Abstract
A meta-analysis of studies investigating the diagnostic accuracy of enzyme-linked immunosorbent assays (ELISA) for antibodies against tissue transglutaminases (tTG) of various origins in celiac disease (CD) diagnosis was carried out. Twenty-one studies, with untreated CD patients and healthy/CD-free controls, were included in the meta-analysis. The diagnostic accuracy was estimated using a summary receiver operating characteristic (SROC) curve and pooled sensitivity (Se) and specificity (Sp). Multiple assays within a study were treated by considering all the assays within a study and by analyzing the most popular assay (i.e., the commercial anti-tTTG ELISA most frequently utilized in the papers in which multiple assays were included). The SROC curve indicated the absence of heterogeneity, and the superiority of recombinant human tTG (rh-tTG) and purified human tTG (ph-tTG) compared to guinea pig-tTG (gp-tTG). The sensitivities (most popular assay) for rh-tTG, ph-tTG, and gp-tTG were 94%, 90%, and 92%, respectively, and the specificities were 97%, 92%, and 96%, respectively. A sensitivity analysis (exclusion of studies with bias) altered the results of ph-tTG: Se, 95%; Sp, 98%. The sensitivities (all individual assays) for rh-tTG, ph-tTG, and gp-tTG were 94%, 94%, and 91%, respectively, and the specificities were 95%, 94%, and 89%, respectively. Human tTG ELISA is sensitive and specific, and it can be used for mass screening. Sensitivity analysis showed that ph-tTG might perform better.
Due to the high prevalence of oligosymptomatic celiac disease (CD), the use of serological diagnostic assays becomes extremely useful in clinical practice, eliminating the performance of needless intestinal biopsies. Among them, assays of immunoglobulin A (IgA) antibodies against tissue transglutaminase (tTG) present with the most promising diagnostic characteristics (1, 8, 25, 29, 34). Enzyme-linked immunosorbent assays (ELISAs) using recombinant human tTG (rh-tTG), purified human tTG (ph-tTG), and guinea pig tTG (gp-tTG) as substrates had been developed, and a plethora of relevant commercial kits are available (8, 25, 29, 36). Despite, however, the fact that these kits present with sufficient sensitivities and specificities, published studies have showed inconsistent effects. Therefore, an estimate of the overall accuracy of each tTG type test and a comparison between them seem imperative.
The primary aim of this meta-analysis was to assist in the interpretation of the anti-tTG in testing for CD and to assess the relative diagnostic accuracy of rh-tTG, ph-tTG, and gp-tTG ELISAs in the diagnosis of CD by evaluating sensitivity and specificity on the basis of a formal analysis of the available studies.
MATERIALS AND METHODS
Study identification.
The MEDLINE database (January 1999 to March 2005) was searched for clinical studies assessing the utility of anti-tTG ELISA in the diagnosis of CD. The search used the following strategy: (celiac disease) AND (tissue transglutaminase OR anti tissue transglutaminase OR anti-tTG OR tTG) AND ([sensitivity and specificity] OR diagnostic test). An author and an experienced librarian in medical literature independently reviewed each abstract in detail to determine the eligibility of each reference to potentially meet the search strategy. The bibliographies of the retrieved articles were also searched. Only articles in English were considered in the meta-analysis; published abstracts and conference proceedings were not considered. The agreement level was also reported.
Study selection.
Two investigators (the two authors) independently examined all full articles identified in the search procedure. Studies with (i) consecutive untreated celiac disease patients diagnosed at least by intestinal biopsy, (ii) more than 10 participants enrolled, (iii) data sufficient to estimate both sensitivity and specificity, (iv) ELISAs with rh-tTG, ph-tTG, or gp-tTG as the antigen, and (v) controls free of CD were included in the meta-analysis. Disagreements were resolved by discussing the full articles.
Data abstraction and sensitivity analysis.
Two investigators, blinded to study details, independently abstracted data from each study, including study setting and technical details of the assay, threshold, validity of study design, and 2 by 2 contingency tables (disease status and test outcome) needed to calculate the sensitivity and specificity. Interrate agreements for the study validity criteria were evaluated using the kappa statistic. Disagreements were resolved by discussing the full articles.
A study is considered biased when healthy controls found anti-tTG positive had been submitted to small intestinal biopsy, since this procedure may result in an overestimate of sensitivity. Sensitivity analyses (i.e., exclusion of specific studies from the analysis) were carried out for studies with bias and for studies consisting of healthy controls only. Sensitivity analysis is a typical procedure in a meta-analysis which investigates the effect of excluded studies in the meta-analysis results. In addition, for studies including IgA-deficient patients, a sensitivity analysis was carried out.
Estimation of diagnostic accuracy.
In the meta-analysis, multiple assays within a study were treated, considering all the assays within a study independently. Since multiple assays within a study might be correlated, the analysis was also performed considering the most popular assay (i.e., the commercial anti-tTG ELISA most frequently utilized in the papers in which multiple assays were included), and subsequent sensitivity analyses were performed based on this setting.
Sensitivity (Se) and specificity (Sp) were calculated from contingency tables abstracted from each study. Fixed effect (FE) and random effect (RE) pooled estimates for sensitivity and specificity were calculated independently (28). Random effects tend to provide wider confidence intervals (CI) and are generally preferable, especially in the presence of between-study heterogeneity.
An analysis based on a summary receiver operating characteristic (SROC) curve was also performed. The SROC curve deals with the problem of different thresholds among studies and is used to estimate the overall diagnostic accuracy of different tests. The sensitivity and specificity for the single-test threshold identified for each study were used to plot the unweighted SROC curve (6, 13, 14, 33). The SROC curve can be fitted with linear regression of the logits of the sensitivity and specificity by the equation D = a + b × S, where D = logit(Se) − logit(1 − Sp) = log(OR) and S = logit(Se) + logit(1 − Sp). The OR (odds ratio) represents the odds of a positive test result among disease persons relative to the odds of a positive test result among nondisease persons, i.e., it is a measure of the discrimination power of the test, and S is a measure of the threshold for classifying a test as positive. The closer the coefficient b is to 0, the more evidence of a lack of significant heterogeneity with respect to OR exists, and then a symmetric SROC curve is produced and a common log(OR) determines the entire SROC curve (13). The unweighted SROC curve reflects the effect of between-study variability in the estimation of test accuracy (4, 6, 13, 14, 33). If b differs from 0, the OR for the association between the test and reference changes for different points on the ROC curve, i.e., it is dependent on the threshold used. The area under the SROC curve (AUC) is related to the common log(OR), and it approaches 1 as the log(OR) gets larger (33).
The SROC curve can be fit weighted by the inverse of the variance of the logarithm of the ORs of the individual studies. However, this weighted analysis may produce biased estimates, and the identification of a proper weighting is currently under investigation (13) and is therefore not considered in this meta-analysis.
The SROC curve shows the tradeoff between sensitivity and specificity for varying thresholds; therefore, by fixing the specificity, the corresponding value on the SROC curve provides an estimate of the pooled sensitivity (pSe). The fixed value of specificity can be the pooled random effects estimate calculated independently (4, 24). The independent estimates of sensitivity and specificity are usually reliable when they are close to the SROC curve (24). Based on the SROC curve, the Q* metric was also derived. At Q*, sensitivity equals specificity (Se = Sp), where Se = exp(a/2)/[1 + exp(a/2)] and 1 − Sp = 1/[1 + exp(a/2)], and represents the diagnostic threshold at which the probability of a correct diagnosis is constant for all subjects (22, 33). In comparing the relative diagnostic value of rh-tTG, ph-tTG, and gp-tTG, all of the above diagnostic metrics should be considered. However, metrics based on the SROC curve [AUC, pSE, Q*, and D = log(OR)] might be more valuable, and they provide a guide as to whether the independent estimates of Se and Sp can be used.
The analyses were carried out using Meta-Test (J. Lau, Boston, MA), R system, and Compaq Visual Fortran90 IMSL subroutines.
RESULTS
Studies identified.
The literature review identified 115 titles of potential relevant articles. After review, 32 titles were judged to be potentially relevant. The search had good agreement between the two reviewers: k = 0.74 (95% CI, 0.61 to 0.83). The abstracts of these articles were reviewed, and then 28 studies published as full articles were selected for further review. Of these 28 articles, 21 met the inclusion criteria and were analyzed (Table 1).
TABLE 1.
Summary characteristics of studies included in the meta-analysis
| Author, yr | No. of patients with CD, no. of controls | Median or mean (range or 95% CI) age (yr) of patients with CD, controls | No. of female/no. of male patients with CD, controls | tTG type | Manufacturer or assay | Cutoff value | Se (%) | Sp (%) |
|---|---|---|---|---|---|---|---|---|
| Sugai, 2000 | 79, 42 (18 diseased) | 37 (17-68), 35 (18-66) | 61/18 | gp | INOVA | 20 AU/ml | 92 | 98 |
| 39/21 | ||||||||
| Martini, 2002 | 101, 190 (101 diseased) | 37 (21-72), 38 (20-77) | 61/81 | rh | Pantec | 12.8 AU/ml | 83 | 92 |
| 39/21 | rh | Eurospital | 7.4 AU/ml | 96 | 82 | |||
| rh | Li StarFISH | 19.7 AU/ml | 66 | 91 | ||||
| rh | Pharmacia | 2 AU/ml | 80 | 99 | ||||
| gp | Genesis | 47.5 AU/ml | 76 | 93 | ||||
| Burgin-Wolf, 2002 | 208, 157 diseased | 6.7, 10.1 | 144/64 | rh | Pharmacia | 6 U/ml | 96 | 99 |
| 87/70 | ||||||||
| Basso, 2001 | 38, 34 diseased | (2-16), (1-14) | 28/10 | rh | Eurospital | 5.5 AU | 89 | 100 |
| 19/15 | gp | Medipan | 26 U/ml | 84 | 100 | |||
| ph | INOVA | 20 U | 84 | 100 | ||||
| Arnika | 0 U/ml | 76 | 100 | |||||
| Tesei, 2003 | 250 (203 type IV VAa), 176 diseased | 39 (13-79), 40 (17-83) | 184/66 | rh | Eurospital | 7 AU/ml | 93 | 95 |
| 132/44 | ||||||||
| Carroccio, 2002 | 24 (17 total VA), 183 diseased | 30 (18-80), 46 (17-84) | 14/10 | rh | Eurospital | 100 | 97 | |
| 94/89 | gp | Eurospital | 100 | 92 | ||||
| Wong, 2002 | 49, 34 diseased | >2, >2 | rh | Aesku Lab | 15 U/ml | 71 | 100 | |
| rh | Binding site | 4 U/ml | 98 | 91 | ||||
| rh | Eurospital | 7 AU | 96 | 88 | ||||
| ph | INOVA | 20 U/ml | 98 | 100 | ||||
| ph | Orgentec | 10 U/ml | 100 | 85 | ||||
| rh | Varelisa | 5 U/ml | 100 | 100 | ||||
| gp | Binding site | 4 U/ml | 88 | 91 | ||||
| gp | Eurospital | 5 AU | 98 | 35 | ||||
| gp | Genesis | 10 U/ml | 96 | 77 | ||||
| gp | Immco Diagn. | 20 EU/ml | 92 | 77 | ||||
| gp | Im/pharcology | 25 AU | 100 | 12 | ||||
| gp | INOVA | 20 U/ml | 86 | 100 | ||||
| gp | Medipan | 25 U/ml | 98 | 53 | ||||
| Hansson, 2000 | 22 (11 total VA), 45 (23 diseased) | 3 (1-16), 6 (1-16) | 14/8 | ph | Homemade | 0.06 AU | 100 | 98 |
| 9/13 | gp | Sigma | 0.06 AU | 91 | 98 | |||
| Sblattero, 2000 | 65 (18 total VA), 170 (20 diseased) | (2-60), (18-60) | 34/31 | rh | Homemade | 91 | 99 | |
| 75/95 | gp | Homemade | 82 | 98 | ||||
| Hansson, 2002 | 25, 53 (29 diseased) | 4 (1-16), 5 (1-18) | 27/26 | rh | Pharmacia | 4.7 AU/ml | 100 | 96 |
| Trevisiol, 2002 | 140 (111 stage type 3c-b), 200 healthy | (1-60), 7 (2-14) | 93/47 | rh | Homemade | 100 | 100 | |
| 105/95 | ||||||||
| Blackwell, 2002 | 32, 38 diseased | rh | Binding site | Those provided by the manufacturers | 100 | 84 | ||
| rh | Pharmacia | 91 | 97 | |||||
| ph | INOVA | 88 | 87 | |||||
| ph | Euroimmun | 94 | 92 | |||||
| gp | Binding site | 50 | 38 | |||||
| Johnston, 2003 | 29, 63 diseased | 52.5, 51.8 | 15/14 | gp | Immco Diagn. | 25 U | 86 | 84 |
| 44/19 | ||||||||
| Fabiani, 2001 | 399, 432 (186 diseased) | 13.5 (0.3-87.4), 13.2 (0.4-78.7) | 256/143 | gp | Eurospital | 7 AU | 90 | 96 |
| 212/220 | ||||||||
| Vitoria, 1999 | 27, 34 diseased | 5.02 ± 4.7, 5.9 ± 4.8 | gp | Medipan | 35 U/ml | 100 | 94 | |
| Scoglio, 2003 | 134 (134 subtotal VA), 47 diseased | <18 yrs (100 subjects) <18 yrs (81 subjects) | gp | Homemade | 99 | 66 | ||
| Llorente, 2004 | 61, 64 diseased | Pharmacia | 3.5 AU/ml | 100 | 95 | |||
| Wolters, 2002 | 52, 49 diseased | 4 (1.1, 14.4), 5.1 (0.8, 19.2) | 38/14 | rh | Pharmacia | 99 | 99 | |
| 20/29 | gp | Homemade | 95 | 92 | ||||
| Leon, 2001 | 86, 152 diseased | rh | Pharmacia | 99 | 99 | |||
| gp | Homemade | 95 | 92 | |||||
| Osman, 2002 | 35, 137 (48 diseased) | (2-81), (2-81) | ph | Homemade | 19 U | 100 | 98.6 | |
| gp | Homemade | 85.7 | 99.3 | |||||
| Kumar, 2001 | 34, 267 (161 diseased) | gp | Homemade | 91 | 97 |
VA, villous atrophy.
Eleven studies (8, 10, 11, 15, 17, 19, 27, 29, 30, 31, 32) evaluated only one assay, six studies (9, 12, 18, 23, 26, 35) evaluated two assays, and the remaining four studies (3, 5, 20, 36) evaluated up to 13 assays. In total, 20 rh-tTG assays, 21 gp-tTG assays, and 7 ph-tTG assays were evaluated by the included studies. Thirteen studies concerning the evaluation of rh-tTG included 1,120 celiac patients and 1,500 controls, 15 studies concerning the evaluation of gp-tTG included 1,168 celiac patients and 1,911 controls, and 5 studies concerning the evaluation of ph-tTG included 176 celiac patients and 288 controls. The most popular rh-tTG manufactured assay was the one by Pharmacia (seven studies), and the next most popular was the one of Eurospital (five studies). The most popular gp-tTG assay was the one of Eurospital (three studies), and the most popular ph-tTG assay was the one of INOVA (two studies) (Table 1).
All studies had a sufficient description of the assay and the gold standard used (biopsy), which represented the true presence or absence of CD. In seven studies (5, 17, 18, 19, 27, 32, 36), there was no sufficient description of the individual's characteristics, and only in five studies (9, 12, 26, 31, 30) there was detailed description of the disease status but the authors did not provide the sensitivities and specificities according to disease status. One study (26) included two (3%) patients with IgA deficiency in the disease group. In two studies (5, 31), there was indication of bias, and in one of these two studies (31), the controls were only healthy blood donors. Therefore, these studies were subject to a sensitivity analysis. The agreement level between the reviewers was high, with k = 0.93 (95% CI, 0.87 to 0.95).
Sensitivity and specificity of h-tTG ELISA.
The sensitivity and specificity of each included assay were calculated (Table 1). In rh-tTG, sensitivity ranged from 65% (20) to 100% and specificity ranged from 84% (5) to 100%. In ph-tTG, the lower levels are higher than those for rh-tTG, and sensitivity ranged from 84% (3) to 100% and specificity ranged from 86% (36) to 100%. In gp-tTG, the lowest sensitivity and specificity values were 50% (5) and 12% (36), respectively, and the highest were 100%.
All assays independently.
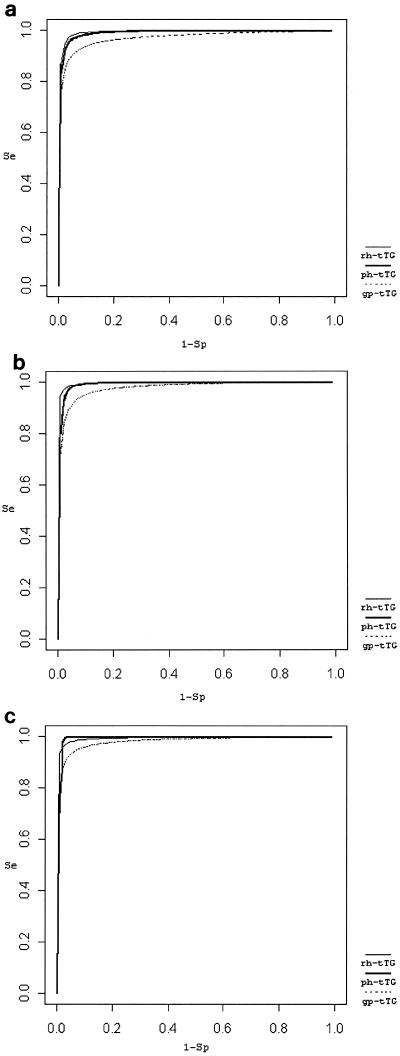
In SROC analysis, the sensitivity and specificity were examined simultaneously, and the trade-off between them is shown in Fig. 1a.
FIG. 1.
Summary ROC curves. (a) rh-tTG (AUC = 0.99, Q* = 0.97), ph-tTG (AUC = 0.99, Q* = 0.96), and gp-tTG (AUC = 0.97, Q* = 0.92), all assays were used independently; (b) rh-tTG (AUC = 0.99, Q* = 0.97), ph-tTG (AUC = 0.99, Q* = 0.97), and gp-tTG (AUC = 0.98, Q* = 0.93), in studies with multiple assays, the most popular assay was chosen in the analysis; (c) rh-tTG (AUC = 0.99, Q* = 0.97), ph-tTG (AUC = 0.99, Q* = 0.98), and gp-tTG (AUC = 0.98, Q* = 0.94), sensitivity analysis for studies showing bias (in studies with multiple assays, the most popular assay was chosen in the analysis).
h-tTG.
The independent RE sensitivity was 94%, and the independent RE specificity was 95%. If a threshold was chosen that the pooled RE specificity was 95%, then the sensitivity was a pSe of 100%. The common diagnostic log(OR) was a D value of 6.70 (95% CI, 5.74 to 7.65). The Q* point at which sensitivity equals specificity has a value of 97%.
ph-tTG.
The diagnostic performance of ph-tTG was similar to that of rh-tTG, the RE sensitivity and specificity were 94% and 94%, respectively. For a specificity of 94%, the SROC curve gives a sensitivity of a pSe of 100%, the Q* was 96%, and the common log(OR) was a D value of 6.33 (95% CI, 4.70 to 7.97).
gp-tTG.
The diagnostic performance of gp-tTG was worse than rh-tTG or ph-tTG. The RE sensitivity and specificity were 91% and 89%, respectively; the pSe was 97%. The common log(OR) (D = 4.99 [95% CI, 4.31 to 5.66]), the AUC, and the Q* were lower than those in h-tTG.
The FE estimates for Se and Sp, the estimated slope b, the intercept a, and the AUC are shown in Table 2.
TABLE 2.
Main analysis results based on multiple assays (manufacturers) and on the most popular assay and sensitivity analysis resultsa
| Assay(s) | tTG type | No. of assays or studies | No. of cases/no. of controls | % Se (95% CI) | % Sp (95% CI) | a (95% CI) | b (95% CI) | AUC | pSe (%) | Q* (%) (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| All | rh | 20 assays | 1,602/2,210 | 94 (90, 96) (RE) | 95 (93, 97) (RE) | 6.70 (5.74, 7.65) | 0.08 (−0.51, 0.68) | 0.99 | 100 | 97 (96, 97) |
| 88 (86, 90) (FE) | 94 (93, 95) (FE) | |||||||||
| ph | 7 assays | 257/362 | 94 (87, 97) (RE) | 94 (88, 97) (RE) | 6.33 (4.70, 7.97) | 0.04 (−1.04, 1.13) | 0.99 | 100 | 96 (94, 98) | |
| 91 (86, 95) (FE) | 94 (89, 95) (FE) | |||||||||
| gp | 21 assays | 1,462/2,115 | 91 (87, 94) (RE) | 89 (81, 94) (RE) | 4.99 (4.31, 5.66) | −0.22 (−0.49, 0.05) | 0.97 | 97 | 92 (91, 94) | |
| 87 (85, 89) (FE) | 87 (85, 89) (FE) | |||||||||
| Most popular | rh | 13 studies | 1,120/1,500 | 94 (92, 96) (RE) | 97 (95, 98) (RE) | 7.20 (5.94, 8.45) | −0.08 (−0.94, 0.78) | 0.99 | 100 | 97 (97, 98) |
| 94 (92, 95) (FE) | 96 (94, 97) (FE) | |||||||||
| ph | 5 studies | 176/288 | 92 (83, 97) (RE) | 96 (90, 99) (RE) | 6.70 (3.54, 9.86) | 0.25 (−2.30, 2.80) | 0.99 | 100 | 97 (94, 99) | |
| 90 (83, 94) (FE) | 95 (91, 97) (FE) | |||||||||
| gp | 15 studies | 1,168/1,911 | 90 (84, 94) (RE) | 92 (86, 96) (RE) | 5.17 (4.26, 6.09) | −0.08 (−0.52, 0.35) | 0.98 | 98 | 93 (91, 95) | |
| 87 (84, 89) (FE) | 90 (89, 92) (FE) | |||||||||
| Most popular (sensitivity analysis) | rh | 11 studies | 948/1,262 | 94 (92, 96) (RE) | 96 (94, 98) (RE) | 6.89 (5.88, 7.90) | −0.16 (−0.82, 0.5) | 0.99 | 99 | 97 (96, 98) |
| 94 (92, 95) (FE) | 96 (94, 97) (FE) | |||||||||
| ph | 4 studies | 144/250 | 95 (82, 97) (RE) | 98 (95, 99) (RE) | 7.66 (5.91, 9.41) | 0.61 (−0.65, 1.87) | 0.99 | 100 | 98 (97, 99) | |
| 91 (83, 95) (FE) | 98 (95, 99) (FE) | |||||||||
| gp | 14 studies | 1,136/875 | 91 (87, 94) (RE) | 93 (87, 96) (RE) | 5.48 (4.81, 6.14) | −0.15 (−0.46, 0.16) | 0.98 | 98 | 94 (93, 95) | |
| 88 (86, 90) (FE) | 91 (90, 93) (FE) |
The independent pooled sensitivity (Se) and pooled specificity (Sp) using RE and FE models, the intercept and slope of the unweighted SROC curve, the AUC of the SROC curve, the pSe derived from SROC using the RE Sp, and the Q* point where sensitivity and specificity are equal are shown.
Most popular assay in studies with multiple assays.
When the meta-analysis considered the most popular assay in studies with multiple assays, the pattern of the relative diagnostic accuracy in the rh-tTG, ph-tTG, and gp-tTG groups remained the same. The derived SROC curves are shown in Fig. 1b.
rh-tTG.
The RE sensitivity and specificity were 94% and 97%, respectively. For a specificity of 97%, the SROC curve produced a sensitivity of a pSe of 100%, the common diagnostic log(OR) was a D value of 7.20 (95% CI, 5.94 to 8.45), and the Q* has a value of 97%.
ph-tTG.
The diagnostic metrics of ph-tTG were slightly lower than those of rh-tTG, but the number of studies concerning ph-tTG was very low for deriving reliable results. The RE sensitivity and specificity were 92% and 96%, respectively. For a specificity of 96%, the SROC curve produced a sensitivity of a pSe of 100%, the common log(OR) was a D value of 6.70 (95% CI, 3.54 to 9.86), and the Q* was 97%.
gp-tTG.
The diagnostic performance of gp-tTG was again less accurate than rh-tTG or ph-tTG. The RE sensitivity and specificity were 90% and 92%, the pSe was 98%, the common log(OR) was a D value of 5.17 (95% CI, 4.26 to 6.09), and the Q* was 93% (91, 95%).
In the sensitivity analysis, excluding the two studies with indications of bias (5, 31), one of which included only healthy blood donors as controls (31), the results changed considerably only for ph-tTG: the ph-tTG produced better diagnostic metrics than the rh-tTG and gp-tTG. The RE sensitivity of ph-tTG was 95%, and its RE specificity was 98%. The pSe was 100%, the log(OR) was 7.66 (95% CI, 5.91 to 9.441), and the Q* was 98 (Fig. 1c). However, in all three tTG categories (rh-tTG, ph-tTG, and gp-tTG) the slope b was not close to 0 but was still not significant (Table 2). The sensitivity analysis for the study with only healthy blood donors as controls (31) and for the study with IgA-deficient patients (33) did not change the pattern and magnitude of the effects.
DISCUSSION
The present meta-analysis attempted to identify the current published literature regarding the use of rh-tTG, ph-tTG, and pg-tTG with ELISA for the diagnosis of celiac disease and to provide a quantitative assessment of their overall performance. Evaluating and summarizing test accuracy from published articles is a complex task with many methodological pitfalls and biases, and the relevant methodology is currently under development. In this context, we used the most appropriate and available methods for meta-analyzing data on test accuracy. One of the main problems we confronted in this attempt was the handling of multiple assays (which might be correlated) within studies. The choice of the most popular assay, which we used, provides a partial solution, although information is omitted from the analysis. By analyzing all assays independently, the effect of large studies with a single assay in estimating the diagnostic metrics is limited compared to studies with multiple assays and fewer participants.
The published studies have had different design settings, which may question the synthesis of information, and therefore, an assessment of the generalizability of results is required (7, 16, 21, 28). In this meta-analysis, the CD patients and the controls were well defined with similar inclusion criteria, although they unavoidably cover a wide spectrum of disease in terms of severity and other manifestations (e.g., cases with low- and high-grade pathology).
The meta-analysis (based on all individual assays or on the most popular assay) demonstrated that ELISAs using human tTG as a substrate are highly sensitive and specific and they have a better discrimination power and diagnostic accuracy than those using guinea pig tTG. ELISAs using rh-tTG and ph-tTG are comparable, although the sensitivity analysis showed that the latter may perform better. This effect may be attributed to the presence of vector (Escherichia coli or other) material in the preparations of recombinant tTG. However, the number of studies evaluating the performance of ELISAs using ph-tTG is limited, and the sensitivity results should be interpreted cautiously. Although sensitivity estimates differ across studies in terms of absolute values, the studies do not show significant heterogeneity in terms of diagnostic power. The SROC curve analysis showed that the diagnostic performance of the various studies closely follows a satisfactory defined path of trade-off between sensitivity and specificity (7, 16, 21, 28, 33, 37). The independent estimates of sensitivity and specificity can be reliable estimates (perhaps not for the rh-tTG studies using the most popular assay), since they are relatively close to the SROC curve. However, any variations in sensitivity and specificity might be due to differences in thresholds used by the authors and to variability in the prevalence of CD (ranging from 14% to 58%).
A more rigorous analysis could be performed if each author reported a receiver operating characteristic curve that presents the performance of a test more thoroughly than do single values of sensitivity and specificity. However, the major constraint to valid meta-analysis is publication bias and the difficulty in assessing the quality of the primary studies (13). However, it has been shown (2) that quality does not affect the magnitude of effects in published studies. In this meta-analysis, sensitivity analyses were carried out for studies with indications of bias, those involving healthy controls only and/or patients with IgA deficiency.
In conclusion, this meta-analysis proved that ELISAs detecting IgA antibodies against rh-tTG and ph-tTG, but not gp-tTG, are working sufficiently in the initial diagnostic approach of CD.
Acknowledgments
We thank Erdos Hedi, librarian at the Institute for Advanced Study, Budapest, Hungary, for expert assistance with computer-based literature searches.
REFERENCES
- 1.Alaedini, A., and P. H. R. Green. 2005. Narrative review: coeliac disease: understanding a complex autoimmune disorder. Ann. Intern. Med. 142:289-298. [DOI] [PubMed] [Google Scholar]
- 2.Balk, E. M., P. A. Bonis, H. Moskowitz, C. H. Schmid, J. P. Ioannidis, C. Wang, and J. Lau. 2002. Correlation of quality measures with estimates of treatment effect in meta-analyses of randomized controlled trials. JAMA 287:2973-2982. [DOI] [PubMed] [Google Scholar]
- 3.Basso, D., N. Gallo, G. Guariso, M. Pittoni, M. G. Piva, and M. Plebani. 2001. Role of anti-transglutaminase (anti-tTG), anti-gliadin, and anti-endomysium serum antibodies in diagnosing coeliac disease: a comparison of four different commercial kits for anti-tTG determination. J. Clin. Lab. Anal. 15:112-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battaglia, M., H. Bucher, M. Egger, F. Grossenbacher, C. Minder, and D. Pewsner. 2002. The Bayes library of diagnostic studies and reviews, 2nd ed. University of Basel, Basel, Switzerland.
- 5.Blackwell, P. J., P. G. Hill, and G. K. Holmes. 2002. Autoantibodies to human tissue transglutaminase: superior predictors of coeliac disease. Scand. J. Gastroenterol. 37:1282-1285. [DOI] [PubMed] [Google Scholar]
- 6.Boissel, J. P., and M. Cucherat. 1998. The meta-analysis of diagnostic test studies. Eur. Radiol. 8:484-487. [DOI] [PubMed] [Google Scholar]
- 7.Brown, M. 2003. Turbidimetric D-dimer test in the diagnosis of pulmonary embolism: a metaanalysis. Clin. Chem. 49:1846-1853. [DOI] [PubMed] [Google Scholar]
- 8.Burgin-Wolff, A., I. Dahlbom, F. Hadziselimovic, and C. J. Petersson. 2000. Antibodies against human tissue transglutaminase and endomysium in diagnosing and monitoring coeliac disease. Scand. J. Gastroenterol. 6:685-691. [DOI] [PubMed] [Google Scholar]
- 9.Carroccio, A., G. Vitale, L. Di Prima, N. Chifari, S. Napoli, C. La Russa, G. Gulotta, M. R. Averna, G. Montalto, S. Mansueto, and A. Notarbartolo. 2002. Comparison of anti-transglutaminase ELISAs and an anti-endomysial antibody assay in the diagnosis of coeliac disease: a prospective study. Clin. Chem. 48:1546-1550. [PubMed] [Google Scholar]
- 10.Fabiani, E., and C. Catassi. 2001. The serum IgA class anti-tissue transglutaminase antibodies in the diagnosis and follow up of coeliac disease. Results of an international multi-centre study. International Working Group on Eu-tTG. Eur. J. Gastroenterol. Hepatol. 13:659-665. [DOI] [PubMed] [Google Scholar]
- 11.Hansson, T. 2002. Recombinant human tissue transglutaminase for diagnosis and follow-up of childhood coeliac disease. Pediatr. Res. 51:700-705. [DOI] [PubMed] [Google Scholar]
- 12.Hansson, T., I. Dahlbom, J. Hall, A. Holtz, L. Elfman, A. Dannaeus, and L. Klareskog. 2000. Antibody reactivity against human and guinea pig tissue transglutaminase in children with coeliac disease. J. Pediatr. Gastroenterol. Nutr. 30:379-384. [DOI] [PubMed] [Google Scholar]
- 13.Irwig, L., A. N. Tosteson, C. Gatsonis, J. Lau, G. Colditz, T. C. Chalmers, and F. Mosteller. 1994. Guidelines for meta-analyses evaluating diagnostic tests. Ann. Intern. Med. 120:667-676. [DOI] [PubMed] [Google Scholar]
- 14.Irwig, L., P. Macaskill, P. Glasziou, and M. Fahey. 1995. Meta-analytic methods for diagnostic test accuracy. J. Clin. Epidemiol. 40:119-130. [DOI] [PubMed] [Google Scholar]
- 15.Johnston, S. D., S. A. McMillan, J. S. Collins, T. C. Tham, N. I. McDougall, and P. Murphy. 2003. A comparison of antibodies to tissue transglutaminase with conventional serological tests in the diagnosis of coeliac disease. Eur. J. Gastroenterol. Hepatol. 15:1001-1004. [DOI] [PubMed] [Google Scholar]
- 16.Kowalski, J., X. M. Tu, G. Jia, and M. Pagano. 2001. A comparative meta-analysis on the variability in test performance among FDA-licensed enzyme immunosorbent assays for HIV antibody testing. J. Clin. Epidemiol. 54:448-461. [DOI] [PubMed] [Google Scholar]
- 17.Kumar, V., M. Jarzabek-Chorzelska, J. Sulej, M. Rajadhyaksha, and S. Jablonska. 2001. Tissue transglutaminase and endomysial antibodies-diagnostic markers of gluten-sensitive enteropathy in dermatitis herpetiformis. Clin. Immunol. 98:378-382. [DOI] [PubMed] [Google Scholar]
- 18.Leon, F., C. Camarero, R. R. Pena, P. Eiras, L. Sanchez, M. Baragano, M. Lombardia, A. Bootello, and G. Roy. 2001. Anti-transglutaminase IgA ELISA: clinical potential and drawbacks in coeliac disease diagnosis. Scand J. Gastroenterol. 36:849-853. [DOI] [PubMed] [Google Scholar]
- 19.Llorente, M. J., M. Sebastian, M. J. Fernandez-Acenero, G. Serrano, and S. Villanueva. 2004. IgA antibodies against tissue transglutaminase in the diagnosis of coeliac disease: concordance with intestinal biopsy in children and adults. Clin. Chem. 50:451-453. [DOI] [PubMed] [Google Scholar]
- 20.Martini, S., G. Mengozzi, G. Aimo, L. Giorda, R. Pagni, and C. S. Guidetti. 2002. Comparative evaluation of serologic tests for coeliac disease diagnosis and follow-up. Clin. Chem. 48:960-963. [PubMed] [Google Scholar]
- 21.Midgette, A. S., T. A. Stukel, and B. Littenberg. 1993. A meta-analytic method for summarizing diagnostic test performances: receiver-operating-characteristic-summary point estimates. Med. Decis. Making 13:253-257. [DOI] [PubMed] [Google Scholar]
- 22.Moses, L. E., D. Shapiro, and B. Littenberg. 1993. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat. Med. 12:1293-1316. [DOI] [PubMed] [Google Scholar]
- 23.Osman, A. A., T. Richter, M. Stern, K. Conrad, J. Henker, C. Brandsch, K. P. Zimmer, and T. Mothes. 2002. Production of recombinant human tissue transglutaminase using the baculovirus expression system and its application for serological diagnosis of coeliac disease. Eur. J. Gastroenterol. Hepatol. 14:1217-1223. [DOI] [PubMed] [Google Scholar]
- 24.Owens, D. K., M. Holodniy, A. M. Garber, J. Scott, S. Sonnad, L. Moses, B. Kinosian, and J. S. Schwartz. 1996. Polymerase chain reaction for the diagnosis of HIV infection in adults. Ann. Int. Med. 124:803-815. [DOI] [PubMed] [Google Scholar]
- 25.Reif, S., and A. Lerner. 2004. Tissue transglutaminase-the key player in coeliac disease: a review. Autoimmun. Rev. 3:40-45. [DOI] [PubMed] [Google Scholar]
- 26.Sblattero, D., T. Not, and R. Marzari. 2000. Human recombinant tissue transglutaminase ELISA: an innovative diagnostic assay for coeliac disease. Am. J. Gastroenterol. 95:1253-1257. [DOI] [PubMed] [Google Scholar]
- 27.Scoglio, R., G. Di Pasquale, G. Pagano, M. C. Lucanto, G. Magazzu, and C. Sferlazzas. 2003. Is intestinal biopsy always needed for diagnosis of coeliac disease? Am. J. Gastroenterol. 98:1325-1331. [DOI] [PubMed] [Google Scholar]
- 28.Sotiriadis, A., G. Makrydimas, and J. P. A. Ioannidis. 2003. Diagnostic performance of intracardiac echogenic foci for Down syndrome: a meta-analysis. Obstet. Gynecol. 101:1009-1016. [DOI] [PubMed] [Google Scholar]
- 29.Sugai, E., G. Selvaggio, H. Vazquez, M. Viola, R. Mazure, B. Pizarro, E. Smecuol, D. Flores, S. Pedreira, E. Maurino, J. C. Gomez, and J. C. Bai. 2000. Tissue transglutaminase antibodies in coeliac disease: assessment of a commercial kit. Am. J. Gastroenterol. 95:2318-2322. [DOI] [PubMed] [Google Scholar]
- 30.Tesei, N., E. Sugai, H. Vazquez, E. Smecuol, S. Niveloni, R. Mazure, M. L. Moreno, J. C. Gomez, E. Maurino, and J. C. Bai. 2003. Antibodies to human recombinant tissue transglutaminase may detect coeliac disease patients undiagnosed by endomysial antibodies. Aliment. Pharmacol. Ther. 17:1415-1423. [DOI] [PubMed] [Google Scholar]
- 31.Trevisiol, C., A. Ventura, V. Baldas, A. Tommasini, D. Santon, S. Martelossi, G. Torre, I. Berti, A. Spano, S. Crovella, A. Amoroso, D. Sblattero, R. Marzari, and A. Bradbury. 2002. A reliable screening procedure for coeliac disease in clinical practice. Scand. J. Gastroenterol. 37:679-684. [DOI] [PubMed] [Google Scholar]
- 32.Vitoria, J. C., A. Arrieta, C. Arranz, A. Ayesta, A. Sojo, N. Maruri, and M. D. Garcia-Masdevall. 1999. Antibodies to gliadin, endomysium, and tissue transglutaminase for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 29:571-574. [DOI] [PubMed] [Google Scholar]
- 33.Walter, S. D. 2002. Properties of the summary receiver operating characteristic (SROC) curve for diagnostic test data. Stat. Med. 21:1237-1256. [DOI] [PubMed] [Google Scholar]
- 34.Watson, R. G. 2005. Diagnosis of coeliac disease. BMJ 330:739-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolters, V., A. F. Vooijs-Moulaert, H. Burger, R. Brooimans, J. De Schryver, G. Rijkers, and R. Houwen. 2002. Human tissue transglutaminase enzyme linked immunosorbent assay outperforms both the guinea pig based tissue transglutaminase assay and anti-endomysium antibodies when screening for coeliac disease. Eur. J. Pediatr. 161:284-287. [DOI] [PubMed] [Google Scholar]
- 36.Wong, R. C., R. J. Wilson, R. H. Steele, G. Radford-Smith, and S. Adelstein. 2002. A comparison of 13 guinea pig and human anti-tissue transglutaminase antibody ELISA kits. J. Clin. Pathol. 55:488-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yiannaki, E. E., E. Zintzaras, A. Analatos, C. Theodoridou, G. N. Dalekos, and A. E. Germenis. 2004. Evaluation of a microsphere-based flow cytometric assay for diagnosis of coeliac disease. J. Immunoassay Immunochem. 25:345-357. [DOI] [PubMed] [Google Scholar]