Abstract
Mycobacterium ulcerans disease (Buruli ulcer) is a skin-ulcerating infection common in some parts of the tropics. We have investigated cytokine secretion after stimulation of whole blood from Buruli ulcer (BU) patients in a region of endemicity in Ghana with M. ulcerans sonicate or culture filtrate antigens to investigate the development of the response over time and its specificity by comparison with the response to Mycobacterium tuberculosis sonicate in human immunodeficiency virus-negative tuberculosis patients. Significant gamma interferon (IFN-γ) production in response to whole-blood stimulation with M. ulcerans sonicate was detected in patients with ulcers, which was higher than that in patients with nodules but similar to subjects with healed BU. The mean IFN-γ response in household contacts of BU patients was not significantly different from that in healthy control subjects from an area of nonendemicity. Results in patients with untreated, smear-positive pulmonary tuberculosis and tuberculosis patients on treatment for more than 2 weeks showed that BU patients responded better to M. ulcerans antigens than tuberculosis patients. In contrast, interleukin-10 results were higher in patients with active M. ulcerans disease than in those with healed lesions, but the pattern of response was similar to that seen in tuberculosis. A similar pattern of cytokine secretion was found using M. tuberculosis sonicate as an antigen. Neither of the two culture filtrate antigens of M. ulcerans appeared to be more specific than M. ulcerans sonicate. In the early stages of M. ulcerans disease there was a mixed Th1 and Th2 cytokine response, but the Th1 response emerged as the dominant type.
Mycobacterium ulcerans disease (Buruli ulcer) is a skin-ulcerating infection common in some parts of the tropics (17). The histopathology is a panniculitis with extensive necrosis of subcutaneous fatty tissue containing abundant extracellular acid-fast bacilli, and in later stages, there is an inflammatory response with granulomas (5), suggesting that cell-mediated immunity plays a role in healing. In one of the few studies giving an insight into the natural history of the disease, about a third of early lesions healed spontaneously over 12 weeks, so it is likely that there is a significant cellular immune response (17). Studies of the delayed hypersensitivity response to intradermal injection of M. ulcerans sonicate (burulin) in patients with M. ulcerans disease showed that those with early disease did not react, whereas a positive response was elicited from 50% of patients with healing lesions, indicating T-cell sensitization (19).
More recently, it was shown that peripheral blood mononuclear cells (PBMC) from Australian subjects with past or current M. ulcerans disease produce less gamma interferon (IFN-γ) after stimulation with live M. ulcerans or live Mycobacterium bovis than PBMC from healthy tuberculin-positive individuals, suggesting T-cell anergy to mycobacterial antigens (8). Using reverse transcription and PCR, they showed that, after stimulation with live M. ulcerans or M. bovis, PBMC of Buruli ulcer patients expressed mainly the Th2 cytokines interleukin-4 (IL-4), IL-5, IL-6, and IL-10, whereas unaffected contacts responded with Th1 cytokines IFN-γ and IL-12 (9). In similar experiments using PBMC from patients in French Guyana stimulated with killed M. ulcerans or M. bovis, patients with nodular forms of the disease had predominantly Th1 cytokine profiles, while those with ulcerative forms of the disease had a mainly Th2 cytokine profile (15). Westenbrink et al. showed higher IFN-γ production after stimulation with tuberculin in patients with healing Buruli ulcer lesions than in controls, but no specific M. ulcerans antigens were used in that study (23).
We have investigated cytokine secretion after stimulation with M. ulcerans sonicate or culture filtrate antigens of whole blood from Buruli ulcer patients in a region of endemicity in Ghana to investigate the development of the response over time and its specificity by comparison to the response to Mycobacterium tuberculosis sonicate in tuberculosis patients.
MATERIALS AND METHODS
Patients and control subjects.
Recruitment of patients and control subjects took place between September 2003 and September 2004 after subjects had given informed consent (from parent or guardian when below 18 years). The study protocol was approved by the ethics review committees at the School of Medical Science, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana, and St. George's Hospital in London, United Kingdom. There were 44 confirmed cases of active M. ulcerans disease (mean age, 12 years), 29 healed M. ulcerans disease subjects (mean age, 21 years), 27 household contacts who had lived with an M. ulcerans disease patient for at least 3 months (mean age, 21 years), and 30 healthy control school children from an area of nonendemicity (mean age, 9 years) (Table 1). Thirty newly diagnosed smear-positive, human immunodeficiency virus (HIV)-negative pulmonary tuberculosis patients (mean age, 34 years) and 29 HIV-negative tuberculosis patients who had been receiving antimycobacterial treatment for 2 to 12 weeks (mean age, 30 years) were recruited from the Chest Clinic at Komfo Anokye Teaching Hospital, Kumasi. Patients with active and healed Buruli ulcer were recruited at St. Martin's Catholic Hospital, Agroyesum, Nkawie Government Hospital, Nkawie, Ghana, and Tepa Government Hospital at Tepa, Ghana. Using clinical notes, subjects were traced whose M. ulcerans disease had been healed for at least 1 year (median period, 3 years; range, 1 to 13 years). None of the patients with M. ulcerans disease had a history of tuberculosis or leprosy or of close contact with cases of either disease.
TABLE 1.
Characteristics of patients and controls
| Result for:
|
||||||
|---|---|---|---|---|---|---|
| Parameter | Buruli ulcer patients
|
Tuberculosis patients
|
Household contactsa | NECb | ||
| Active Buruli ulcer | Healed Buruli ulcer | Untreated | Partially treatedc | |||
| No. of subjects | 44 | 29 | 30 | 29 | 27 | 30 |
| Age (yr) | 12 ± 4 | 21 ± 3 | 34 ± 4 | 30 ± 4 | 21 ± 4 | 9 ± 1 |
| No. of M/no. of Fd | 18/16 | 16/13 | 14/16 | 16/13 | 11/16 | 15/15 |
Healthy controls from an area of nonendemicity.
Subjects lived with an M. ulcerans disease patient for at least 3 months.
Subjects had been receiving antimycobacterial treatment for more than 2 weeks.
M, males; F, females.
Antigens.
M. ulcerans sonicate antigen was prepared from M. ulcerans 1, an M. ulcerans isolate of African origin. A loopful of M. ulcerans colonies cultivated on Lowenstein Jensen slopes was transferred into 10 ml of Sauton's medium (2 mM MgSO4, 10 mM citric acid, 3 mM K2HPO4, 30 mM asparagine, 0.005% ferric ammonium citrate, 520 mM glycerol, pH adjusted to 7.2 with ammonia, autoclaved for 20 min at 121°C) and incubated at 30°C. The 10-ml M. ulcerans culture was passaged into 50 ml and subsequently into 500 ml of Sauton's medium with shaking (speed, 100 rpm; New Brunswick Scientific Co., Inc., Edison, N.J.). After 4 weeks, the bacterial pellet, obtained after centrifugation of M. ulcerans 1 cultures at 4°C for 30 min at 18,000 × g, was washed twice with phosphate-buffered saline in 500 ml polycarbonate tubes and centrifuged at 4°C for 30 min at 18,000 × g (10,500 rpm) in a Sorvall Plus centrifuge as described elsewhere (22). The pellet was suspended in 35 ml sterile water and sonicated with a Branson 250 Sonifier at 50% duty cycle using a small probe in a cup-horn container: four cycles of 15 min with continuous cooling, interspersed with 5-min breaks cooling on ice. The M. ulcerans sonicate was aliquoted in 2-ml portions and lyophilized. Ag423 and Ag424 were obtained from cultures of M. ulcerans 1 after cultivation in Sauton's protein-free medium. Culture supernatant was filtered through a closed system using a 1.2-μm filter (Excelon PP capsules; Schleicher and Schuell) and then with a sterile 0.2-μm filter (sterile capsule 0.45 μm/0.2 μm; Sartorius AG). The culture filtrate was concentrated 10 times from a 3-liter volume by ultrafiltration using a Sartocon Micro ultrafiltration unit with a 5-kDa molecular mass cut off. The concentrated culture filtrate proteins were precipitated with ammonium sulfate (0 to 45%) at pH 6.2 for Ag423 and pH 4.0 for Ag424 and stored at −20°C. Proteins were quantified using the bicinchoninic acid method with Pierce reagents. The concentration of Ag423 was 1.2 mg/ml and that of Ag424 was 0.4 mg/ml. M. tuberculosis sonicate was prepared using a procedure similar to that for M. ulcerans sonicate from M. tuberculosis 1 (myc4514; RIVM [National Institute of Public Health and Environmental Protection], Bilthoven, The Netherlands).
Whole-blood assay.
Twelve milliliters of venous blood was obtained by venesection from M. ulcerans patients and controls in sodium heparin Vacutainer tubes (Becton Dickinson, United Kingdom). The whole-blood assay was performed in a laboratory at Komfo Anokye Teaching Hospital, Kumasi. Whole blood was aseptically distributed, 1 ml per well in duplicate in 24-well tissue culture plates (Falcon; Becton-Dickinson, United Kingdom). Cell cultures were incubated with sonicates, culture filtrate antigens, or phytohemagglutinin (PHA) at a final concentration of 10 μg per ml. One set of wells was unstimulated to serve as background controls. Gentamicin was added to each well at 10 μg/ml (Sigma, United Kingdom). The plates were gently swirled 10 times clockwise and 10 times anticlockwise on a flat surface and incubated at 37°C with 5% CO2 for 24 h. Plasma supernatants (200 to 300 μl per well) were collected and stored at −70°C. Enzyme-linked immunosorbent assays (ELISA) for IL-10 and IFN-γ were carried out on samples that were clear and nonhemolyzed.
ELISA.
OptEIA sets for human IL-10- and human IFN-γ-containing components necessary to develop enzyme-linked immunosorbent assays and recommended buffers and solutions were obtained from BD Biosciences, Pharmingen (San Diego, Calif.). The manufacturer's recommended assay procedure was used.
Calculation of ELISA results and statistical analysis.
The mean absorbance of duplicate standards, samples, and controls was calculated for each plate, and the mean zero standard absorbance was subtracted. Results were analyzed with GraphPad Prism 4 software (GraphPad Software, Inc.), and a standard curve (best fit) was plotted with IL-10 or IFN-γ concentration (pg/ml). Values for unstimulated cultures were subtracted from those for stimulated cultures as background. Results for IL-10 and IFN-γ were presented as means of duplicates. The lower detection limits were 4.7 pg/ml for the IFN-γ and 7.8 pg/ml for the IL-10. Results were validated by a significant response to PHA stimulation.
Descriptive results of cytokines were expressed as means and standard errors of the means. Means for subjects and control groups were compared using analysis of variance with the Tukey-Kramer multiple comparison test. A P value of less than 0.05 was considered significant.
RESULTS
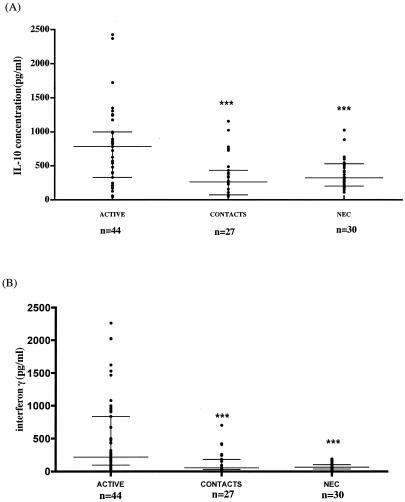
Figure 1 shows that both IL-10 and IFN-γ production in response to M. ulcerans 1 sonicate were significantly higher in active M. ulcerans disease patients (IL-10, 831 ± 123 pg/ml; IFN-γ, 498.4 ± 88 pg/ml [mean ± standard error]) than in control subjects living in an area of nonendemicity (NEC) (IL-10, 387 ± 40 pg/ml [P < 0.01]; IFN-γ, 78.25 ± 9.1 pg/ml [P < 0.001]), who were the least likely to show a positive response. The response of household contacts was similar to that of NEC, but several contacts had higher IFN-γ responses, raising the possibility that these individuals had been exposed to the organism.
FIG. 1.
IL-10 (A) and IFN-γ (B) production in active Buruli ulcer patients, household contacts, and NEC after stimulation of whole blood with an M. ulcerans 1 sonicate. ***, P values of <0.05, referring to comparison with active M. ulcerans disease patients. The horizontal lines represent the median and the interquartile range. Each dot represents a subject.
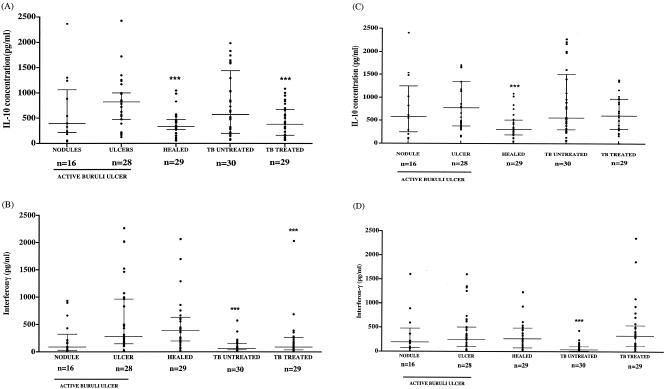
Patients with active M. ulcerans disease were divided into those with early disease (nodules or plaques, with or without edema), those with established ulcers, and subjects with healed lesions, and they were compared to patients with active tuberculosis. All blood samples were stimulated with M. ulcerans sonicate, M. tuberculosis sonicate, and PHA. Samples from all patients responded to PHA. Figures 2A and 2B show that both IL-10 and IFN-γ production in response to M. ulcerans sonicate were higher in patients with ulcers than in those with nodules, but IFN-γ production remained high in patients with healed lesions, whereas IL-10 production was significantly lower in this group. The IL-10 response in patients with untreated tuberculosis was similar to that of patients with ulcerative M. ulcerans disease, but it was significantly lower in patients receiving antimycobacterial drug therapy. In contrast, the IFN-γ response to M. ulcerans sonicate was significantly lower in both groups of tuberculosis patients than in ulcerative M. ulcerans disease patients. However, the IFN-γ response to M. tuberculosis sonicate was similar in M. ulcerans disease and tuberculosis patients (Fig. 2D), suggesting that M. ulcerans sonicate was more specific than M. tuberculosis sonicate. The IL-10 response to M. tuberculosis sonicate was similar to that to M. ulcerans sonicate in M. ulcerans disease, but the treated tuberculosis patients responded better to this antigen preparation with no significant difference from the M. ulcerans ulcer patients (Fig. 2C).
FIG. 2.
IL-10 (A and C) and IFN-γ (B and D) production after stimulation of whole blood with an M. ulcerans 1 sonicate (A and B) or M. tuberculosis sonicate (C and D) in active and healed Buruli ulcer patients, patients with untreated TB, and patients with tuberculosis receiving antimycobacterial drugs (treated). The horizontal lines represent the median and interquartile range. ***, P values of <0.05, referring to comparison with ulcerative M. ulcerans disease.
Both IL-10 and IFN-γ responses to M. ulcerans culture filtrate antigens Ag423 and Ag424 were lower than those to the mycobacterial sonicate antigens. The responses to Ag424 were lower than those to Ag423, and there were no significant differences between any of the patient groups (P > 0.05).
The development of the cytokine response during M. ulcerans disease is demonstrated in Fig. 2. There was wide variation in both IL-10 and IFN-γ responses to M. ulcerans sonicate (Fig. 2A and 2B), but there was no correlation between IL-10 and IFN-γ responses (Spearman r = 0.13; P = 0.46) in individuals. Thus, low IFN-γ cannot be attributed to high IL-10 production.
DISCUSSION
This is the first investigation of the immune response to M. ulcerans antigens in patients with M. ulcerans disease from Africa where the disease is endemic in particular areas. A broad spectrum of patients was included as well as household contacts, control subjects from an area of nonendemicity, and patients with tuberculosis. The whole-blood antigen stimulation technique was a useful tool when blood samples were collected several miles from the laboratory. Similar whole-blood assays with short incubations have been used to investigate exposure to M. tuberculosis in low-risk populations without active tuberculosis (1-3, 6, 11, 13, 14, 16, 20).
Patients with active or healed M. ulcerans disease mounted a significant IFN-γ response to M. ulcerans antigens compared with NEC with the lowest exposure to M. ulcerans. There was no evidence of specific systemic immunosuppression for M. ulcerans, since Buruli ulcer patients produced higher levels of IFN-γ than tuberculosis (TB) patients in response to M. ulcerans sonicate, and levels of response to M. tuberculosis sonicate were similar to those of TB patients (Fig. 2C). There was no reason to believe that these tuberculosis patients were immunocompromised, and they were known to be HIV negative. Newly diagnosed untreated M. tuberculosis patients also had elevated IL-10 responses to M. ulcerans antigens which were higher than those of patients receiving antimycobacterial treatment. There was cross-reactivity between M. ulcerans and M. tuberculosis antigens with respect to IL-10 responses of patients with M. ulcerans disease, but with regard to IFN-γ, M. ulcerans patients responded more strongly to M. ulcerans sonicate than tuberculosis patients so M. ulcerans sonicate may have contained antigens which were not present in M. tuberculosis sonicate and which conferred partial specificity. Disappointingly, neither of the culture filtrate antigens showed increased specificity.
These results are different from the findings of Gooding et al. (8, 9) and Prevot et al. (15). Gooding et al. showed that 4 active and 10 healed (8) or 1 active and 22 healed (9) Buruli ulcer patients had low IFN-γ responses after stimulation of PBMC with live M. ulcerans isolates and that there was low expression of Th1 cytokines in PBMCs using reverse transcription and PCR. There were many differences between the above and the present studies, including the antigen preparations used and whether whole-blood or PBMCs were tested, but none of these clearly explains the discrepancies. Live M. ulcerans was used for the stimulation assays by Gooding et al. (8, 9), but they chose that preparation because it was thought to stimulate the strongest response. Theoretically M. ulcerans could secrete mycolactone during incubation, leading to inhibition of cytokine secretion, but this would be expected to apply to IL-10 also and it could not account for reduced responses to M. bovis or Ag85. There were differences in the patient populations in that the Australians were both Caucasians and Aborigines, which is unlikely to be a significant factor, and they were mostly patients with healed lesions, whereas in the present study, there were large numbers of patients with each disease presentation as well as household contacts and control subjects from areas of nonendemicity. There was a wide range of responses between individuals in all the disease groups. The patients were very uncertain about the duration of their disease, so it was not possible to demonstrate a significant relationship between duration of disease and level of IFN-γ response, but this may have been a factor nevertheless. Prevot et al. found that nodular forms (n = 4) of Buruli ulcer produced significantly higher levels of IFN-γ than ulcerative forms (n = 9), but this is the only study to have found this (15). If the IFN-γ response develops at different rates in different individuals, it is essential to include adequate numbers to obtain a balanced view, and undoubtedly, the number of subjects in the present study was higher than in either of the other studies.
Although the IFN-γ response was low in most patients with nodules, the response in some was above the median for the ulcer group (Fig. 2B). These lesions might have healed spontaneously, as observed in 30% of patients in the placebo arm of the investigation of clofazamine treatment of early lesions (17), and this could be investigated in a prospective study, but selective treatment based on IFN-γ response may be impractical in an African setting.
A similar pattern of low IFN-γ response to M. ulcerans sonicate apart from a small number of individuals was observed among household contacts (Fig. 1B), suggesting that some people in areas of endemicity develop an immune response without any clinical disease. This is highly likely, since it happens with other mycobacterial infections, including tuberculosis (12), but it is difficult to speculate how this occurs in M. ulcerans infection, since the mode of transmission remains uncertain.
This study showed that blood samples from patients with active M. ulcerans disease produced significantly more IL-10 after M. ulcerans antigen stimulation than NEC or household contacts (Fig. 2A). The IL-10 response developed more rapidly from the nodular to the ulcerative stage than the IFN-γ response, but it fell after healing. Prevot et al. also demonstrated significantly higher IL-10 responses in ulcerative than in nodular M. ulcerans disease using whole killed M. ulcerans as an antigen, but IL-10 responses to M. bovis BCG and Ag85 were close to the detection limit of their assay in both nodules and ulcers (15). Ag85 and M. bovis were not tested in the present studies, but there was a good IL-10 response to M. tuberculosis sonicate, which was similar to that induced by M. ulcerans sonicate (Fig. 2C).
Prevot et al. suggested that an elevated IL-10 response was the cause of reduced IFN-γ levels in Buruli ulcer patients, since IL-10 is known to be a powerful down-regulator of IFN-γ production (15). In the present studies, patients with nodular disease did show low IFN-γ responses in the presence of higher IL-10 responses, but patients with ulcerative disease had the highest IL-10 responses and, at the same time, showed high IFN-γ levels. There was no inverse correlation between IFN-γ and IL-10 (Spearman r = 0.13), suggesting that IFN-γ production was not suppressed by IL-10.
The pattern of cytokine secretion in the present study suggested that the immune response was predominantly TH2 in early nodular lesions when the IL-10 response was higher than that of IFN-γ but in the later, ulcerative stage it was both TH1 and TH2, with only the TH1 response persisting in patients with healed lesions. A similar pattern of an early transient TH2 response has been observed in M. tuberculosis infection. When tuberculosis was first diagnosed, production of IFN-γ in peripheral blood after M. tuberculosis antigen stimulation was low, but there was a high IL-10 response (4, 10, 18). Later, a Th1 response dominated by IFN-γ production emerged in most patients. Indeed, in our studies, IFN-γ responses were higher in treated than in untreated tuberculosis, but this difference was not significant, probably as a result of the variable duration of treatment in our group of patients.
There were limitations to this study. White blood cell and differential counts were not done, which might account for some variation in cytokine responses, but van Crevel et al. demonstrated that IL-10 and IFN-γ in an ex vivo whole-blood system did not correlate with absolute T-lymphocyte or monocyte counts (21). Also, the differences in ages of patients and controls could bias the results.
The contribution of mycolactone to pathogenesis of human M. ulcerans lesions is likely to be profound, since subcutaneous injection of the toxin into guinea pigs causes necrotic lesions similar to those resulting from injection of live organisms (7). Pahlevan showed that it had specific immunosuppressive effects in vitro, such as inhibition of IL-1, tumor necrosis factor alpha, IL-2, and IL-10, but it has also been shown to cause cell cycle arrest and cell death by apoptosis so it is likely to interfere with generation of the immune response locally in infected tissues. However, the present study did not show any evidence of systemic immunosuppression, suggesting that it may be necessary to investigate local immune responses in the infected tissues to observe any effects of mycolactone.
Acknowledgments
This study was supported by a grant from the Wellcome Trust. R.P. has a Wellcome Trust Training Research Fellowship Award for Research into Infectious Diseases for Scientists from Tropical and Developing Countries.
We thank Edwin Ampadu, Ghana National Buruli ulcer program, Kofi Asare, Ashanti regional director of health services, Nsiah Asare, chief executive Komfo Anokye Teaching Hospital (KATH), George Bedu-Addo, Department of Medicine (KATH), Acquiline Sagoe, Director of Nursing (KATH), Beatrice Appau, District Director (Atwima), and Elizabeth Adentwe, District Director (Tepa), for their assistance with this study.
REFERENCES
- 1.Bellete, B., J. Coberly, G. L. Barnes, C. Ko, R. E. Chaisson, G. W. Comstock, and W. R. Bishai. 2002. Evaluation of a whole-blood interferon-gamma release assay for the detection of Mycobacterium tuberculosis infection in 2 study populations. Clin. Infect. Dis. 34:1449-1456. [DOI] [PubMed] [Google Scholar]
- 2.Brock, I., M. E. Munk, A. Kok-Jensen, and P. Andersen. 2001. Performance of whole blood IFN-gamma test for tuberculosis diagnosis based on PPD or the specific antigens ESAT-6 and CFP-10. Int. J. Tuberc. Lung Dis. 5:462-467. [PubMed] [Google Scholar]
- 3.Brock, I., K. Weldingh, T. Lillebaek, F. Follmann, and P. Andersen. 2004. Comparison of tuberculin skin test and new specific blood test in tuberculosis contacts. Am. J. Respir. Crit. Care Med. 170:65-69. [DOI] [PubMed] [Google Scholar]
- 4.Dieli, F., G. Friscia, C. Di Sano, J. Ivanyi, M. Singh, R. Spallek, G. Sireci, L. Titone, and A. Salerno. 1999. Sequestration of T lymphocytes to body fluids in tuberculosis: reversal of anergy following chemotherapy. J. Infect. Dis. 180:225-228. [DOI] [PubMed] [Google Scholar]
- 5.Dodge, O. G. 1964. Mycobacterial skin ulcers in Uganda: histopathological and experimental aspects. J. Pathol. Bacteriol. 88:169-174. [DOI] [PubMed] [Google Scholar]
- 6.Fietta, A., F. Meloni, A. Cascina, M. Morosini, C. Marena, P. Troupioti, P. Mangiarotti, and L. Casali. 2003. Comparison of a whole-blood interferon-gamma assay and tuberculin skin testing in patients with active tuberculosis and individuals at high or low risk of Mycobacterium tuberculosis infection. Am. J. Infect. Control 31:347-353. [DOI] [PubMed] [Google Scholar]
- 7.George, K. M., L. Pascopella, D. M. Welty, and P. L. Small. 2000. A Mycobacterium ulcerans toxin, mycolactone, causes apoptosis in guinea pig ulcers and tissue culture cells. Infect. Immun. 68:877-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gooding, T. M., P. D. Johnson, D. E. Campbell, J. A. Hayman, E. L. Hartland, A. S. Kemp, and R. M. Robins-Browne. 2001. Immune response to infection with Mycobacterium ulcerans. Infect. Immun. 69:1704-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gooding, T. M., P. D. Johnson, M. Smith, A. S. Kemp, and R. M. Robins-Browne. 2002. Cytokine profiles of patients infected with Mycobacterium ulcerans and unaffected household contacts. Infect. Immun. 70:5562-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jo, E. K., J. K. Park, and H. M. Dockrell. 2003. Dynamics of cytokine generation in patients with active pulmonary tuberculosis. Curr. Opin. Infect. Dis. 16:205-210. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, P. D., R. L. Stuart, M. L. Grayson, D. Olden, A. Clancy, P. Ravn, P. Andersen, W. J. Britton, and J. S. Rothel. 1999. Tuberculin-purified protein derivative-, MPT-64-, and ESAT-6-stimulated gamma interferon responses in medical students before and after Mycobacterium bovis BCG vaccination and in patients with tuberculosis. Clin. Diagn. Lab Immunol. 6:934-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufmann, S. H. 2002. Protection against tuberculosis: cytokines, T cells, and macrophages. Ann. Rheum. Dis. 61(Suppl. 2):ii54-ii58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazurek, G. H., P. A. LoBue, C. L. Daley, J. Bernardo, A. A. Lardizabal, W. R. Bishai, M. F. Iademarco, and J. S. Rothel. 2001. Comparison of a whole-blood interferon gamma assay with tuberculin skin testing for detecting latent Mycobacterium tuberculosis infection. JAMA 286:1740-1747. [DOI] [PubMed] [Google Scholar]
- 14.Mori, T., M. Sakatani, F. Yamagishi, T. Takashima, Y. Kawabe, K. Nagao, E. Shigeto, N. Harada, S. Mitarai, M. Okada, K. Suzuki, Y. Inoue, K. Tsuyuguchi, Y. Sasaki, G. H. Mazurek, and I. Tsuyuguchi. 2004. Specific detection of tuberculosis infection: an interferon-gamma-based assay using new antigens. Am. J. Respir. Crit. Care Med. 170:59-64. [DOI] [PubMed] [Google Scholar]
- 15.Prevot, G., E. Bourreau, H. Pascalis, R. Pradinaud, A. Tanghe, K. Huygen, and P. Launois. 2004. Differential production of systemic and intralesional gamma interferon and interleukin-10 in nodular and ulcerative forms of Buruli disease. Infect. Immun. 72:958-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravn, P., M. E. Munk, A. B. Andersen, B. Lundgren, L. N. Nielsen, T. Lillebaek, I. J. Soerensen, P. Andersen, and K. Weldingh. 2004. Reactivation of tuberculosis during immunosuppressive treatment in a patient with a positive QuantiFERON-RD1 test. Scand. J. Infect. Dis. 36:499-501. [DOI] [PubMed] [Google Scholar]
- 17.Revill, W. D., R. H. Morrow, M. C. Pike, and J. Ateng. 1973. A controlled trial of the treatment of Mycobacterium ulcerans infection with clofazimine. Lancet ii:873-877. [DOI] [PubMed] [Google Scholar]
- 18.Song, C. H., H. J. Kim, J. K. Park, J. H. Lim, U. O. Kim, J. S. Kim, T. H. Paik, K. J. Kim, J. W. Suhr, and E. K. Jo. 2000. Depressed interleukin-12 (IL-12), but not IL-18, production in response to a 30- or 32-kilodalton mycobacterial antigen in patients with active pulmonary tuberculosis. Infect. Immun. 68:4477-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanford, J. L., W. D. Revill, W. J. Gunthorpe, and J. M. Grange. 1975. The production and preliminary investigation of Burulin, a new skin test reagent for Mycobacterium ulcerans infection. J. Hyg. (London) 74:7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Streeton, J. A., N. Desem, and S. L. Jones. 1998. Sensitivity and specificity of a gamma interferon blood test for tuberculosis infection. Int. J. Tuberc. Lung Dis. 2:443-450. [PubMed] [Google Scholar]
- 21.van Crevel, R., J. van der Ven-Jongekrijg, M. G. Netea, W. de Lange, B. J. Kullberg, and J. W. van der Meer. 1999. Disease-specific ex vivo stimulation of whole blood for cytokine production: applications in the study of tuberculosis. J. Immunol. Methods 222:145-153. [DOI] [PubMed] [Google Scholar]
- 22.Verbon, A., S. Kuijper, H. M. Jansen, P. Speelman, and A. H. Kolk. 1990. Antigens in culture supernatant of Mycobacterium tuberculosis: epitopes defined by monoclonal and human antibodies. J. Gen. Microbiol. 136:955-964. [DOI] [PubMed] [Google Scholar]
- 23.Westenbrink, B. D., Y. Stienstra, M. G. Huitema, W. A. Thompson, E. O. Klutse, E. O. Ampadu, H. M. Boezen, P. C. Limburg, and T. S. van der Werf. 2005. Cytokine responses to stimulation of whole blood from patients with buruli ulcer disease in Ghana. Clin. Diagn. Lab. Immunol. 12:125-129. [DOI] [PMC free article] [PubMed] [Google Scholar]