Abstract
The mechanisms by which probiotic bacteria affect the immune system are unknown yet, but many of them are attributed to an increase in the innate or in the acquired immune response. To study the influence of the probiotic bacterium Lactobacillus casei in the expression of receptors involved in the innate immune response, this bacterium was orally administered to BALB/c mice. After, they were sacrificed; the small intestine and intestinal fluids were collected to measure secretory immunoglobulin A (IgA) specific for L. casei. Mononuclear cells from Peyer's patches were isolated to determine the CD-206 and TLR-2 receptors. In histological slices we determined the number of IgA+, CD4+, CD8+, and CD3+ cells and two cytokines (interleulin-5 [IL-5] and IL-6). CD-206 and TLR-2 increased with respect to the untreated control. We did not observe an increase in the T population or in the IL-5-positive cells. IgA+ cells and IL-6-producing cells increased after 7 days of L. casei administration. We did not find specific antibodies against L. casei. The main immune cells activated after oral L. casei administration were those of the innate immune response, with an increase in the specific markers of these cells (CD-206 and TLR-2), with no modification in the number of T cells.
Host defense against foreign challenge is elicited by the immune system, which consists of the innate and the acquired immune systems that induce both the systemic and the mucosal immune responses.
The innate and adaptive immune systems are two interdependent parts of a single integrated immune system. At the gut mucosal level, the innate immune response not only provides the first line of defense against pathogenic microorganisms but also provides the biological signals that instruct the adaptive immune system to elicit a response. Noncommensal and probiotic bacteria are also able to induce a gut mucosal immune response (26).
The cells that play a critical role in initiating the innate immune response are the macrophages and the dendritic cells, which are specialized phagocytes that participate in the cellular and molecular clearance as well as in the defense against infection. These phagocytic cells have developed a receptor system call pattern recognition receptors, which are able to recognize molecular patterns associated with pathogens present in the surface. These receptors are activated by pathogens (2).
One of the most intensely studied families of pattern recognition receptors is the Toll-like receptor (TLR) family. TLRs play a central role in alerting antigen-presenting cells to the presence of pathogenic material (5). TLRs can activate the innate immune response, mainly inflammation, before the adaptive immune response (1, 9, 22).
TLR-2 recognizes a variety of microbial components such as lipoproteins/lipopeptides from various pathogens, peptidoglycans, and lipoteichoic acid from gram-positive bacteria. It has been reported that TLR-2 is able to recognize lipopolysaccharide (LPS) preparations from enterobacteria such as Leptospira interrogans, Porphyromonas gingivalis, and Helicobacter pylori (11, 33). These LPS structurally differ from the typical LPS of gram-negative bacteria recognized by TLR-4 in the number of acyl chains in the lipid A component, which presumably confers differential recognition (24).
Another family of receptors implicated in pattern recognition is the mannose receptor family. The mannose receptor CD-206 is the best characterized and was first identified as a receptor involved in the clearance of self antigens such as endogenous proteins, including myeloperoxidase, lysosomal hydrolases, and some hormones that contain sulfated carbohydrate groups (19). This receptor binds carbohydrate groups containing mannosyl/fucosyl residues and a terminal lectin domain that binds sulfated carbohydrate groups and is able to recognize a number of microbial proteoglycans. The mannose receptor also facilitates the uptake of mannosylated antigens by dendritic cells in vitro for presentation to the acquired immune system.
At present there is much evidence concerning the role of probiotics, especially lactic acid bacteria (LAB), in the maintenance of health or in the prevention of disease.
The probiotic consumptions had been useful in the treatment of many types of diarrhea, including antibiotic-associated diarrhea in adults, travelers' diarrhea, and diarrheal diseases in young children caused by rotaviruses (12, 25, 32).
Probiotics may exert a beneficial effect on allergic reaction and in lactose intolerance, and probiotics have also been attributed other effects, such as the increase of nutrient bioavailability, the decrease of the serum cholesterol concentrations, and the improvement of urogenital health.
Probiotics such as lactobacilli and bifidobacteria in fermented or culture-containing dairy foods may play a role in reducing the risk of colon cancer (4, 36).
Another property attributed to probiotics is modulation of the host's immune response. Some of their effects have been attributed to an increase in the innate immune response and others to an increase in the acquired immune response; however, the mechanisms through which these probiotic LAB, orally administered, influence the gut immune system and produce immunostimulative effects are still unknown.
Probiotic microorganisms are defined as “live microbial feed supplements that beneficially affect the host animal by improving its intestinal microbial balance” (7). In humans, organisms of the Lactobacillus genus are most commonly used as probiotics, either as single species or in mixed cultures with other bacteria.
The immunological properties of probiotic bacteria have been studied previously (35) and showed that certain LAB, such as Lactobacillus casei, Lactobacillus rhamnosus, and Lactobacillus plantarum enhance both systemic and mucosal immunity. Food containing probiotic bacteria are able to stimulate the immunoglobulin A (IgA) immune response (17). In vitro studies have shown that several LAB strains promote the immunopotentiator capacity of cells of the innate immune system, including macrophages (18).
In a previous work,we demonstrated that the oral administration of LAB stimulated the gut immune cells to release inflammatory (tumor necrosis factor alpha, gamma interferon [IFN-γ], and interleukin-12 [IL-12]) and regulatory (IL-4, IL-10) cytokines. This effect was dose and strain dependent. Some strains induced regulatory and proinflammatory cytokines, while others increased the intestinal inflammatory response (27). The induction of an immune response by lactobacilli implies certain effects on the gut immune system, with interactions between bacteria and the epithelial and/or immune cells. In our laboratory, we demonstrated that lactobacilli can stimulate the immune system by different pathways of internalization. The LAB antigen uptake can be carried out by M or FAE cells from the Peyer's patches or by specialized epithelial cells (M cells) from the villi of the small intestine (15). These observations are important to explain the variations in the different responses induced in the host (28). We previously demonstrated that the whole bacteria cannot be introduced through the epithelial cells and that only the antigenic particles or products of degradation of the bacteria are able to make contact with the immune cells. We also showed that the viability of the probiotic bacteria was an important condition for a better stimulation of the gut immune system (20).
In another study, we demonstrated by in vitro assays that the interaction of the probiotic strain L. casei CRL 431 with the epithelial cells is through TLR-2 and that the release of IL-6 is induced as a consequence of this interaction (34).
These previous results led us to the present study with the aim to determine the main immune mechanisms induced by the probiotic strain L. casei CRL 431 at the intestinal level using conventional mice as an experimental model.
As consequence of these previous results, in this study we studied the expression of two receptors (CD-206 and TLR-2) present in the surface of macrophages and dendritic cells that are involved in innate immunity and the way in which orally administered L. casei affects this expression. We also analyzed the participation of the acquired immune system in the gut measuring specific secretory IgA (S-IgA) against L. casei epitopes. The markers of the T population (CD4, CD8, and CD3) and two cytokines (IL-5 and IL-6) also released by the T population were analyzed.
MATERIALS AND METHODS
Bacterial strain and culture conditions.
Lactobacillus casei CRL 431 used in this study was obtained from the CERELA culture collection (San Miguel de Tucumán, Argentina). L. casei was cultured in sterile Man Rogosa Sharpe (MRS) broth. The cells were harvested by centrifugation at 5,000 × g for 10 min and then washed three times with sterile saline solution.
Mice.
BALB/c mice weighing 25 to 30 g (6 weeks of age) were obtained from the inbred colony maintained at CERELA. Each experimental and control group for each period of the assay consisted of 5 mice. The animals were fed balanced rodent food and water ad libitum.
L. casei administration.
The mice were divided into four experimental groups. In three of them, mice were housed in individual boxes and given L. casei CRL 431 for 2, 5, or 7 consecutive days at 108 UFC/ml/mouse/day. The cells were suspended in sterile 10% (vol/vol) nonfat milk and administered at 1% (vol/vol) in the drinking water. The mice in the control group received 10% nonfat milk in the drinking water under the same conditions as the test groups. The volume drank, controlled daily, was 2.5 to 3 ml in both experimental and control groups.
Histological samples.
At the end of each administration period, the animals were killed, and the small intestine was removed for histological preparations following Sainte-Marie's (31) technique for paraffin inclusion.
Determination of CD-206 (mannose receptor)- and TLR2-positive cells in slices of the small intestine.
The CD-206- and TLR-2-positive cells were measured on histological slices from the small intestine of mice that received L. casei for 2, 5, or 7 days and for the same periods in the control group. After deparaffinization with an immersion in xylene and rehydration in ethanol, paraffin sections (4 μm) were incubated with a 1% blocking solution of bovine serum albumin (BSA) and Hank's balanced saline solution (HBSS) for 30 min at room temperature. They were washed in HBSS. Mouse anti-human CD-206 (BD Biosciences Pharmingen) monoclonal antibodies (diluted 1: 200 in HBSS) or rabbit anti-mouse TLR-2 (eBioscience) polyclonal antibodies (diluted 1:300) were applied to the sections for 60 min at room temperature. This incubation was followed by two washes with HBSS. The sections were then treated for 45 min with a dilution of the rabbit anti-mouse or goat anti-rabbit antibody conjugated with fluorescein isothiocyanate (FITC; Jackson Immuno Research Labs, Inc.) at room temperature and washed in HBSS twice. The number of fluorescent cells was counted in 30 fields of vision at a magnification of ×1,000. Results were expressed as the mean number of positive fluorescent cells per 10 fields.
Isolation of immune cells from Peyer's patches and CD-206 and TLR-2 determination.
After each feeding period with L. casei, the small intestine of each mouse was removed and the intestinal fluid was collected for enzyme-linked immunosorbent assays (ELISA). The intestinal tissue was examined for the presence of Peyer's patches, which were excised in HBSS with added fetal bovine serum. The epithelium cells were separated with an HBSS-fetal bovine serum solution containing EDTA. The mononuclear cells (sediment) were incubated with Dispase DNase solution, and the mononuclear cells were recovered. These cells were collected from the supernatant, washed with HBSS and then with RPMI 1640 medium (Sigma, St. Louis, Mo.). These leukocytes (total cells) were adjusted to 4 × 106 to 5 × 106 cells/ml in RPMI 1640. Twenty microliters of this cellular suspension was placed in each well of an immunofluorescence slide. The cells were fixed with formalin (ICC fixation buffer; PharMingen) and incubated with a 1% blocking solution of BSA-phosphate-buffered saline (PBS). The activity of the endogenous peroxidase was blocked with H2O2-methanol solution. The cells were then incubated with avidin and biotin blocking solution, followed by incubation with mouse anti-human CD-206 (BD Biosciences Pharmingen) monoclonal antibodies (diluted 1:200 in HBSS) or with rabbit anti-mouse TLR-2 (eBioscience USA) polyclonal antibodies (diluted 1:300). Then they were incubated with goat anti-mouse antibody or with goat anti-rabbit antibody conjugated with biotin-SP (Jackson Immuno Research Labs, Inc.). Vectastaine Elite ABC solution (Vector Labs) was added to the cells, which were then incubated with 3,3-diaminobenzidine tetrahydrochloride. The results were expressed as percentages of positive cells (counted at a magnification of ×1,000 in light microscopy).
Immunofluorescence assay for IgA-secreting cells and CD4+, CD8+, and CD3+ T lymphocytes.
The number of IgA+ cells and CD4+, CD8+, and CD3+ T lymphocytes was determined by direct immunofluorescence assays in the histological samples from the small intestine for each period of time assayed and for both test and control groups. IgA+ cells were determined as follows: the slides were incubated with α-chain monospecific antibody conjugated with FITC (Sigma, St. Louis, MO). For CD4+, CD8+, and CD3+ T lymphocyte determinations, monoclonal antibodies conjugated with FITC were used (Cedarlane, Ottawa, Canada). The number of fluorescent cells was counted in 30 fields of vision as seen at a magnification of ×1000 using a fluorescent light microscope. The results are expressed as numbers of positive cells in 10 fields of vision.
Immunofluorescence assays for the detection of IL-5- and IL-6-producing cells.
The cytokine-positive cells were measured on histological slices from the small intestine of the mice from the test and control groups. After deparaffinization with an immersion in xylene and rehydration in ethanol, paraffin sections (4 μm) were incubated with a 1% blocking solution of BSA and HBSS for 30 min at room temperature. They were washed in saponin-HBSS and incubated with normal goat serum (diluted 1:50) for 30 min. Goat anti-mouse IL-5 and rabbit anti-mouse IL-6 (R & D Systems, Inc., and Peprotech, Inc., Rocky Hill, NJ, respectively) polyclonal antibodies (diluted 1:100 in saponin-HBSS) were applied to the sections for 75 min at room temperature. This incubation was followed by two washes with saponin-HBSS. The sections were then treated for 45 min with a dilution of the rabbit anti-goat or goat anti-rabbit antibody conjugated with FITC (Jackson Immuno Research Labs, Inc.) at room temperature and washed in saponin-HBSS. The number of fluorescent cells was counted in 30 fields of vision at a magnification of ×1,000. Results were expressed as mean numbers of positive fluorescent cells per 10 fields.
Determination of specific IgA in intestinal fluids.
Specific antibodies in mouse intestinal fluid were measured by an ELISA technique using Nunc-Immuno plates (MaxiSorb F96; A/S, Nunc, Roskilde, Denmark). Plates were coated by overnight incubation at 4°C with 50 μl of a suspension of L. casei CRL 431 (108 UFC/ml). Nonspecific protein-binding sites were blocked with 250 μl of PBS (pH 7.2) containing 5% nonfat dry milk. Dilutions of 1/4 in PBS of the test and control samples of intestinal fluid were prepared in the ELISA plates (100 μl per well), which were then incubated at room temperature for 2 h. After washing with PBS-0.05% Tween, the plates were incubated at room temperature with affinity-purified, peroxidase-conjugated goat polyclonal antibodies specific for the alpha chain of mouse IgA (Sigma, St. Louis, MO) diluted 1/1,000. The plates were developed with o-phenylene-diamine (Sigma) in citrate-phosphate buffer (pH 5). Reactions were determined with 100 μl of H2SO4 (2 N). The optical density was read at 493 nm by using a Versa Max microplate reader (Molecular Devices).
Statistical analysis.
All results in this article are means of three independent trials ± standard deviation (SD). Student's t test was used to assess the statistical significance of the differences between test and control groups.
RESULTS
CD-206- and TLR-2-positive cells in the small intestine of mice fed with L. casei.
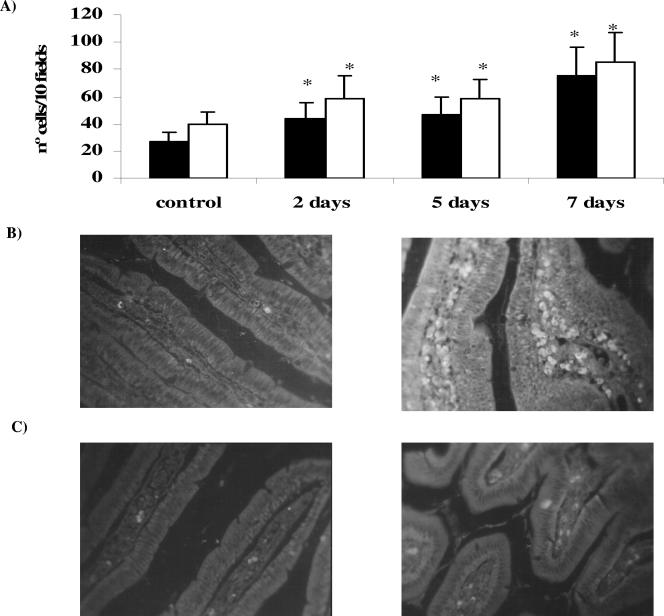
Figure 1A and B show that, in the animals with L. casei administration for 2, 5, or 7 consecutive days, the number of CD-206+ cells in the small intestine increased significantly in comparison with the control group (27 ± 9). The increase in the expression of this receptor was more important after 7 days of L. casei administration (70 ± 21).
FIG. 1.
Determination of CD-206 and TLR-2 receptors in immune cells in lamina propria of the small intestine of mice given L. casei. (A) Effect of L. casei administration on the number of CD-206+ and TLR-2+ cells. ▪, CD-206+ cells; □, TLR-2+ cells. (B) Microphotography of CD-206+ control cells and cells receiving 7 days of L. casei administration. (C) Microphotography of TLR-2+ control cells and cells receiving 7 days of L. casei administration. Animals received L. casei CRL 431 for 2, 5, or 7 consecutive days. The positive cells were determined on histological sections from the small intestine of the test and control groups by immunofluorescence assays. Values are the means of the results from 3 mice ± SD. *, significant differences between test and control groups (P < 0.001).
The number of TLR-2+ cells in the lamina propria of mice given L. casei increased significantly for the three periods of administration (68 ± 9, 62 ± 1, and 95 ± 17 for 2, 5, and 7 days, respectively) compared with results for the control group (40 ± 9), with this enhancement being greater for 7 days of L. casei administration (Fig. 1A and C).
Identification of CD-206 and TLR-2 in immune cells isolated from Peyer's patches.
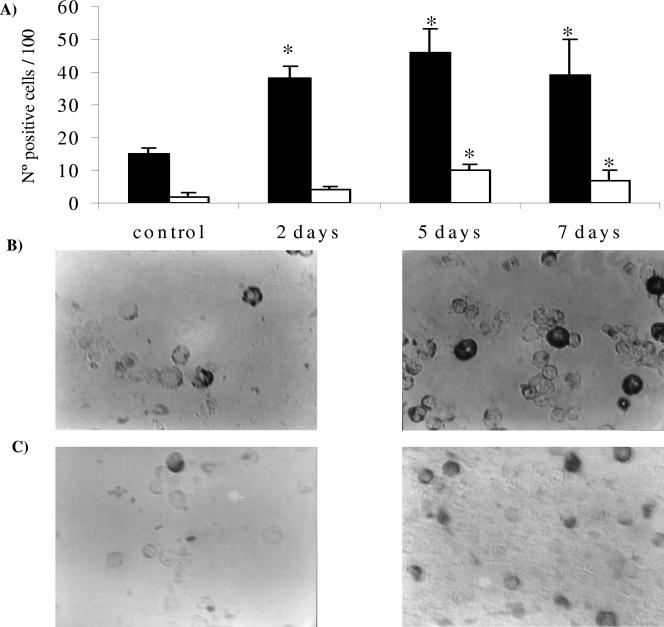
Macrophages and dendritic cells obtained from Peyer's patches after L. casei administration showed an important increase in the number of CD-206-positive cells (38 ± 4, 46 ± 7, and 39 ± 11) in comparison with the control (15 ± 2) for all periods assayed. These results are expressed in Fig. 2A and B.
FIG. 2.
Determination of CD-206 and TLR-2 receptors in immune cells isolated from Peyer's patches. (A) Effect of L. casei administration on the number of CD-206+ and TLR+ cells. ▪, CD-206+ cells; □, TLR-2+ cells. (B) Microphotography of CD-206+ control cells and cells receiving 5 days of L. casei administration. (C) Microphotography of TLR-2+ control cells and cells receiving 5 days of L. casei administration. The immune cells were purified from the Peyer's patches of mice that received L. casei for 2, 5, or 7 days and from the control group. Cells were determined by means of the immunoperoxidase technique. The values are the means of the results from 3 mice ± SD. *, significant differences between test and control groups (P < 0.001).
The effect of L. casei administration on TLR-2 expression can be observed in Fig. 2A and C. The increase in this receptor was significant for 5 and 7 days of L. casei administration compared with the control, while for 2 days of administration, we found no effect in the expression of this receptor, with the values being similar to those of the control group. The expression of TLR-2 was lower than that of CD-206 in these immune cells isolated from the Peyer's patches.
T-lymphocyte markers and IgA+ cells in the small intestine.
L. casei administration showed no effect on the number of the CD4, CD8, and CD3 markers of the T lymphocytes. The values obtained for all these markers were similar to those of the control group for all periods of L. casei administration. These results, expressed in Table 1, would indicate that L. casei did not influence the T-cell population of the lamina propria in the small intestine. With regard to IgA+ cells determined in the lamina propria of the small intestine, we observed a significant increase (P < 0.001) in these cells (230 ± 20) compared with the control (118 ± 20) only for 7 days of L. casei administration (Table 1).
TABLE 1.
Influence of L. casei administration on number of IgA+ cells and CD markers of T populationa
| Treatment group | No. of cells of type:
|
|||
|---|---|---|---|---|
| CD3 | CD4 | CD8 | IgA | |
| 2 days | 25 ± 5 | 25 ± 5 | 34 ± 9 | 124 ± 19 |
| 5 days | 42 ± 11 | 22 ± 6 | 22 ± 6 | 133 ± 15 |
| 7 days | 43 ± 9 | 22 ± 4 | 26 ± 7 | 230 ± 23* |
| Control | 43 ± 10 | 32 ± 8 | 28 ± 7 | 118 ± 17 |
CD3-, CD4-, CD8-, and IgA-positive cells were determined in the lamina propria of the small intestines of mice administered L. casei (2, 5, or 7 days) and in the control group by direct immunofluorescence assays. Values are the means of results for 3 mice ± SD. *, significant differences between test and untreated control (P < 0.001).
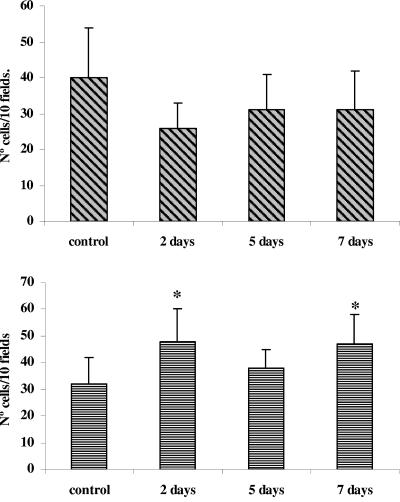
IL-5 and IL-6 determination.
The determination of IL-5-positive cells in the lamina propria of the small intestine of mice administered L. casei showed that this lactobacillus did not influence IL-5 production. The values obtained were similar to or even lower than (2 days of L. casei administration) those of the control group (40 ± 14) (Fig. 3, top). With regard to the number of IL-6-producing cells, we observed an increase in the mice administered L. casei for 2 and 7 days compared with the untreated control (32 ± 10). These results are shown in Fig. 3, bottom.
FIG. 3.
Determination of IL-5+ and IL-6+ cells in the lamina propria of the small intestines of mice receiving L. casei administration. (Top) Effect of L. casei on IL-5 production; (bottom) effect of L. casei on IL-6 production. Positive cells for these cytokines were determined on histological sections from the small intestine of the test and control groups by indirect immunofluorescence assays. Values are means of results for 3 mice ± SD. Significant differences were calculated in comparison with the untreated control group (*, P < 0.001).
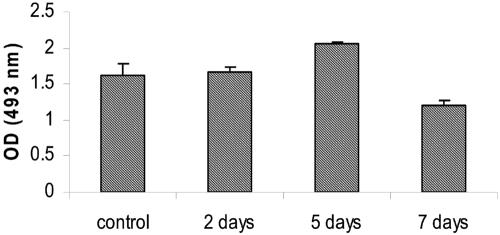
Specific S-IgA against L. casei determination.
The administration of L. casei did not induce specific S-IgA against its own epitopes. Figure 4 expresses the results obtained.
FIG. 4.
Determination of S-IgA against L. casei CRL 431 epitopes. S-IgA specific for L. casei CRL 431 cell wall epitopes was determined using ELISA in the intestinal fluid from mice that received a suspension of L. casei for 2, 5, or 7 consecutive days. Values are the mean optical densities (OD) for 3 mice ± SD. No significant differences in the test and control group were found.
DISCUSSION
The epithelial surface of the gut has intimate contact with the commensal intestinal microflora, which plays a crucial role in the anatomical, physiological, and immunological development of the host. This epithelial surface of the gastrointestinal tract is also confronted with a range of different microorganisms from the outside environment; however, it is protected by physiological and immune barriers. To maintain homeostasis in the mucosae, different defense mechanisms are involved in permanent and effective surveillance. One of these mechanisms is the secretory immune system through IgA antibodies (6). The protective microflora prevents the invasion and colonization of pathogenic microorganisms through the IgA antibodies and the competition for receptors and/or metabolic substrates.
It is known that the microflora of the gut stimulates the proliferation of epithelial cells (10) and that colonization of the gut with commensal microflora influences the development of the immune system (23).
Epithelial cells are important as the first line of defense because they are in constant contact with the bacteria and the bacterial products on their apical surface and because they are in close proximity with adjacent immune cells on their basolateral side. Epithelial cells can differentiate between pathogenic and nonpathogenic bacteria, probably by means of the recognition of conserved structure components in the bacteria (3); this fact is important for the tolerance of the epithelium to the normal microbial flora.
Nonpathogenic probiotic bacteria present in food can also influence the behavior of the gut mucosal immune system. When these microorganisms make contact with the epithelial cells and with the immune cells in Peyer's patches or lamina propria of the intestine, the associated immune cells such as monocytes/macrophages and dendritic cells initiate the innate immune response against these antigenic stimulations.
The basic functions of the mucosal immune system include protection against pathogens, prevention of the penetration by foreign antigens, induction of oral tolerance, and maintenance of mucosal homeostasis. The main difference between the mucosal and systemic immunities is that in the former the mechanisms of innate immunity and the activation of B cells for mucosal immunity are more important than the adaptive immune response involving the T-cell population (29). The knowledge of the different requirements to induce an effective immune response at the mucosal sites would help to define immunotherapeutic approaches and to prevent diseases. Probiotic bacteria would be a good choice to improve the mucosal immune system if the mechanisms through which they work are known.
The above considerations led us to determine whether or not the probiotic bacteria L. casei CRL 431 is able to induce or favor innate immunity. In a previous work using in vitro assays, we determined that this strain of L. casei interacts with the epithelial cells through TLR-2, thus inducing IL-6 release (34). In this study, we measured the expression of TLR-2 and CD-206 (mannose receptor) in dendritic cells and macrophages isolated from the Peyer's patches and in the lamina propria of the small intestine. The results obtained with the mannose receptor (Fig. 1 and 2) showed a significant increase in the number of positive cells for this receptor for all periods of L. casei administration, either in lamina propria or in isolated cells of the Peyer's patches. This receptor is mainly implicated in the homeostatic system for the clearance of endogenous molecules, but its participation in the context of the innate immune response needs to be clarified. However, it is possible that this receptor, in isolation or in association with other receptors, could affect the activation state of dendritic cells and macrophages. The mannose receptor also facilitates the uptake in vitro of the mannosylated antigens by dendritic cells for presentation to T cells, thus influencing the adaptive immune response mediated by Th1 during pathological conditions (21). When we analyzed the expression of TLR-2 (Fig. 1 and 2), we found an increase in the TLR-2 expressed in these cells after L. casei administration, either in Peyer's patches or in the lamina propria of the small intestine. We believe that the increase in CD-206 and TLR-2 is due to upregulation of these markers, in agreement with our previous studies (20) where we demonstrated that probiotic bacteria or their antigenic particles can be internalized by dendritic cells (DC) or macrophages and would induce signals to increase the number of CD-206 and TLR-2 receptors. However, we cannot exclude that the increase in these receptors may be due to the influx of cells. Many evidences indicate that in Peyer's patches there are two types of DC, myeloid (mDC) and plasmacytoid DC (13). mDC cells express TLR-2 and TLR-4 (16). The increase in the number of TLR-2-positive cells could indicate an activation of the mDC population in Peyer's patches. It is possible that the effect of L. casei on the immune cells involves TLR-2 as well, as was reported for the effect of this LAB on epithelial cell activation (34). It is well demonstrated in vitro that T cells in the presence of mDC secrete a large amount of IFN-γ with little IL-4, IL-5, and IL-10 (30) and that, in contrast, when T cells are cultured with plasmacytoid DC, they secrete a large amount of IL-4, IL-5, and IL-10 and only a small amount of IFN-γ, characteristic of a Th2 response (14).
In a previous paper, we demonstrated the profile of cytokines induced by L. casei (27), except for IL-6 and IL-5. When we analyzed the number of positive cells for IL-5 (Fig. 3, top) in the small intestine of mice fed L. casei, we did not find an increase in this interleukin compared to the control. After L. casei stimulation, we measured the receptors CD4, CD8, and CD3, characteristic of the T-cell population. No increases in the proliferation or clonal expansion of these cells were found in the lamina propria of the small intestine (Table 1). The results obtained for IL-5 and without increases in the T-cell population allow us to suggest that the increases for the IL-10+ cells reported in this model could be produced by other cells such as macrophages and dendritic cells (27).
The anti-inflammatory role of IL-6 in the enhancement of IgA secretion has been well demonstrated. This cytokine has the ability to induce the terminal development of B cells in plasmatic cells, which express IgA (8).
The intestinal epithelial cells produced an important number of cytokines, such as IL-6, as we demonstrated in a previous work (34). IL-6 is also produced by macrophages and T cells.
When we determined the effect of L. casei administration on the number of IL-6-positive cells, we found a significant increase in their number and the effect was dose dependent. We do not have an explanation for the down regulation found for IL-6 on day 5, and it is matter for further studies. The IL-6 was probably released by epithelial cells or macrophages (Fig. 3, bottom). This increase is in agreement with the IgA+ cells measured in the lamina propria of the small intestine after 7 days of L. casei administration (Table 1).
We did not detect S-IgA specific for L. casei in the intestinal fluid (Fig. 4). These findings could mean that non-antigen presentation to Th2 cells produced antibody against their own epitopes and that the nonadaptive immune response for antibody production was induced.
In the present paper, we demonstrated that the interaction between L. casei and the immune cells associated with the gut induced an increase in the number of CD-206 and TLR-2 receptors in the cells implicated mainly in the innate immune response (macrophages and dendritic cells). The increases obtained for IL-6+ cells allow us to suggest that they could be related to the enhancement of the IgA B-cell population.
The results obtained would indicate that, in the activation of the gut immune system by the probiotic strain L. casei CRL 431, the main immune cells implicated are those involved in the innate immune response (macrophages and dendritic cells) and that the T-cell population would be less involved in the immune activation observed. The transmission of the signals induced in the immune cells of the innate immunity after probiotic stimulation through CD-206 or TLR-2 receptors to the other immune cells present in the mucosal sites will allow us to determine the exact mechanisms by which the probiotic microorganisms work and to develop adjuvant strategies to increase the surveillance of the gut mucosal immune system without alterations in the intestinal homeostasis.
This is a first report demonstrating by in vivo studies in conventional animals that the main immune mechanism induced by the probiotic strain L. casei CRL 431 is the innate immunity with an influence in the clonal expansion of the IgA B-cell population.
Acknowledgments
This work was financially supported by grants 26/D231-2001/2003 from Consejo de Investigaciones Universidad Nacional de Tucumán (CIUNT) (Argentina), PIP 02176 from CONICET (Argentina), and PICT 00/10068 from FONCYT (Argentina).
REFERENCES
- 1.Ahmad-Nejad, P., H. Hacker, M. Rutz, S. Bauer, R. M. Vabulas, and H. Wagner. 2002. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur. J. Immunol. 32:1958-1968. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, K. V. 2000. Toll signaling pathways in the innate immune response. Curr. Opin. Immunol. 12:13-19. [DOI] [PubMed] [Google Scholar]
- 4.de Moreno de LeBlanc, A., J. Valdez, and G. Perdigón. 2004. Inflammatory immune response. Eur. J. Inflamm. 2:21-31. [Google Scholar]
- 5.Dunzendorfer, S., H. K. Lee, K. Soldau, and P. S. Tobias. 2004. TLR4 is the signaling but not the lipopolysaccharide uptake receptor. J. Immunol. 22:33-54. [DOI] [PubMed] [Google Scholar]
- 6.Fagarasan, S., and T. Honjo. 2003. Intestinal IgA synthesis: regulation of front-line body defences. Nat. Rev. Immunol. 3:63-72. [DOI] [PubMed] [Google Scholar]
- 7.Fuller, R. 1989. Probiotics in man and animals. J. Appl. Bacteriol. 66:365-378. [PubMed] [Google Scholar]
- 8.Goodrich, M. E., and D. W. McGee. 1999. Effect of intestinal epithelial cell cytokines on mucosal B-cell IgA secretion: enhancing effect of epithelial-derived IL-6 but not TGFbeta on IgA+ B cells. Immunol. Lett. 67:11-14. [DOI] [PubMed] [Google Scholar]
- 9.Heil, F., et al. 2003. The Toll-like receptor 7 (TLR7)-specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily. Eur. J. Immunol. 33:2987-2997. [DOI] [PubMed] [Google Scholar]
- 10.Heyman, M., A. M. Crain-Denoyelle, G. Corthier, J. L. Morgat, and J. F. Desjeux. 1986. Postnatal development of protein absorption in conventional and germ-free mice. Am. J. Physiol. 251:G326-G331. [DOI] [PubMed] [Google Scholar]
- 11.Hirschfeld, M., et al. 2001. Signaling by Toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect. Immun. 69:1477-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isolauri, E., M. Juntunen, T. Rautanen, P. Sillanaukee, and T. Koivula. 1991. A human Lactobacillus strain (Lactobacillus casei sp. Strain GG) promotes recovery from acute diarrhea in children. Pediatrics 88:90-97. [PubMed] [Google Scholar]
- 13.Iwasaki, A., and B. L. Kelsall. 2000. Localization of distinct Peyer's patch dendritic cells subset and their recruitment by chemokines macrophages inflammatory protein (MIP)-3 alpha, MIP3-beta, and secondary lymphoid organ chemokine. J. Exp. Med. 191:1381-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwasaki, A., and B. L. Kelsall. 1999. Freshly isolated Peyer's patches, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J. Exp. Med. 190:229-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang, M., M.-N. Kweon, K. Iwatani, M. Yamamoto, K. Terahara, C. Sasakawa, T. Suzuki, T. Nochi, Y. Yokota, P. D. Rennert, T. Hiroi, H. Tamagawa, H. Iijima, J. Kunisawa, Y. Yuki, and H. Kiyono. 2004. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc. Natl. Acad. Sci. USA 101:6110-6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarrossay, D., G. Napolitani, M. Colonna, F. Sallusto, and A. Lanzavecchia. 2001. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur. J. Immunol. 31:3388-3393. [DOI] [PubMed] [Google Scholar]
- 17.Kaila, M., E. Isolauri, E. Soppi, E. Virlanen, S. Laine, and H. Arvilommi. 1992. Enhancement of the circulating antibody secreting cell response in human diarrhoea by a human Lactobacillus strain. Pediatr. Res. 32:141-144. [DOI] [PubMed] [Google Scholar]
- 18.Kato, I., T. Yokokura, and M. Mutai. 1983. Macrophage activation by Lactobacillus casei in mice. Microbiol. Immunol. 27:611-618. [DOI] [PubMed] [Google Scholar]
- 19.Lee, S. J., S. Evers, D. Roeder, A. F. Parlow, J. Risteli, L. Risteli, Y. C. Lee, T. Feizi, H. Langen, and M. C. Nussenzweig. 2002. Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science 295:1898-1901. [DOI] [PubMed] [Google Scholar]
- 20.Maldonado Galdeano, C., and G. Perdigón. 2004. Role of viability of probiotic strains in their persistence in the gut and in mucosal immune stimulation, J. Appl. Microbiol. 97:673-681. [DOI] [PubMed] [Google Scholar]
- 21.Mansour, M. K., L. S. Schlesinger, and S. M. Levitz. 2002. Optimal T cell responses to Cryptococcus neoformans mannoprotein are dependent on recognition of conjugated carbohydrates by mannose receptors. J. Immunol. 168:2872-2879. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto, M., K. Funami, M. Tanabe, H. Oshiumi, M. Shingai, Y. Seto, A. Yamamoto, and T. Seya. 2003. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J. Immunol. 171:3154-3162. [DOI] [PubMed] [Google Scholar]
- 23.Moreau, M. C., and V. Gaboriau-Routhiau. 2000. Influence of resident intestinal microflora on the development and functions of the intestinal-associated lymphoid tissue, p. 69-104. In R. Fuller and G. Perdigón (ed.), Probiotics 3. Immunomodulation by the gut microflora a probiotics. R. Kluer Academic Publishers, London, United Kingdom.
- 24.Netea, M. G., M. van Deuren, B. J. Kullberg, J. M. Cavaillon, and W. M. Van der Maer. 2002. Does the shape of lipid A determine the interaction of LPS with Toll-like receptors? Trends Immunol. 23:135-139. [DOI] [PubMed] [Google Scholar]
- 25.Oksanen, P. J., S. Salminen, M. Saxelin, P. Hamalainen, A. Ihantola-Vormisto, L. Muurasniemi-Isoviita, S. Nikkari, T. Oksanen, I. Porsti, and E. Salminen. 1990. Prevention of travelers diarrhea by Lactobacillus GG. Ann. Med. 22:53-56. [DOI] [PubMed] [Google Scholar]
- 26.Perdigón, G., R. Fuller, and R. Raya. 2001. Lactic acid bacteria and their effect on the immune system. Curr. Issues Intest. Microbiol. 2:27-42. [PubMed] [Google Scholar]
- 27.Perdigón, G., C. Maldonado Galdeano, J. C. Valdez, and M. Medici. 2002. Interaction of lactic acid bacteria with the gut immune system. Eur. J. Clin. Nutr. 56(Suppl. 4):21-26. [DOI] [PubMed] [Google Scholar]
- 28.Perdigón, G., R. Fuller, and M. Medina. 2005. The influence of the lactic acid bacteria and other resident microflora on the immune system of the growing animal, p. 351-375. In W. H. Holzapfel and P. J. Naughton (ed.), Microbial ecology in growing animals. Elsevier Publishing, Inc., London, United Kingdom.
- 29.Revaz, V., and D. Nardelli-Haefliger. 2005. The importance of mucosal immunity in defense against epithelial cancers. Curr. Opin. Immunol. 17:175-179. [DOI] [PubMed] [Google Scholar]
- 30.Rissoan, M. C., V. Soumelis, and N. Kadowaki. 1999. Reciprocal control of T helper cell and dendritic cell differentiation. Science 283:1183-1186. [DOI] [PubMed] [Google Scholar]
- 31.Sainte-Marie, G. 1962. A paraffin embedding technique for studies employing immunofluorescence, J. Histochem. Cytochem. 10:150-156. [Google Scholar]
- 32.Siitonen, S., H. Vapaatalo, S. Salminen, A. Gordin, M. Saxelin, R. Wikberg, and A. L. Kirkkola. 1990. Effect of Lactobacillus GG yoghurt in prevention of antibiotic associated diarrhoea. Ann. Med. 22:57-59. [DOI] [PubMed] [Google Scholar]
- 33.Smith, M. F., Jr., A. Mitchell, G. Li, S. Ding, A. M. Fitzmaurice, K. Ryan, S. Crowe, and J. B. Goldberg. 2003. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-jB activation and chemokine expression by epithelial cells. J. Biol. Chem. 278:32552-32560. [DOI] [PubMed] [Google Scholar]
- 34.Vinderola, G., C. Matar, and G. Perdigón. 2005. Role of the epithelial cells in the immune effects mediated by gram-positive probiotic bacteria. Involvement of Toll-like receptors. Clin. Diagn. Lab. Immunol. 12:1075-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vintiñi, E., S. Alvarez, M. Medina, M. Medici, M. V. de Budeguer, and G. Perdigón. 2000. Gut mucosal immunostimulation by lactic acid bacteria. Biocell 24:223-232. [PubMed] [Google Scholar]
- 36.Wollowski, I., G. Rechkemmer, and B. L. Pool-Zobel. 2001. Protective role of probiotics and prebiotics in colon cancer. Am. J. Clin. Nutr. 73:451S-455S. [DOI] [PubMed] [Google Scholar]