Abstract
The objective of this study was to investigate the influence of exogenous reproductive hormones on the local and systemic production of specific immunoglobulin A (IgA) and IgG antibodies after vaginal vaccination with recombinant cholera toxin subunit B (CTB). Three groups of women using either progesterone-containing intrauterine devices (n = 9), oral contraceptives (n = 8), or no hormonal contraceptive methods (n = 9) were vaginally immunized twice, 2 weeks apart. Cervical secretions, vaginal fluids, and serum were collected before and after vaccination. Total and CTB-specific IgA and IgG antibodies in genital secretions and serum were analyzed by enzyme-linked immunosorbent assay. A majority of the women presented strong CTB-specific IgA and IgG antibody responses in cervicovaginal secretions after vaccination, whereas the antitoxin responses in serum were weaker. Exogenously administered steroid hormones did not seem to have any impact on the production of specific antibodies. Both the frequencies and the magnitudes of IgA and IgG antitoxin responses in genital secretions were comparable among the three immunization groups. An association, in particular for IgA, was found between the magnitudes of the CTB-specific antibody responses in cervical secretions and vaginal fluids after vaccination. The sensitivities and positive predictive values of vaginal antibody analyses to reflect responses in cervical secretions were also high, suggesting that vaginal fluids alone might be used for evaluation of genital immune responses in large-scale vaccination studies in the future.
Sexually transmitted diseases (STDs) represent a major global health problem. To halt the spread of STDs, interest has focused on defining mucosal vaccination strategies which could elicit pathogen-specific neutralizing antibodies in secretions of the genital tract (18, 29). Mucosal immunity in the female genital tract is influenced by cytokines, immunoglobulins (Igs), and reproductive hormones (23). Understanding the interactions of Igs and sexual steroids is important for the development of vaccination strategies against STDs.
The best-defined component of the mucosal immune system is locally produced immunoglobulin A (IgA), which consists of protease-resistant Ig dimers and incorporated J-chains adapted to function in the external environment of the mucosa (6). In the female genital tract, a considerable quantity of the IgA antibodies is produced in the cervix and a smaller amount in the fallopian tubes (22). The single-layer epithelium of the endocervix, the fallopian tubes, and the uterus express the secretory component (SC), which is necessary for the transportation of locally produced polymeric IgA into genital secretions (26). In contrast, the multilayer epithelium of the vagina does not stain for SC (21). Genital tract secretions contain higher levels of IgG than IgA antibodies. However, the origin of the IgG antibodies in cervicovaginal secretions has not been fully clarified, and the mechanism by which IgG reaches the luminal secretions remains unclear. Data suggest that the majority of IgG results from the transudation of serum antibodies (2, 12), although it is possible that some IgG may be actively transported or locally produced in the mucosa (37).
Sexual steroids seem to play a critical role in the regulation of local immunity in the genital tracts of humans (5, 7, 11, 20, 23). Estrogens upregulate the expression of SC in vitro, thereby increasing the transport of secretory Igs into the lumen (25). Immunohistochemical studies have demonstrated that both the number of IgA-producing plasma cells and the level of IgA within the endometrial glands in the cervix increase during the late secretory phase of the menstrual cycle, when the progesterone level is high (4). The levels of Igs in genital tract secretions of women are also subjected to considerable hormone-dependent variations during the menstrual cycle, with the lowest levels occurring around the time of ovulation (27, 30, 31). Furthermore, in oral contraceptive users there seems to be an association between the levels of Igs in cervical secretions and the amount of progesterone ingested (8).
The present study was undertaken to examine whether sexual steroids used for contraception might have an influence on the local and systemic IgA and IgG antibody responses in women after vaginal immunization with an inactivated cholera vaccine containing recombinant cholera toxin B subunit (CTB). The individual CTB-specific antibody responses in cervical secretions were also compared with responses in vaginal fluids and in serum to evaluate whether there is a simpler approach to the assessment of cervical antibody responses after mucosal vaccination. CTB is one of the best-characterized mucosal antigens with regard to both safety and immunogenicity in humans (3, 13, 14). No side effects have been reported after vaginal administration of CTB (17, 19, 35, 36). Recently, studies with mice have demonstrated that ganglioside-binding toxins including CTB might accumulate in the olfactory nerve and bulb when given intranasally (34). Whether such interactions occur in humans is not known.
MATERIALS AND METHODS
Subjects.
Twenty-six healthy women of reproductive age gave oral and written informed consent to participate in the study, which was approved by the Human Research Ethical Committee at the medical faculty of Göteborg University, Göteborg, Sweden. None of the volunteers had previously been vaccinated against cholera or had traveled to areas where cholera or enterotoxigenic Escherichia coli is endemic during the 5 years preceding the study. Entry criteria for all women consisted of a normal gynecological examination, a normal Papanicolaou (Pap) smear, and a negative PCR test for Chlamydia trachomatis. All women had used their current contraceptive method for at least 3 months prior to the start of the study.
Three groups of women using either progesterone-containing intrauterine devices (IUDs), oral contraceptive pills (OCPs), or no pharmacological contraceptive (NPC) methods were recruited for the study. The IUD group included nine women, 32 to 49 years old (mean age, 38 years), who used a levonorgestrel-containing IUD (Mirena; Schering, Germany) which releases 20 μg levonorgestrel per 24 h and induces amenorrhea in 80 to 90% of the users 1 year after insertion (1). All women in the IUD group were amenorrheic. The OCP group consisted of eight women, 18 to 41 years old (mean age, 27 years), using an oral contraceptive pill which contained 30 μg ethinyl estradiol and 150 μg levonorgestrel (seven women used Follimin [Wyeth Lederle Nordiska, Solna, Sweden], and one woman used Neovletta [Schering Nordiska, Jarfalla, Sweden]). The pills were taken once daily for 3 weeks, followed by 1 tablet-free week. The NPC group included nine women, 27 to 48 years old (mean age, 39 years), who did not use any pharmacological contraceptive method. All women in the NPC group had regular menstrual periods.
Vaccination.
Each volunteer was vaginally immunized with two doses of a licensed, inactivated B-subunit-whole-cell (B-WC) cholera vaccine (Dukoral; SBL Vaccin AB, Stockholm, Sweden) administered at 2-week intervals. The vaccine contained 1 mg of recombinant CTB and 1011 inactivated cholera vibrios per dose (10). Each dose (3 ml) of the vaccine was mixed with 650 mg of a biologically inert polysaccharide (Eldexomer; batch 020; Perstorp Pharma, Perstorp, Sweden). The freshly made vaccine-gel mixture was deposited in the posterior fornix of the vagina, and the women remained in a horizontal position for 10 min after each vaccination (35, 36). The first immunization was given at a random time for the IUD group and on day 10 after the start of the withdrawal-bleeding for the OCP group. For the NPC group, the first immunization was initiated on day 10 in the menstrual cycle, i.e., 10 days after the last menstrual bleeding had started.
Collection of specimens.
Cervical secretions, vaginal fluids, and serum were collected immediately before the first immunization and 14 days after the second immunization. Samples of cervical secretions were obtained with a syringe (Aspiglaire; Biotechnologies International, Aigle, France), and the volumes were recorded. Before determination of Ig contents and specific antibodies, the cervical samples were diluted 1:10 with phosphate-buffered saline (PBS) and treated with bromelain (Sigma Chemical Company, St Louis, MO) at 25 μg ml−1 to solubilize the mucus (36). The bromelain-treated specimens were stored at −70°C until analysis. Vaginal fluids were collected by using two highly absorbent ophthalmic surgical sponges (Weck-cel; Medtronic Solan, Jacksonville, FL), which were placed on the mucosal surface in the posterior fornix of the vagina for 5 min (17). The fluid volume absorbed into each sponge was determined by weighing the sponge plus an Eppendorf tube before and after placement on the vaginal mucosa. The vaginal samples were diluted 1:10 with PBS and treated with bromelain as described above. Thereafter, the sponge was removed from the Eppendorf tube, placed in the top compartment of a Micro-spin filter tube (batch 24133; Alltech, Deerfield, Ill.), and centrifuged at 3,000 × g for 3 min. Following this initial extraction, a second centrifugation of the sponge was performed at 4,000 × g for 3 to 5 min depending on the viscosity of the sample. Vaginal fluids eluted from the two sponges were pooled and stored at −70°C until analysis. Serum specimens were stored in aliquots at −20°C until analysis.
Determination of total immunoglobulin and specific antibodies.
The total IgA and IgG contents in cervical secretions and vaginal fluids were determined by a modified enzyme-linked immunosorbent assay (ELISA) (3, 33). CTB-specific IgA and IgG antibodies in genital secretions as well as in serum were determined by the GM1 ELISA method as previously described (32). The CTB-specific IgA and IgG antibody activities (in units per microgram) in genital secretions were determined by dividing the ELISA Ig titer (in units per milliliter) by the total Ig concentration (in micrograms per milliliter) in the specimens to compensate for the variation in Ig contents in specimens collected on different days. Responders were defined as having a >2-fold increase in specific antibody activities between pre- and postvaccination specimens (15, 35). In serum, a twofold or greater increase in end point titers between pre- and postvaccination specimens was used to signify seroconversion at a P value of ≤0.05 (14, 15).
Statistical methods.
The GraphPad Prism computer program (GraphPad Software Inc., San Diego, CA) was used for calculations and statistical comparisons. All antibody titers were log10 transformed to normalize the sample distribution. The frequencies of antibody responses in the different immunization groups were compared by using Fisher's exact test. To determine the statistical significance of differences in the magnitudes of antibody responses between the three immunization groups, one-way analysis of variance was used. Statistical significance was set at a P value of ≤0.05. The association between the CTB-specific immune responses in cervical secretions and the responses in vaginal fluids and in serum was determined by using linear regression analyses.
RESULTS
Antibody responses in genital secretions and serum.
The influence of exogenous reproductive hormones on the antibody responses in genital secretions and serum after two vaginal immunizations with CTB was studied for three groups of women using either progesterone-containing intrauterine devices (IUD group), oral contraceptive pills (OCP group), or no pharmacological contraceptive methods (NPC group). Cervical secretions and vaginal fluids were collected prior to the first vaccination and 14 days after the second vaccination, i.e., on the same day of the contraceptive or menstrual cycle. Secretion volumes did not differ significantly between samples collected on these two occasions. Therefore, geometric means for secretion volumes and concentrations of Igs in the various immunization groups were calculated using all specimens collected at 1-month intervals, and the results are presented in Table 1. Significantly lower volumes of cervical secretions were obtained for the OCP group than for the IUD and NPC groups (P < 0.01), but the concentrations of IgA and IgG antibodies displayed no differences among the groups. Similar amounts of vaginal fluids were achieved in the three groups of women (Table 1). The content of IgA antibodies was higher in the IUD group than in the OCP and NPC groups (P < 0.001 and P < 0.05, respectively), whereas no differences in total IgG were noted among the groups (Table 1).
TABLE 1.
Volumes and total immunoglobulin concentrations in undiluted genital secretions collected from women using different types of contraceptive methods
| Genital specimen and parameter | Immunization group
|
||
|---|---|---|---|
| IUD (n = 9) | OCP (n = 8) | NPC (n = 9) | |
| Cervical secretion | |||
| Vol (μl)a | 152 (113-205) | 39 (30-52) | 196 (154-249) |
| IgA concn (μg ml−1)b | 225 (150-338) | 149 (78-284) | 144 (85-243) |
| IgG concn (μg ml−1)b | 540 (366-796) | 508 (243-1,062) | 288 (137-607) |
| Vaginal fluid | |||
| Vol (μl)c | 204 (171-244) | 140 (119-175) | 133 (98-179) |
| IgA concn (μg ml−1) | 292 (218-393) | 76 (41-142) | 116 (65-207) |
| IgG concn (μg ml−1) | 1,187 (800-1,762) | 506 (245-1,040) | 502 (282-891) |
Cervical secretions were collected with a syringe. Volumes are expressed as geometric means (95% confidence intervals) of pre- and postvaccination specimens for the whole group.
Geometric means (95% confidence intervals) of total immunoglobulin concentrations in specimens collected before and after two vaginal vaccinations for the whole group.
Vaginal fluids eluted from two ophthalmic sponges were pooled. Volumes are expressed as geometric means (95% confidence intervals) of pre- and postvaccination specimens for the whole group.
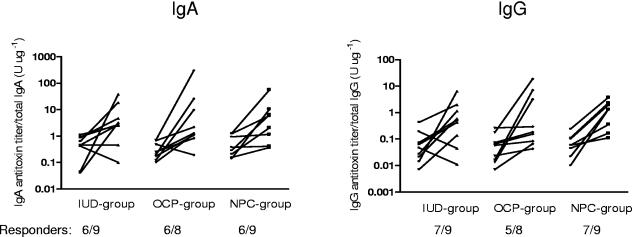
Before vaccination, the CTB-specific IgA and IgG antibody activities in genital secretions as well as in serum were low and displayed no differences among the three immunization groups (Fig. 1). A majority of the volunteers demonstrated, regardless of contraceptive use, significant increases in CTB-specific IgA antibody titers in cervical secretions after two vaginal doses of CTB (Fig. 1). The magnitude of the IgA antitoxin responses was higher in the OCP group (13.7-fold) than in the IUD and NPC groups (7.6-fold and 7.3-fold, respectively), but the difference was not statistically significant. The vaccination also induced CTB-specific IgG antibody responses in most of the volunteers (Fig. 1). The IgG antitoxin titer increase was slightly lower in the IUD group (7.4-fold) than in the OCP and NPC groups (13-fold and 11.2-fold, respectively).
FIG. 1.
Antitoxin antibody responses in cervical secretions after two vaginal doses of recombinant CTB in women using different types of contraceptive methods. The numbers of responders are given below the x axes. Responders were defined as having a >2-fold increase in the ratio of the CTB-specific antibody titer to the total-immunoglobulin concentration between pre- and postvaccination specimens.
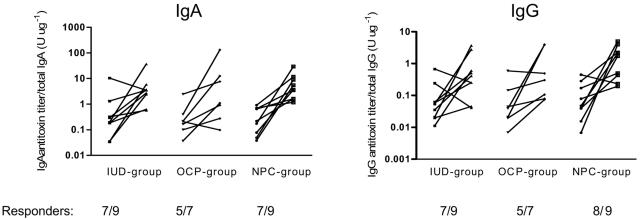
In accordance with findings for cervical secretions, CTB-specific IgA and IgG antibody titer increases in vaginal fluids were found in most of the volunteers after vaccination (Fig. 2). The increases in IgA antitoxin titers were higher for the NPC group (19.3-fold) than for the IUD and OCP groups (10.9-fold and 10.5-fold, respectively), but the differences were not statistically significant. Also, the CTB-specific IgG antibody responses were higher in the NPC group (15.8-fold) than in the IUD and OCP groups (6.3-fold and 7.8-fold, respectively).
FIG. 2.
Antitoxin antibody responses in vaginal fluids after two vaginal doses of recombinant CTB in women using different types of contraceptive methods. The numbers of responders are given below the x axes. Responders were defined as having a >2-fold increase in the ratio of the CTB-specific antibody titer to the total-immunoglobulin concentration between pre- and postvaccination specimens. Specimens from one volunteer in the OCP group contained too low IgA and IgG concentrations (≤40 μg ml−1) and were therefore excluded from further analyses.
In general, the antitoxin responses in serum were weaker than the responses in genital secretions. The highest numbers of IgA as well as IgG antitoxin responses to vaginally given CTB were found in the NPC group (Table 2). The increases in serum antitoxin titers were also somewhat higher in the NPC group than in the IUD and OCP groups.
TABLE 2.
Serum antibody responses after two vaginal doses of recombinant CTB in women using different types of contraceptive methods
| Immune response | Immunization group
|
||
|---|---|---|---|
| IUD | OCP | NPC | |
| IgA antitoxin | |||
| Frequencya | 5/9 | 3/8 | 7/9 |
| Prevaccination titerb | 85 (44-161) | 34 (12-93) | 41 (22-73) |
| Postvaccination titerc | 219 (103-468) | 85 (40-181) | 300 (94-955) |
| Fold increased | 2.6 | 2.5 | 7.3 |
| IgG antitoxin | |||
| Frequency | 4/9 | 2/8 | 5/9 |
| Prevaccination titer | 127 (70-232) | 138 (77-246) | 153 (103-227) |
| Postvaccination titer | 238 (119-479) | 306 (156-603) | 546 (205-1492) |
| Fold increase | 1.9 | 2.2 | 3.6 |
Number of responders per number tested. Responders were defined as having a twofold or greater increase in CTB-specific titers between pre- and postvaccination specimens.
Geometric mean CTB-specific antibody titer (95% confidence interval) before vaccination for the whole group of volunteers.
Geometric mean CTB-specific antibody titer (95% confidence interval) 14 days after the second vaccination for the whole group of volunteers.
Geometric mean titer increase in relation to the prevaccination titers for all subjects.
Reflection of cervical antibody responses in vaginal fluids and serum.
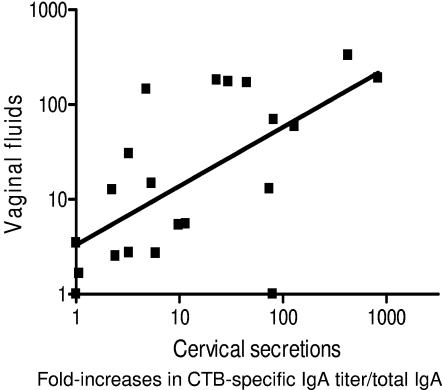
For the 25 individuals presenting both cervical and vaginal samples after vaccination (data from the three immunization groups were pooled), the relationship between the magnitudes of individual immune responses in the two types of samples was calculated by using linear regression analyses. An association between the CTB-specific IgA antibody responses in cervical secretions and vaginal fluids was found (R2 = 0.43; beta coefficient ± standard error [SE] = 0.62 ± 0.15; P < 0.001) (Fig. 3). The association between the IgG antitoxin responses was somewhat weaker (R2 = 0.20; beta coefficient ± SE = 0.42 ± 0.17; P < 0.023). The frequencies of CTB-specific antibody responses in cervical secretions and vaginal fluids were also compared by using a four-field contingency table. The sensitivities of vaginal IgA and IgG analyses to reflect antitoxin responses in cervical secretions were 94% and 84%, respectively. The positive predictive values for such analyses were also high, 89% for IgA antitoxin and 80% for IgG antitoxin.
FIG. 3.
Association between increases in CTB-specific IgA antibody titers per total-IgA levels in cervical secretions and corresponding increases in vaginal fluids from 25 volunteers after two vaginal immunizations with recombinant CTB.
We also investigated the relationship between the CTB-specific antibody responses in cervical secretions and serum for the 26 individuals presenting both types of specimens after vaccination (data from the three immunization groups were pooled). A moderate association between the IgA antitoxin responses in cervical secretions and serum was found (R2 = 0.21; beta coefficient ± SE = 0.32 ± 0.13; P < 0.018). A similar association between the IgG antitoxin responses was noted (R2 = 0.31; beta coefficient ± SE = 0.33 ± 0.10; P = 0.003). The sensitivities of serum IgA and IgG analyses to reflect antitoxin responses in cervical secretions were low, 53% and 56%, respectively. The positive predictive values for such analyses were 77% for IgA antitoxin and 100% for IgG antitoxin.
DISCUSSION
The female endocervix is considered to be the most important organ involved in local immunity in the genital tract, since it contains higher numbers of IgA- and IgG-secreting cells than the ectocervix, the fallopian tubes, and the vagina (8, 23). For evaluation of novel STD vaccine approaches, it is important to elucidate factors that might influence the local immune responses in the cervix. A number of studies have suggested that reproductive hormones might affect the production of antibodies in the female reproductive tract (7, 8, 16, 19, 23, 27, 31). It has been shown that in ovulating women the concentrations of total IgA and IgG in cervical secretions decrease around the time of ovulation but then rebound during the luteal phase, approaching Ig levels present during the follicular phase of the menstrual cycle (8, 24, 27, 31). Franklin and Kutteh (8) reported a fourfold difference between the preovulatory and periovulatory levels of total IgA and IgG during a 9-day evaluation of cervical antibodies. When the evaluation period was extended to 23 days, Nardelli-Haefliger et al. (27) found an even greater decline (approximately seven- to ninefold) in median IgG levels between the follicular and periovulatory phases of the menstrual cycle. In the present study, cervical secretions were obtained 4 days prior to ovulation (mid-follicular phase) from the women in the NPC group, and the levels of total IgA and IgG were in accordance with previous findings (8).
Monophasic OCPs did not seem to have any influence on the Ig levels in genital secretions. The concentration of IgA antibodies in our OCP users was higher than that reported from a previous study (27), whereas the IgG concentrations were similar. The use of progesterone-containing IUDs, on the other hand, seemed to increase the concentration of IgA antibodies in genital secretions. Interestingly, in triphasic OCP users, levels of IgA have also been found to increase significantly in cervical mucus, paralleling the increase in oral ingestion of norethindrone (8).
Vaginal vaccination has been shown to be superior to both oral and rectal vaccination for induction of antibody responses in cervical secretions (17, 36). Previously, we reported that biweekly vaginal vaccinations with inactivated B-WC cholera vaccine on days 10 (mid-follicular phase) and 24 (mid-luteal phase) of the menstrual cycle induced stronger CTB-specific antibody responses in cervical secretions than random biweekly vaginal vaccinations (16). The progesterone level is low on day 10 and high on day 24 of the cycle, whereas the estrogen level is high at both time points. Kozlowski et al. (19) did evaluate the outcome of monthly vaginal vaccination in relation to the phase of the menstrual cycle during which the B-WC cholera vaccine was given. Vaginal vaccinations performed during either the follicular or the luteal phase were equally effective in inducing CTB-specific antibody responses in genital tract secretions. However, only vaginal vaccination performed during the follicular phase could consistently induce cervical IgA antibodies against the bacterial lipopolysaccharide components of the vaccine. This finding suggests that the environment in the female genital tract might be more favorable for uptake of antigens or induction of immune responses during the follicular phase. Recently, the influence of the menstrual cycle and oral contraceptive use on the levels of vaccine-specific IgG antibodies in cervical secretions was examined for women who had been parenterally immunized with human papillomavirus 16 virus-like particles (27). For ovulating women, a seven- to ninefold decline in cervical titers of specific IgG was recorded from the follicular to the periovulatory phase of the menstrual cycle. In contrast, the specific IgG antibody levels among OCP users were relatively constant throughout the contraceptive cycle. The present study is, to our knowledge, the first to examine the effects of exogenously administered reproductive hormones on the induction of vaccine-specific IgA and IgG antibodies in the female genital tract after vaginal vaccination. In accordance with our previous studies (16, 35, 36), a majority of the women demonstrated significant increases in CTB-specific IgA and IgG antibody titers in genital secretions after vaginal administration of recombinant CTB, whereas the antitoxin responses in serum were weaker. Despite the potency of exogenous steroids given, which normally affects a number of physiological functions in women, we were not able to detect any differences in the frequencies and magnitudes of CTB-specific immune responses among women using progesterone-containing IUDs, OCPs, or no hormonal contraceptive methods. However, since most other antigens are less immunogenic than CTB, it could be argued that other, weaker antigens might have displayed different results.
For the evaluation of local immune responses in the female genital tract after various routes of vaccination, cervical secretions and/or vaginal fluids have been used. Vaginal fluid is considered to be a transudate of serum leading to a dilution of approximately 40% (for IgA) to 50% (for IgG) of the antibody concentrations in cervical secretions (19). In the present study, the levels of IgA and IgG were comparable in cervical secretions and vaginal fluids. The use of different collection procedures might have affected our results. Cervical secretions were obtained with a syringe, whereas vaginal fluids were collected with Weck-cel sponges. Cervical secretions are not regarded as ideal specimens for assessment of genital immune responses in large-scale immunization studies, since the collection is time-consuming, requires gynecological assistance, and at times yields small amounts of secretions (9, 28). Vaginal fluid, on the other hand, is an easily accessible secretion. Our results indicate that cervical antibody responses are well reflected in vaginal fluids. An association, in particular for IgA, was found between the magnitudes of the CTB-specific immune responses in cervical secretions and vaginal fluids after vaccination. The sensitivities and positive predictive values of vaginal IgA and IgG analyses to reflect cervical IgA and IgG antibody responses were also high. These findings are consistent with a recent report showing that the CTB-specific IgA and IgG activities measured in vaginal fluids were highly correlated with those in cervical secretions after vaginal immunization with B-WC cholera vaccine.
In summary, we have shown that a majority of the women presented strong CTB-specific IgA and IgG antibody responses in genital secretions after vaginal vaccination. The use of potent reproductive hormones did not seem to have any influence on the induction of CTB-specific antibody responses in the female genital tract. These findings may be of relevance for the administration of future vaccines against STDs to fertile women. Our results also indicate that cervical antibody responses are well reflected in vaginal fluids, suggesting that vaginal fluids alone might be used for the evaluation of local immune responses in the genital tract in large-scale vaccination studies.
Acknowledgments
This study was supported by grants from the Swedish Society of Medicine and the medical faculty of Göteborg University. The financial support given to the Göteborg University Vaccine Institute by the Knut and Alice Wallenberg Foundation is gratefully acknowledged.
We gratefully acknowledge Kerstin Andersson for excellent technical assistance; Anna Glantz, Ann-Kristin Bokström, and Katja-Stenström-Bohlin for assistance in recruiting volunteers; and Perstorp Pharma for providing the Eldexomer.
REFERENCES
- 1.Andersson, J. and G. Rybo. 1990. Levonorgesterol releasing intrauterine devices in the treatment of menorrhagia. Br. J. Obstet. Gynaecol. 97:690-694. [DOI] [PubMed] [Google Scholar]
- 2.Belec, L., C. Tevi-Benissan, X. S. Lu, T. Prazuck, and J. Pillot. 1995. Local synthesis of IgG antibodies to HIV within the female and male genital tracts during asymptomatic and pre-AIDS stages of HIV infection. AIDS Res. Hum. Retrovir. 11:719-729. [DOI] [PubMed] [Google Scholar]
- 3.Bergquist, C., E.-L. Johansson, T. Lagergård, J. Holmgren, and A. Rudin. 1997. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect. Immun. 65:2676-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjercke, S., and P. Brandtzaeg. 1993. Glandular distribution of immunoglobulins, J chain, secretory component, and HLA-DR in the human endometrium throughout the menstrual cycle. Hum Reprod. 8:1420-1425. [DOI] [PubMed] [Google Scholar]
- 5.Brabin, L. 2001. Hormonal markers of susceptibility to sexually transmitted infections: are we taking them seriously? BMJ 323:394-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradtzaeg, P. 1985. The role of J chain and secretory component in receptor-mediated glandular and hepatic transport of immunoglobulins in man. Scand. J. Immunol. 22:111-146. [DOI] [PubMed] [Google Scholar]
- 7.Brandtzaeg, P. 1997. Mucosal immunity in the female genital tract. J. Reprod. Immunol. 36:23-50. [DOI] [PubMed] [Google Scholar]
- 8.Franklin, R. D., and W. H. Kutteh. 1999. Characterization of immunoglobulins and cytokines in human cervical mucus: influence of exogenous and endogenous hormones. J. Reprod. Immunol. 42:93-106. [DOI] [PubMed] [Google Scholar]
- 9.Hildesheim, A., M. C. Bratti, R. P. Edwards, M. Schiffman, A. C. Rodriguez, R. Herrero, M. Alfaro, L. A. Morera, S. V. Ermatinger, B. T. Miller, and P. A. Crowley-Nowick. 1998. Collection of cervical secretions does not adversely affect Pap smears taken immediately afterward. Clin. Diagn. Lab. Immunol. 5:491-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmgren, J., A.-M. Svennerholm, M. Jertborn, J. Clemens, D. A. Sack, R. Salenstedt, and H. Wigzell. 1992. An oral B subunit: whole cell vaccine against cholera. Vaccine 10:911-914. [DOI] [PubMed] [Google Scholar]
- 11.Ildgruben, A. K., I. M. Sjöberg, and M. L. Hammarström. 2003. Influence of hormonal contraceptives on the immune cells and thickness of human vaginal epithelium. Obstet. Gynecol. 102:571-582. [DOI] [PubMed] [Google Scholar]
- 12.Jalanti, R., and H. Isliker. 1977. Immunoglobulins in human cervico-vaginal secretions. Int. Arch. Allergy Appl. Immunol. 53:402-408. [DOI] [PubMed] [Google Scholar]
- 13.Jertborn, M., I. Nordström, A. Kilander, C. Czerkinsky, and J. Holmgren. 2001. Local and systemic immune responses to rectal administration of recombinant cholera toxin B subunit in humans. Infect. Immun. 69:4125-4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jertborn, M., A.-M. Svennerholm, and J. Holmgren. 1992. Safety and immunogenicity of an oral recombinant cholera B subunit-whole cell vaccine in Swedish volunteers. Vaccine 10:130-132. [DOI] [PubMed] [Google Scholar]
- 15.Jertborn, M., A.-M. Svennerholm, and J. Holmgren. 1986. Saliva, breast milk, and serum antibody responses as indirect measures of intestinal immunity after oral cholera vaccination or natural disease. J. Clin. Microbiol. 24:203-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson, E. L., L. Wassen, J. Holmgren, M. Jertborn, and A. Rudin. 2001. Nasal and vaginal vaccinations have differential effects on antibody responses in vaginal and cervical secretions in humans. Infect. Immun. 69:7481-7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozlowski, P. A., S. Cu-Uvin, M. R. Neutra, and T. P. Flanigan. 1997. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect. Immun. 65:1387-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozlowski, P. A., S. Cu-Uvin, M. R. Neutra, and T. P. Flanigan. 1999. Mucosal vaccination strategies for women. J. Infect. Dis. 179(Suppl. 3):S493-S498. [DOI] [PubMed] [Google Scholar]
- 19.Kozlowski, P. A., S. B. Williams, R. M. Lynch, T. P. Flanigan, R. R. Patterson, S. Cu-Uvin, and M. R. Neutra. 2002. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J. Immunol. 169:566-574. [DOI] [PubMed] [Google Scholar]
- 20.Kutteh, W. H., and R. D. Franklin. 2001. Quantification of immunoglobulins and cytokines in human cervical mucus during each trimester of pregnancy. Am. J. Obstet. Gynecol. 184:865-872. [DOI] [PubMed] [Google Scholar]
- 21.Kutteh, W. H., K. D. Hatch, R. E. Blackwell, and J. Mestecky. 1988. Secretory immune system of the female reproductive tract. I. Immunoglobulin and secretory component-containing cells. Obstet. Gynecol. 71:56-60. [PubMed] [Google Scholar]
- 22.Kutteh, W. H., and J. Mestecky. 1994. Secretory immunity in the female reproductive tract. Am. J. Reprod. Immunol. 31:40-46. [DOI] [PubMed] [Google Scholar]
- 23.Kutteh, W. H., Z. Moldoveanu, and J. Mestecky. 1998. Mucosal immunity in the female reproductive tract: correlation of immunoglobulins, cytokines, and reproductive hormones in human cervical mucus around the time of ovulation. AIDS Res. Hum. Retrovir. 14(Suppl 1):S51-S55. [PubMed] [Google Scholar]
- 24.Kutteh, W. H., S. J. Prince, K. R. Hammond, C. C. Kutteh, and J. Mestecky. 1996. Variations in immunoglobulins and IgA subclasses of human uterine cervical secretions around the time of ovulation. Clin. Exp. Immunol. 104:538-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menge, A. C., and J. Mestecky. 1993. Surface expression of secretory component and HLA class II DR antigen on glandular epithelial cells from human endometrium and two endometrial adenocarcinoma cell lines. J. Clin. Immunol. 13:259-264. [DOI] [PubMed] [Google Scholar]
- 26.Mestecky, J., C. Lue, and M. W. Russell. 1991. Selective transport of IgA. Cellular and molecular aspects. Gastroenterol. Clin. N. Am. 20:441-471. [PubMed] [Google Scholar]
- 27.Nardelli-Haefliger, D., D. Wirthner, J. T. Schiller, D. R. Lowy, A. Hildesheim, F. Ponci, and P. De Grandi. 2003. Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus-like particles. J. Natl. Cancer Inst. 95:1128-1137. [DOI] [PubMed] [Google Scholar]
- 28.Rohan, L. C., R. P. Edwards, L. A. Kelly, K. A. Colenello, F. P. Bowman, and P. A. Crowley-Nowick. 2000. Optimization of the weck-Cel collection method for quantitation of cytokines in mucosal secretions. Clin. Diagn. Lab. Immunol. 7:45-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell, M. W., and J. Mestecky. 2002. Humoral immune responses to microbial infections in the genital tract. Microbes Infect. 4:667-677. [DOI] [PubMed] [Google Scholar]
- 30.Schumacher, G. F. B. 1973. Soluble proteins in cervical mucus, p. 201-221. In R. J. Blandau and K. Moghissi (ed.), The biology of the cervix. University of Chicago Press, Chicago, Ill.
- 31.Shrier, L. A., F. P. Bowman, M. Lin, and P. A. Crowley-Nowick. 2003. Mucosal immunity of the adolescent female genital tract. J. Adolesc. Health 32:183-186. [DOI] [PubMed] [Google Scholar]
- 32.Svennerholm, A.-M., J. Holmgren, R. Black, M. Levine, and M. Merson. 1983. Serologic differentiation between antitoxin responses to infection with Vibrio cholerae and enterotoxin-producing Escherichia coli. J. Infect. Dis. 147:514-522. [DOI] [PubMed] [Google Scholar]
- 33.Svennerholm, A.-M., M. Jertborn, L. Gothefors, A. M. Karim, D. A. Sack, and J. Holmgren. 1984. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B subunit-whole cell vaccine. J. Infect. Dis. 149:884-893. [DOI] [PubMed] [Google Scholar]
- 34.van Ginkel, F. W., R. J. Jackson, Y. Yuki, and J. R. McGhee. 2000. The mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J. Immunol. 165:4778-4782. [DOI] [PubMed] [Google Scholar]
- 35.Wassen, L., and M. Jertborn. 2005. Kinetics of local and systemic immune responses after vaginal immunization with recombinant cholera toxin B subunit in humans. Clin. Diagn. Lab. Immunol. 12:447-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wassen, L., K. Schön, J. Holmgren, M. Jertborn, and N. Lycke. 1996. Local intravaginal vaccination of the female genital tract. Scand. J. Immunol. 44:408-414. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida, M., S. M. Claypool, J. S. Wagner, E. Mizoguchi, A. Mizoguchi, D. C. Roopenian, W. I. Lencer, and R. S. Blumberg. 2004. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity 20:769-783. [DOI] [PubMed] [Google Scholar]