the introduction of VZV vaccination and also for determining a cutoff for immunity or protection from disease in the absence of any clinically derived criteria.
A particular problem encountered with VZV is that, when using a population antibody concentration profile in healthy adults to determine a cutoff for susceptibility, very few negative sera are found. In adults, VZV population immunity is typically in excess of 90% (13). To overcome this problem, we have applied special mathematical techniques (9) to model the distribution of VZV quantitative data so that the optimal positive/negative cutoff point can be estimated using maximum likelihood.
The aim of this study was to validate a highly sensitive immunoassay for measuring VZV IgG levels in adult populations, determine a cutoff for VZV immunity which is statistically valid, and provide baseline data of the distribution of VZV IgG concentrations in a vaccine-naive adult population.
MATERIALS AND METHODS
Samples.
The sera tested were collected from different sources and included a panel of 446 samples from London blood donors collected in 2001, 222 samples collected from southwest England regional healthcare workers in 2001, and 66 samples collected from northwest England regional laboratory staff in 1999. An additional panel of 30 negative sera collected from routine southwest regional VZV screening activities was also included. A further panel of 337 London antenatal samples was also used in a supplementary analysis comparing TRFIA and Behring VZV enzyme immunoassay. Finally, a panel of 298 sera collected during 2004 that had been tested for VZV IgG or IgM was assembled from a London Hospital routine laboratory.
VZV IgG TRFIA.
This assay used VZV enzyme-linked immunosorbent assay-grade antigen (The Binding Site, Birmingham, United Kingdom) which was a sucrose density gradient centrifugation-purified extract of human embryo lung-cultured VZV strain Ellen. The coating concentration of antigen was that which gave a europium count of 400,000 to 600,000 with British standard VZV antibody (NIBSC, South Mimms, United Kingdom) at a concentration of 50 mIU/ml.
DELFIA microtiter plates (Perkin Elmer, Cambridge, United Kingdom) were coated with antigen at concentrations of 1.0 to 2.0 μg/ml (depending on batch) prepared in 0.05 M carbonate/bicarbonate buffer, pH 9.6. The plates were stored overnight at 4°C and washed four times with DELFIA wash buffer (Perkin Elmer, United Kingdom) using a DELFIA plate washer (Perkin Elmer, United Kingdom). Sera for testing were diluted 1 in 50 in DELFIA assay buffer (Perkin Elmer, United Kingdom), and 100 μl was loaded into appropriate wells. A standard curve was run on each plate, prepared from British standard VZV antibody diluted in DELFIA assay buffer at concentrations ranging from 50 mIU/ml to 0.39 mIU/ml. The plates were sealed and incubated in a humid chamber for 2 h at 37°C and then washed four times, as before. Europium-labeled anti-human IgG conjugate (Perkin Elmer, United Kingdom) diluted 1 in 500 in DELFIA assay buffer was added at 100 μl per well using a multichannel pipette. The plates were then incubated for 1 h at 37°C and washed four times, as before, and 150 μl DELFIA enhancement solution (Perkin Elmer, United Kingdom) was added to all wells. Following 15 min of rotating incubation at room temperature, in the dark, the plates were read using a DELFIA 1234 reader (Perkin Elmer, United Kingdom), and data were analyzed using Multicalc software, version 2000 (Wallac Oy, Finland). Interpolated antibody concentrations were expressed in mIU/ml.
Diamedix VZV IgG EIA.
Test kits for the Diamedix VZV IgG enzyme immunoassay (EIA) were obtained from Launch Diagnostics Ltd. (New Ash Green, United Kingdom). The manufacturer's instructions were followed. A Labsystems Wellwash 4 Mk 2 (Thermo Life Sciences Ltd., Basingstoke, United Kingdom) was used to wash plates. Absorbance at 450 nm, using a reference wavelength of 620 nm, was measured using a Tecan Sunrise photometer (Tecan Instruments, Reading, United Kingdom). VZV antibody concentrations were expressed as enzyme units per ml (EU/ml). Antibody levels less than 15.0 EU/ml were interpreted as VZV IgG negative, those between 15.0 EU/ml and 19.9 EU/ml were interpreted as equivocal, and levels 20 EU/ml or greater were presumed immune to VZV.
Dade Behring Enzygnost anti-VZV IgG EIA.
Dade Behring Enzygnost anti-VZV IgG test kits were obtained from Dade Behring United Kingdom Ltd. (Milton Keynes, United Kingdom). The manufacturer's instructions were followed. The kit reference serum and test samples were prediluted 1 in 21 in sample buffer. Plates were washed using a Wellwash 4 Mk 2 (Thermo Life Sciences) and read photometrically (Tecan Sunrise) at 450 nm using a 620-nm reference wavelength. The calculated absorbance of each sample was determined by subtraction of the optical density at 620 nm from that at 450 nm followed by subtraction of the calculated absorbance of the respective control antigen well. The concentration (mIU/ml) of VZV antibody in the sample, based on the WHO international standard for VZV immunoglobulin, could then be determined through application of a manufacturer's derived correction factor. The Behring EIA had a limit of detection of 50 mIU/ml, and sera with antibody levels less than 50 mIU/ml were classified as negative. Equivocal sera were identified as having antibody levels of 50 to 110 mIU/ml.
Merck glycoprotein EIA.
The assay used was based on that described by Wasmuth and Miller (21), and a standard operating procedure confidential to Merck, Sharp, and Dohme Research Laboratories was followed. Serum samples were diluted 1 in 50 in sample diluent containing globulin-depleted goat serum (G9023; Sigma, United Kingdom), and 100 μl was added to alternate columns of VZV glycoprotein and MRC5 control antigen-coated wells of a Nunc Maxisorp (Life Technologies, Paisley, United Kingdom) microtiter plate. Dilutions of a reference serum supplied by Merck, Sharp, and Dohme Research Laboratories and the British standard for varicella-zoster antibody (NIBSC 90/690) were also run on the same plate together with a negative-control serum. The plate was then sealed with foil and incubated for 90 min in a humid atmosphere using a 37°C water bath. It was then washed four times with 300 μl wash buffer (phosphate-buffered saline containing 0.05% Tween 20) using a multichannel Finnpipette (ThermoLifeSciences). Goat anti-human IgG alkaline phosphatase conjugate (AH10305; Biosource International, Camarillo, CA) was prepared at a dilution of 1 in 5,000 in sample diluent, and 100 μl was added to all wells. The plate was then incubated for 60 min as before and washed four times as described previously. Substrate which had been prepared 10 min prior to addition to the plate by dissolving two tablets of 4-nitrophenylphosphate disodium salt hexahydrate substrate (N9389; Sigma) in 10 ml buffer (1 M diethanolamine/0.5 mM MgCl2) was added at 100 μl/well. The plate was covered with foil and left at room temperature for approximately 45 min prior to addition of 50 μl stop reagent (3 N NaOH) to all wells. Absorbance at a wavelength of 405 nm was then read using an Anthos 2001 plate reader.
The concentration (mIU/ml) of antibodies to VZV glycoprotein in samples was determined by interpolation from a standard curve of British standard VZV antibody. The cutoff used was ≤10 mIU/ml for antibody negativity. A standard curve of the Merck reference serum was also drawn and used for calibration purposes.
Statistical analysis and modeling.
Initially censored observations were excluded from the analysis. The data were assumed to follow three normal distributions, one for negative, one for positive, and one for high-positive results. Using mixture modeling, the estimates of these distributions were calculated by maximum likelihood methods (9). Let ni be the frequency in one of i = 1,2,…,k serum categories (where “1” is 0.9 to 1 mIU/ml and “2” is 1 to 1.1 mIU/ml, etc.) and λi be the expected count of that category. Then the maximum likelihood estimates can be calculated by minimizing the deviance D, i.e., the difference between the likelihood of the fitted model (LC) and the likelihood of the full model, i.e., when ni = λi (LF). The deviance for this model is given by
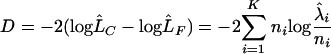 |
The optimal positive/negative cutoff point was then estimated to minimize the discrepancy between the specificity and sensitivity on the panel based on the model estimates (9). The analysis was then redone including censored observations to investigate the influence of these observations on the cutoff estimate.
Microsoft Excel 2000 was used.
RESULTS
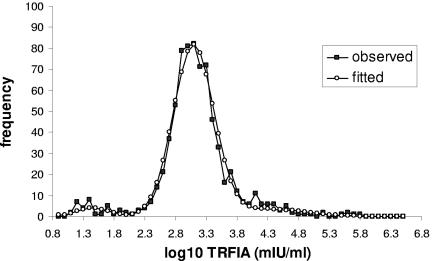
A total of 719 samples were used for the analysis of distribution of VZV IgG levels in a population of adults. Of these, 15 samples were censored (reported as “>1,000,000”) and were initially excluded from the analysis. The geometric mean antibody level for this population was 1,365.0 mIU/ml. For determination of a cutoff antibody level, the population sample of adult sera was supplemented with 30 known “negative” sera from our routine laboratory collection and mixture modeling was applied. The 3-normal-distribution mixture model fitted the data satisfactory (deviance = 30.03). The observed and the fitted mixture models are shown in Fig. 1. The estimated optimal cutoff point was 1.97, i.e., 93.33 mIU/ml. The same analysis was repeated including the censored observations (after given an arbitrary value of 2 × 1,000,000), with little effect on the positive/negative cutoff.
FIG. 1.
Observed and fitted mixture model distributions of serum VZV IgG levels (mIU/ml) measured in 749 individuals.
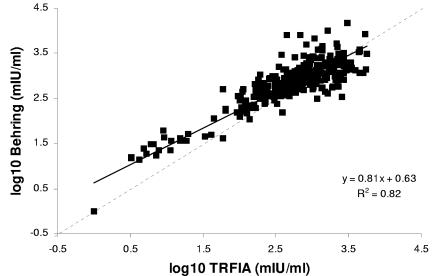
The estimated TRFIA cutoff (93.3 mIU/ml) was then used to classify TRFIA results into positive and negative on a separate panel of 337 antenatal sera which had also been tested by Behring EIA. Four Behring EIA equivocal sera were identified. According to the Behring EIA interpretative guidelines, equivocal sera could either be “negative” for patients at risk of infection or “positive” for organ donors. Assuming the Behring equivocal sera to indicate adults in need of vaccination (antibody negative), the sensitivity and specificity of the Behring EIA in relation to TRFIA were 98.4% and 80.7%, respectively. A scatterplot was constructed (Fig. 2) to compare TRFIA results to those obtained with the Behring EIA, and the correlation coefficient (r) for the two assays was 0.90.
FIG. 2.
Scatterplot comparing VZV IgG levels measured by TRFIA and Behring EIA in 337 antenatal blood samples.
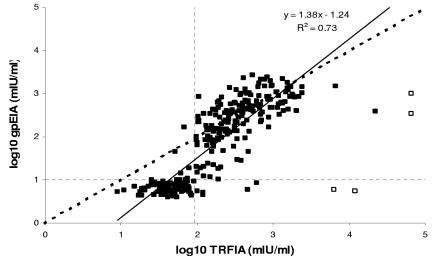
Finally, the TRFIA was evaluated against the Merck glycoprotein EIA (gpEIA) and Diamedix VZV EIA using a panel of 298 sera collected from a London Hospital routine laboratory. The sera were tested blind by TRFIA. A scatterplot comparing the VZV IgG levels by the gpEIA and TRFIA is shown in Fig. 3. An outlier investigation was carried out by calculating the “standardized residuals,” i.e., the distance between each observation and its expected estimate given by the model taking into account the model variability. Four samples with standardized residuals outside the range of ±3 were considered outliers and excluded from the regression line calculations. The correlation coefficient was 0.85 after fitting a linear model. The sensitivity and specificity of TRFIA compared to gpEIA were 97.8% and 93.5%, respectively, with a positive predictive value of 96.3% and negative predictive value of 96.2%.
FIG. 3.
Scatterplot comparing VZV IgG levels measured by TRFIA and Merck glycoprotein EIA for 298 sera collected from a London Hospital routine laboratory. The dotted lines denote the cutoff points, while the bold dotted line denotes the line of equivalence (y = x). Open squares denote potential outliers.
The sensitivity and specificity of Diamedix VZV EIA compared to TRFIA were 76.4% and 97.1%, respectively, if equivocal sera were treated as negative and 95.9% and 93.3%, respectively, if equivocal sera were treated as positive.
DISCUSSION
EIAs have been extensively used to measure VZV antibody following natural infection. A number of studies (2, 6, 20) have shown EIAs to lack sensitivity and specificity compared to FAMA, particularly when vaccine-induced antibody is measured. Typically, commercial VZV EIAs use extracts of VZV-infected cells as their capture antigen (12). It has been shown that EIAs using purified glycoprotein antigens (gpEIAs) can have equivalent, or even greater, sensitivity and specificity than FAMA or EIAs using whole-cell antigen (18). The greater sensitivity and specificity of gpEIAs is likely to be a consequence of the more purified and concentrated nature of the antigen used. The Merck assay used in our study is a gpEIA which has been extensively evaluated (11, 18, 21) and is considered an optimal reference assay alternative to FAMA or enhanced neutralization assays (10). Unfortunately, gpEIAs are not commercially available, and the Merck assay is restricted to a small number of specialist testing centers.
In this study, we have shown a VZV time-resolved fluorescence immunoassay to have sensitivity (97.8%) and specificity (93.5%) equivalent to those of the Merck gpEIA. A significant advantage of the TRFIA is that it uses purified whole-cell antigen extract which is much more readily obtained than glycoprotein antigen. We believe that the loss in sensitivity due to use of cell extract antigen is compensated for by the inherent gains in sensitivity achieved through use of fluorescence decay measurement of lanthanide chelates which is the detection system used in TRFIA (15). Time resolution of the decay measurement filters out nonspecific fluorescence which may produce high background levels in conventional fluorescence-based assays.
An important aspect of the TRFIA development process was to generate a valid cutoff to differentiate individuals possessing specific IgG (who have therefore experienced VZV infection at some time) from those who have not experienced VZV. Because, at present, in the United Kingdom, VZV vaccination is specifically targeted at susceptible healthcare care personnel working in “at risk” areas, it seemed appropriate that the population used to determine the cutoff was representative. We therefore assembled a panel of sera from 719 healthy adults; however, due to the low number of VZV IgG-negative sera, we had to add on a further 30 negative serum samples from various sources. Using the population mixture modeling technique, a cutoff level of 93.3 mIU/ml was calculated. It is important that this cutoff is only applicable to healthy adults. For young children and vaccinees, a further study will have to be performed to estimate a cutoff. There is no agreed level of VZV IgG which can be used to describe individuals as susceptible or immune to VZV, and the calculation of a specific value in international units is an important first step in addressing this issue.
The performance of two commercial assays in relation to TRFIA was also investigated. The Diamedix VZV EIA showed good specificity (93.3% to 97.1%) in relation to TRFIA; however, its sensitivity was significantly lower (76.4%) compared to TRFIA if equivocal test results were treated as negative. According to the manufacturer, if the result for a serum is equivocal, a further specimen should be collected and testing repeated in parallel. If the second sample is also equivocal, the patient should be considered negative for primary or recent infection and have equivocal antibody status. The lack of sensitivity of the Diamedix EIA may be attributable to the cutoff used failing to distinguish between true negative and low-positive sera. Vyse and colleagues (24) have also shown that the current cutoff for the Diamedix EIA is set so that true negatives together with sera containing low levels of VZV IgG are treated as susceptible.
The Behring VZV EIA had a sensitivity of 98.4% and specificity of 80.7% compared to TRFIA. A direct comparison of the sensitivity and specificity of the Behring EIA in relation to the Diamedix EIA cannot be made, as different populations of sera were tested. According to the manufacturer, the Behring EIA can be used to check whether a VZV vaccination has been successful, and therefore, TRFIA, which appears to be as sensitive but more specific, may prove useful for this indication as well. According to Robertson and colleagues (19), the cutoff of the Behring EIA has been determined by measuring VZV IgG levels in 168 immune adults and 41 children aged between 1 and 3 years. The optical density (OD) values were shown to be bimodally distributed, and the cutoff was generated by computing the OD lower limit in antibody positives and the OD upper limit in antibody negatives.
From the data presented, we conclude that TRFIA is a highly sensitive and specific assay for detection of VZV IgG in naturally infected adults. The performance of TRFIA in relation to Merck gpEIA and Behring EIA would suggest it has potential for application to measuring VZV IgG levels following vaccination where it has been shown that the VZV IgG produced is significantly less than that following natural infection (3, 7). A major advantage of the VZV TRFIA is that a whole-cell purified antigen can be used to a similar effect as the glycoprotein antigen used in the Merck EIA. A further benefit of the TRFIA is that the reagents used (cell purified antigen, assay buffer, wash buffer, europium-labeled conjugate, and enhancement solution) undergo manufacturers' internal control procedures and are readily available through a worldwide network of suppliers. Fluorometric readers with the software capability to time resolve fluorescence decay are increasingly available and fall within the procurement budgets of many large laboratories. Although more expensive than traditional VZV reference antibody methodologies (FAMA, virus neutralization) the TRFIA technique requires less staff skill and time and can be automated to run hundreds of sera per day.
We have seen in other areas of serology, for example, diphtheria antitoxin determination (23), that assays using time-resolved fluorescence technology have performed as well as traditional virological methodologies. The VZV TRFIA clearly has the potential to be adopted as an alternative to traditional methods for sensitive detection of VZV IgG, and we have already initiated further collaborative validation studies. In anticipation of other laboratories adopting the technique, we hope to see further studies which may aid in determining appropriately defined cutoffs for positive and negative samples. Crucial to the success of this endeavor is the availability of internationally accepted standardized reference serum preparations containing known, agreed amounts of VZV IgG.
Acknowledgments
We thank Alan Shaw of Merck & Co., Inc. (Whitehouse Station, New Jersey), for help and advice with the glycoprotein EIA.
REFERENCES
- 1.Anonymous. 1996. Varicella, p. 251-261. In D. M. Salisbury and N. T. Begg (ed.), Immunisation against infectious disease. HMSO, London, United Kingdom.
- 2.Balfour, H. H., C. K. Edelman, C. L. Dirksen, D. R. Palermo, C. S. Suarez, J. Kelly, J. T. Kentala, and D. D. Crane. 1988. Laboratory studies of acute varicella and varicella immune status. Diagn. Microbiol. Infect. Dis. 10:149-158. [DOI] [PubMed] [Google Scholar]
- 3.Bogger-Goren, S., K. Baba, P. Hurley, H. Yabuuchi, M. Takahashi, and P. L. Ogra. 1982. Antibody response to varicella-zoster virus after natural or vaccine-induced infection. J. Infect. Dis. 146:260-265. [DOI] [PubMed] [Google Scholar]
- 4.Breuer, J. 2001. Vaccination to prevent varicella and shingles. J. Clin. Pathol. 54:743-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brisson, M., W. J. Edmunds, N. J. Gay, B. Law, and G. De Serres. 2000. Modelling the impact of immunization on the epidemiology of varicella zoster virus. Epidemiol. Infect. 125:651-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demmler, G. J., S. P. Steinberg, G. Blum, and A. A. Gershon. 1988. Rapid enzyme-linked immunosorbent assay for detecting antibody to varicella-zoster virus. J. Infect. Dis. 157:211-212. [DOI] [PubMed] [Google Scholar]
- 7.Diaz, P. S., S. Smith, E. Hunter, and A. M. Arvin. 1988. Immunity to whole varicella-zoster virus antigen and glycoproteins I and p170: relation to the immunizing regimen of live attenuated varicella vaccine. J. Infect. Dis. 158:1245-1252. [DOI] [PubMed] [Google Scholar]
- 8.Edmunds, W. J., M. Brisson, N. J. Gay, and E. Miller. 2002. Varicella vaccination: a double edged sword? Commun. Dis. Public Health 5:185-186. [PubMed] [Google Scholar]
- 9.Gay, N. J. 1996. Analysis of serological surveys using mixture models: application to a survey of parvovirus B19. Stat. Med. 15:1567-1573. [DOI] [PubMed] [Google Scholar]
- 10.Krah, D. L., P. J. Provost, and R. W. Ellis. 1995. Combined use of complement and anti-immunoglobulin in an enhanced neutralization assay for antibodies to varicella-zoster virus. J. Virol. Methods 53:176-187. [DOI] [PubMed] [Google Scholar]
- 11.Krah, D. L. 1996. Assays for antibodies to varicella-zoster virus. Infect. Dis. Clin. N. Am. 10:507-526. [DOI] [PubMed] [Google Scholar]
- 12.Larussa, P., S. Steinberg, E. Waithe, B. Hanna, and R. Holzman. 1987. Comparison of five assays for antibody to varicella-zoster virus and the fluorescent-antibody-to-membrane-antigen test. J. Clin. Microbiol. 25:2059-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lerman, Y., G. Chodick, S. Tepper, G. Livni, and S. Ashkenazi. 2004. Seroepidemiology of varicella-zoster virus antibodies among health-care workers and day-care-centre workers. Epidemiol. Infect. 132:1135-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maple, P. A. C., and C. S. Jones. 2002. Time-resolved fluorometric immunoassay for rubella antibody-a useful method for serosurveillance studies. Vaccine 20:1378-1382. [DOI] [PubMed] [Google Scholar]
- 15.McKie, A., A. Vyse, and C. Maple. 2002. Novel methods for the detection of microbial antibodies in oral fluid. Lancet Infect. Dis. 2:18-24. [DOI] [PubMed] [Google Scholar]
- 16.Miller, E., R. Marshall, and J. Vurdien. 1993. Epidemiology, outcome and control of varicella-zoster infection. Rev. Med. Microbiol. 4:222-230. [Google Scholar]
- 17.Preblud, S. R. 1981. Age-specific risks of varicella complications. Pediatrics 68:14-17. [PubMed] [Google Scholar]
- 18.Provost, P. J., D. L. Krah, B. J. Kuter, D. H. Morton, T. L. Schofield, E. H. Wasmuth, J. White, W. J. Miller, and R. W. Ellis. 1991. Antibody assays suitable for assessing immune responses to live varicella vaccine. Vaccine 9:111-116. [DOI] [PubMed] [Google Scholar]
- 19.Robertson, P. W., S. M. Bell, and M. J. Ferson. 1989. A method for determining the cut-off value of a varicella-zoster virus IgG enzyme immunoassay for immune status. J. Virol. Methods 26:115-118. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg, S. P., and A. A. Gershon. 1991. Measurement of antibodies to varicella-zoster virus by using a latex agglutination test. J. Clin. Microbiol. 29:1527-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wasmuth, E. H., and W. J. Miller. 1990. Sensitive enzyme-linked immunosorbent assay for antibody to varicella-zoster virus using purified VZV glycoprotein antigen. J. Med. Virol. 32:189-193. [DOI] [PubMed] [Google Scholar]
- 22.Williams, V., A. A. Gershon, and P. A. Brunell. 1974. Serologic response to varicella-zoster membrane antigens measured by indirect immunofluorescence. J. Infect. Dis. 130:669-672. [DOI] [PubMed] [Google Scholar]
- 23.Von Hunolstein, C., H. Aggerbeck, N. Andrews, G. Berbers, F. Fievet-Groyne, P. A. C. Maple, R. M. Olander, M. Raux, and A. Tischer. 2000. European Sero-Epidemiology Network: standardisation of the results of diphtheria antitoxin assays. Vaccine 18:3287-3296. [DOI] [PubMed] [Google Scholar]
- 24.Vyse, A. J., N. J. Gay, L. M. Hesketh, P. Morgan-Capner, and E. Miller. 2004. Seroprevalence of antibody to varicella zoster virus in England and Wales in children and young adults. Epidemiol. Infect. 132:1129-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]