WHIPPLE'S DISEASE
Overview of the Disease
Whipple's disease (WD) is a rare systemic disease, first described in 1907 by the American pathologist George H. Whipple as an intestinal lipodystrophy. He reported the fatal illness in a patient with weight loss, chronic cough, fever, and accumulation of fat in the intestine, mesenteric lymph nodes, and stool (121a). The hallmark of the disease is the accrual of periodic-acid Schiff (PAS)-stained foamy macrophages in the lamina propria. Although G. H. Whipple suggested a bacterial etiology in his systematic description of the disease in his index patient (121a), it was not until 1961 that intramacrophagic and free-living bacilli were observed in patient duodenal biopsies (16a). The bacteria present a rod-shaped morphology with a symmetric external membrane that is not found among gram-positive bacteria, and yet it differs from the external membrane in gram-negative bacteria because it is devoid of lipopolysaccharide (LPS) (22a). It surrounds a thin homogeneous layer and an internal membrane containing polysaccharides, which are probably responsible for the PAS staining (111). As a result of phylogenetic studies based on the ssr and rpoB gene sequences, the agent, which is now referred to as Tropheryma whipplei, has been placed among the gram-positive bacteria in the Actinobacteria clade. Several environmental studies suggest that T. whipplei is ubiquitous. Indeed, bacterial DNA was detected in 66% of wastewater samples from five different sewage treatment plants in Germany (72a).
In 1997, culture of T. whipplei was achieved in interleukin-4 (IL-4)-deactivated macrophages; however, it was not possible to establish stable subcultures (107a). In 2000, our laboratory successfully cultured T. whipplei from the heart valve specimen of a patient with endocarditis in a human fibroblast cell line (HEL) (98). The ability to culture the organism allowed the generation of antibodies against T. whipplei (98) and enumeration of the complete genome sequence for two strains (7a, 101). Analysis of the genome sequence, which is approximately 0.9 Mb in length, revealed deficiencies in the biosynthetic pathways of 16 amino acids. Addition of the missing amino acids to culture medium permitted the axenic culture of T. whipplei (102a).
WD is considered to be rare, although no valid estimate of the incidence is available. The disease was diagnosed in a set of patients within a familial context (22, 96), which implies an immunogenetic component in the pathogenesis of WD. Several studies have shown a greater prevalence of HLA-B27 antigen in WD patients (26% versus 8% in European and American populations, respectively) (16, 22), but no causal association between HLA-B27 presence and infection susceptibility has been demonstrated. The classic symptoms of WD include weight loss, diarrhea, and chronic arthropathy. However, it is now known that the disease is often multisystemic, including possible cardiac and central nervous system involvement, and has clinical manifestations that may be varied and nonspecific (Table 1). T. whipplei has been detected in 4% of patients with various gastrointestinal diseases and in 7% of presumably healthy control subjects (2). T. whipplei DNA has also been detected in 40% of subgingival and gingival sulcus samples from healthy individuals (124). The disease is characterized by the persistent bacterial infection of affected tissues, resulting in recurring relapses and gradual exacerbation. PCR-based diagnosis of WD, which is accomplished by detection of the genomic material of T. whipplei, is well defined (30). However, in practice, some studies raise concerns about the diagnostic value of T. whipplei PCR alone. Indeed, peripheral blood mononuclear cells from four patients with active WD (confirmed by PAS-positive intestinal biopsies) were negative by PCR for T. whipplei DNA (74). On the contrary, T. whipplei DNA has been detected in people without WD (28). Diagnosis can also be made by examination of biopsy samples using electron microscopy, which allows visualization of the distinctive trilamellar cell wall of T. whipplei. Serologic tests for the diagnosis of WD are not yet established, because antibodies directed against T. whipplei have been measured in subjects without WD as well as in WD patients (27). Thus, although the diagnosis is usually made by duodenal biopsy, it can simply be overlooked in the early stages of WD or in other forms of the disease when gastrointestinal involvement is not clear.
TABLE 1.
Epidemiology and clinical symptoms of Whipple's diseasea
| Epidemiology | Finding |
|---|---|
| Reported cases | 1,000 |
| Mean age | 50 yrs |
| Sex ratio (male/female) | 8 |
| Familial cases | Few |
| Major clinical manifestations | |
| Weight loss | 90% |
| Diarrhea | 75% |
| Arthropathy | 85% |
| Abdominal pain | 60% |
| Other symptoms | |
| Arthralgias, arthritis | 70-90% |
| Anemia | 75-90% |
| Fever | 45% |
| Night sweats | |
| Lymphadenopathy | 40-50% |
| Hyperpigmentation | 40-60% |
| Cardiac manifestations | 35-65% |
| Pulmonary manifestations | 35-60% |
| CNS manifestations | 20-30% |
| Ocular manifestations | 5-15% |
| Splenomegaly | 5-10% |
The Immune Deficiency of WD
Since the initial description of WD, it has been hypothesized that a preexisting immune deficiency acts as a contributing factor in the development of the disease. The clinical presentation of the disease does not evoke an opportunistic infection. The disease does not appear to affect the humoral response, since total immunoglobulin G (IgG) and IgM levels remain normal even though IgA levels are higher before treatment and reach control values thereafter (22, 117). A reduction of B- and T-cell numbers has been observed with a diminution of the CD4+/CD8+ ratio (78). Hence, it is likely that an alteration in the immune response selectively affects some features of the response to T. whipplei while not impairing host defenses against common pathogens. A deficiency of macrophage function has been documented in several studies. Although WD patient macrophages have normal phagocytic activity, they are unable to degrade bacterial antigens efficiently (13, 22). The expression of CD11b, the α chain of the phagocytic receptor CR3 (CD11b/CD18), is reduced in patient macrophages (78). The production of interleukin-12 (IL-12) by monocytes/macrophages from WD patients has been shown to be defective (76). IL-12 is mainly produced by monocytes, macrophages, and dendritic cells and stimulates gamma interferon (IFN-γ) production by NK cells and T lymphocytes. IL-12 is pivotal in the stimulation of Th1 differentiation and IFN-γ-mediated microbicidal competence of macrophages. Hence, reduced IL-12 production leads to diminished IFN-γ production by T cells and defective macrophage activation. The report of a patient with refractory WD who had an apparent response to IFN-γ therapy strengthens this hypothesis (107). In this context, this immune defect appears to be specific to Tropheryma, since patients with WD do not suffer from opportunistic infections, as is commonly reported in IL-12 deficiencies (67). Peripheral blood mononuclear cells from patients with WD had increased production of IL-4 in response to stimulation with phytohemagglutinin and reduced secretion of IFN-γ and IL-2 compared to controls (75). In addition, defective microbicidal activity of macrophages in WD may be intrinsic, because decreased killing of Candida tropicalis by monocytes has been observed in patients in remission (5). Thus, macrophages might be more susceptible to T. whipplei infection because of decreased classically activating cytokines and increased alternatively activating IL-4 (39), indicative of a Th2 immune response.
INTESTINAL MACROPHAGES
T. whipplei organisms are localized within the lamina propria as determined by in situ hybridization: bacterial RNA was mainly detected at the top of the villi and below the epithelial cell basement membrane (32). Dobbins proposed that tissue infiltration with T. whipplei occurred from the lamina propria and not from the lumen (21). After bacteria have been delivered to the subepithelial lamina propria, they encounter intestinal macrophages (68). We will review various aspects of intestinal macrophages with special emphasis on desensitization or anergy that may be essential in the pathophysiology of intestinal WD.
Tissue Distribution, Phenotype, and Differentiation of Macrophages
Macrophages derive from myeloid progenitor cells that first develop into monocytes and enter the blood (69). In the absence of an inflammatory stimulus, monocytes remain in the blood flow and are exposed to numerous hormones and constitutive chemokines involved in homeostasis and in host defenses that influence their fate. Monocytes then adhere and migrate through capillary endothelium to peripheral tissues and lymph nodes, where they differentiate into resident macrophages (61). In contrast, when inflammatory mediators are produced in response to an inciting event, inducible chemokines lead to the recruitment and activation of macrophages in tissues (53). Intestinal macrophages are located primarily in the subepithelial region of the lamina propria and in Peyer's patches. They constitute 10 to 20% of mononuclear cells in the intestinal lamina propria (23). Their local distribution is affected by several factors associated with the ecology of the intestinal flora (20, 38). Intestinal macrophages are also densely distributed in the colorectal mucosa (89), and their distribution at different sites of the gastrointestinal tract has been reported in monkeys (54) and guinea pigs (44), in which intestinal macrophages were mostly found in the small intestine.
Intestinal macrophages differ markedly from blood monocytes (112-114), the cells from which they are derived (14, 43, 105). They express several markers of myeloid lineage, including CD44, CD68, acid phosphatase, nonspecific esterase, and CD33, a member of the sialoadhesin family of sialic acid-dependent cell adhesion molecules (Table 2). They do not express CD14, a major receptor for gram-negative bacteria or their LPS (112); only colonic macrophages express low levels of CD14 (43, 103). The lack of CD14 results from down-regulation of CD14 expression rather than a posttranscriptional control (113). It may account for the low level of mucosal inflammation despite an environment rich in “CD14 ligands.” This is reinforced by the nominal expression of receptors involved in the inflammatory response, such as CR3, Toll-like receptors 1 to 5 (TLR1 to TLR5), and FcγR (CD64, CD32, and CD16). Intestinal macrophages do not express CD89 (113), a transmembrane glycoprotein receptor for monomeric and polymeric IgA1 and IgA2 (85), which are involved in IgA transcytosis across the mucosal epithelium (86). The absence of CD89 prevents IgA-mediated phagocytosis required for protection against environmental pathogens and IgA-mediated release of proinflammatory mediators, including reactive oxygen intermediates, leukotrienes, and prostaglandins (31, 40).
TABLE 2.
Phenotypic characterization of intestinal macrophages and monocytesa
| Compound | Function | Intestinal macrophages | Circulating monocytes |
|---|---|---|---|
| CD11b (CR3) | α Subunit of complement receptor 3 | + | +++++ |
| CD123 (IL-3R) | IL-3 receptor α chain | − | ++ |
| CD13 | Aminopeptidase N | +++++ | +++++ |
| CD14 | Receptor for LPS-LBP complex | − | +++++ |
| CD16 (FcγRIII) | Low-affinity Fc receptor | − | ++ |
| CD25 (IL-2R) | IL-2 receptor α chain | − | + |
| CD25 + LPS | − | +++ | |
| CD32 (FcγRII) | Low-affinity receptor for aggregated Ig | − | +++++ |
| CD33b | Binds sialoconjugates | +++++ | +++++ |
| CD36 | Scavenger receptor | + | +++++ |
| CD44 | Binds hyaluronic acid | ++++ | ++++ |
| CD64 (FcγRI) | High-affinity receptor for IgG | − | +++ |
| CD68 (macrosialin) | Unknown | +++++ | ++ |
| CD89 (FcαR) | IgA receptor | − | +++++ |
| HLA-DR | MHC class II | +++++ | +++++ |
| Acid phosphatase | ++++ | ++++ | |
| Nonspecific esterase | +++++ | +++++ | |
| TLR1-5 | Toll-like receptor | − | +++++ |
Spöttl and coworkers attempted to differentiate monocytes into intestinal-like macrophages in the presence of epithelial cells (115). This work was based on four observations: (i) in normal intestinal mucosa, macrophages form a layer in the subepithelial region of the lamina propria separated by the basement membrane from the epithelial cells (92); (ii) macrophages are concentrated in a band immediately beneath the luminal intestinal epithelium (93); (iii) the mucosal basement membrane is perforated with numerous small pores in vivo, allowing a direct interaction of subepithelial macrophages with intestinal epithelial cells (118); and (iv) components of the basement membrane and extracellular matrix are involved in cell differentiation (49). Using a three-dimensional, organotypic model with primary intestinal fibroblasts embedded in a collagen gel covered with epithelial cells, the authors successfully differentiated monocytes into CD14- and CD11b-negative macrophages within 7 days (115). These macrophages also lacked CD25, the receptor for IL-2, known to stimulate inflammatory functions of monocytes/macrophages. They exhibited decreased IL-1β transcription in response to LPS, likely due to the lack of CD14. A recent study describes the use of stromal-cell-conditioned medium to differentiate monocytes into intestine-like macrophages with a reduced expression of CD14, CD16, chemokine receptor CCR5, and receptors for chemotactic factors, such as C5a and f-met-leu-phe (114). This conditioned medium also caused a diminution of release of IL-1, IL-6, IL-10, and tumor necrosis factor (TNF) and an increase of transforming growth factor-β (TGF-β) release following LPS or IFN-γ stimulation. It is probable that TGF-β plays a role in the diminished release of proinflammatory cytokines. Note that in vivo, intestinal epithelial cells and lamina propria mast cells appeared to be the source of mucosal TGF-β (114).
Intestinal Macrophages and Immunity
Intestinal macrophages may be considered bipolar cells: they induce an effective innate response and they restrict inflammation via anergy. First, phagocytic and bactericidal activities of intestinal macrophages are maintained despite the lack of cell surface innate immunity receptors (114). Indeed, lamina propria macrophages exhibited avid phagocytic activity for bovine serum albumin-coated beads (113), Candida albicans (112), Salmonella enterica serovar Typhimurium, and Escherichia coli (114). Intestinal macrophages are strongly bactericidal: 99% of phagocytosed S. enterica serovar Typhimurium organisms were killed within the first hour (114). They were capable of even greater bactericidal activity than monocytes, as the numbers of live S. enterica serovar Typhimurium or E. coli organisms recovered from lysed macrophages were an order of magnitude less than those recovered from blood monocytes (114). Second, intestinal macrophages contribute to immunity through the secretion of antimicrobial peptides. Antimicrobial peptides are mainly expressed by intestinal epithelial cells, particularly by Paneth cells, but it has recently been shown that cathelin-related antimicrobial peptide (CRAMP) can be expressed by murine macrophages. Its production was increased upon infection with S. enterica serovar Typhimurium, and macrophages lacking CRAMP did not control Salmonella replication, indicating the role of this peptide in the antimicrobial arsenal of macrophages (104). Other proteins with antimicrobial activity have been identified in the normal human colon mucosa; they include ubiquicidin, ribosomal proteins L30, L39, and S19, histones H1.5 and H2B, phospholipase A2, and eosinophil cationic protein (51, 120). Ubiquicidin is expressed by macrophages, and it exhibits antimicrobial activity against Listeria monocytogenes and S. enterica serovar Typhimurium (47). Different histone-derived peptides from evolutionarily diverse species ranging from fish to mammals have antimicrobial properties against enteric bacteria and can neutralize endotoxin (12). A recent work revealed Nod2 protein as a critical regulator of bacterial immunity within the intestine (63). Nod2 is present in macrophages as well as dendritic and Paneth cells and can be induced in enterocytes. This protein is essential in the detection of muramyl dipeptide, a conserved structure in bacterial peptidoglycan, and activation of the adaptive immune system by acting as an adjuvant receptor for antibody production, either directly or by enhancing the production of α-defensins (119, 122).
Despite their role in innate immunity, intestinal macrophages display profound “inflammatory anergy,” which is critical for the homeostasis of normal intestinal mucosa. This immunologic disarmament is likely beneficial for the peripheral nonresponsiveness to the abundant antigens to which the mucosa is continuously exposed (103). It has been shown that bacterial phagocytosis did not stimulate intestinal macrophages to release cytokines (114). Down-regulation of surface receptors is also a strategy to circumvent the inflammatory response. Indeed, intestinal macrophages did not respond to LPS, probably because of the lack of CD14. They did not respond to various stimuli, including Helicobacter pylori urease, heat-killed Staphylococcus aureus, IFN-γ, or phorbol myristate acetate, as revealed by the undetectable or trace level of IL-1, IL-6, IL-12, TNF, or IL-8 (114). The low expression of TLR and FcγR might dampen inflammatory signals. Similarly, the low expression of CR3 may limit the excessive activation of intestinal macrophages, since CR3 recognizes complement components produced by intestinal epithelial cells and microbe products via its lectin site. It is important to note that intestinal macrophages did not release IL-10 or TGF-β, suggesting that the anergy does not result from autocrine production of these anti-inflammatory cytokines.
Anergy of intestinal macrophages may also result from the uptake of apoptotic material in the lamina propria. Hence, Nagashima et al. described the presence of “typical intestinal macrophages” that had round or oval nuclei and relatively abundant cytoplasm containing prominent phagocytosed vesicles or fragments morphologically resembling so-called apoptotic bodies in the lamina propria (89). These apoptotic bodies expressed epithelial cell-associated antigens (CEA, Ber-Ep4, and cytokeratin) (89). Macrophage responses to apoptotic cell engulfment include down-modulation of inflammatory responses (80, 116), and it has been shown that activated monocytes switch from a proinflammatory to an anti-inflammatory state following contact with apoptotic neutrophils (15). Hence, phagocytosis of apoptotic cells by intestinal macrophages might account for the macrophage intestinal anergy.
MACROPHAGES: PIVOTAL CELLS IN WD PATHOPHYSIOLOGY
T. whipplei Specifically Replicates in Monocyte-Derived Macrophages
We recently showed that monocytes and monocyte-derived macrophages have differing susceptibilities to T. whipplei (19). For example, macrophages allowed intracellular bacterial growth, and bacteria started to replicate at day 6. T. whipplei intramacrophagic growth was slow, with a doubling time around 30 h during exponential growth, which is in agreement with another study on T. whipplei (18 h in HEL cells [98]) as well as phylogenetically close bacteria such as mycobacteria (20 h [11]). From day 9, the growth rate decreased slowly and bacteria seemed to enter into stasis after 12 days. T. whipplei replication was associated with macrophage apoptosis. Apoptosis induction was strong (20% of cells became annexin V-positive after 48 h of incubation) and occurred concomitantly to IL-1β release. Macrophage apoptosis is likely a key event for bacterial dissemination of numerous pathogens (Table 3), and one can suspect that, as for other pathogens, apoptosis induction is a critical event in the pathophysiology of WD. Shigella flexneri represents the paradigm of host cellular machinery subversion to induce apoptosis. Following translocation, S. flexneri organisms are phagocytosed by macrophages present in the dome cells of Peyer's patches. Through their type III secretion system, S. flexneri organisms secrete several proteins, including IpaB (invasion plasmid antigen B), which is thought to be essential for invasion of epithelial cells. Furthermore, IpaB interacts with and activates caspase 1, resulting in apoptosis of the infected cell by activation of other downstream caspases (126, 127) and release of mature IL-1 by cleavage of pro-IL-1 (125).
TABLE 3.
Bacteria that induce macrophage apoptosis
| Bacterial pathogen | Reference |
|---|---|
| Actinobacillus actinomycetemcomitans | 58 |
| Aeromonas hydrophila | 34 |
| Bacillus anthracis | 95 |
| Bordetella pertussis | 62 |
| Brucella abortus | 33 |
| Burkholderia cepacia | 52 |
| Chlamydia psittaci | 91 |
| Chlamydia trachomatis | 41 |
| Clostridium difficile | 71 |
| Escherichia coli K-12 | 66 |
| Francisella tularensis | 65 |
| Helicobacter pylori | 37 |
| Leptospira interrogans | 81 |
| Mycobacterium avium | 9 |
| Mycobacterium tuberculosis | 59 |
| Parachlamydia acanthamoeba | 42 |
| Photobacterium damselae subsp. piscicida | 25 |
| Salmonella enterica serovar Typhimurium | 46 |
| Salmonella enterica serovar Typhi | 109 |
| Shigella dysenteriae | 102 |
| Shigella flexneri | 127 |
| Tropheryma whipplei | 19 |
| Yersinia enterocolitica | 83 |
| Yersinia pestis | 90 |
| Yersinia pseudotuberculosis | 84 |
| Vibrio vulnificans | 57 |
In contrast, we showed that monocytes efficiently killed T. whipplei within 3 days (19) and did not undergo apoptosis. In addition, monocytes acquired microbicidal activity which was associated with thioredoxin, a thiol-containing redox molecule (see below) (19). It is likely that thioredoxin and glutaredoxin systems, known to possess anti-poptotic activity for mammalian cells (3), are involved in apoptosis prevention.
IL-16, Critical Cytokine in WD Pathophysiology
We performed microarray experiments to analyze the different behavior of monocytes and macrophages in response to T. whipplei (19). We showed that IL-12 was repressed in both monocytes and macrophages following T. whipplei infection. This result is consistent with other studies reporting decreased IL-12 production in WD (76) and with the hypothesis that a specific immune defect is present in WD. However, IL-12 repression does not explain T. whipplei differential survival in monocytes and macrophages. In contrast, T. whipplei replication in macrophages was associated with IL-16 expression. IL-16 is a proinflammatory cytokine secreted by activated CD4+ and CD8+ peripheral blood lymphocytes (8, 121), eosinophils (70), mast cells (106), monocytes (29), and epithelial cells (7). IL-16 is synthesized in a 631-amino-acid precursor form (pro-IL-16) (6) and is subsequently cleaved at Ser511 by activated caspase 3 to generate the mature 123-amino-acid molecule (123). Mature IL-16 is a chemoattractant for CD4-expressing immune cells, such as T cells (8), monocytes (17), dendritic cells (55, 56), and eosinophils (97). IL-16 is also involved in the production of the proinflammatory cytokines TNF, IL-1β, IL-6, and IL-15 by monocytes (79). The expression of the IL-16-encoding gene was up-regulated, and IL-16 was secreted by macrophages following T. whipplei infection (19). In contrast, IL-16 was not secreted by monocytes that killed T. whipplei. The addition of IL-16 in monocyte cultures stimulated T. whipplei replication. Conversely, anti-IL-16 antibodies completely abolished T. whipplei replication in macrophages. IL-16 seems to stimulate macrophage deactivation, since costimulation of macrophages with IL-16 and T. whipplei up-regulated IL-10- and TGF-β1-encoding gene expression. It is likely that the expression of these two cytokines results from the production of inflammatory cytokines in response to T. whipplei. Both IL-10 and TGF-β1, immunoregulatory cytokines, would be more permissive for bacterial replication. Finally, serum IL-16 levels are associated with the activity of the disease: they were higher in patients than in controls, and as soon as a successful treatment was administrated, IL-16 levels decreased to control values (19). Thus, WD may be added to the list of diseases in which IL-16 is involved, such as inflammatory bowel diseases (36, 45, 60, 82, 110).
T. whipplei Survival and Redox Context
Different mechanisms may be responsible for the killing of T. whipplei by monocytes. Transcriptional profiles of T. whipplei-infected monocytes were similar to those of LPS or IFN-γ classically activated monocytes (19). In humans, T. whipplei is seen intracellularly (99, 100) or as extracellular metabolically active bacteria in the intestinal lumen (32). Studies using infection of HeLa cells showed that T. whipplei survives intracellularly by altering the phagosomal environment. Phagosomes containing bacteria were acidic, and T. whipplei colocalized with lysosome-associated protein 1 but not with cathepsin D, indicating that phagolysosome fusion and maturation were incomplete (35). Interestingly, vacuole acidification was critical to the survival of the organisms, since agents that increased the intravacuolar pH decreased bacterial viability (35).
As reported for other intracellular bacteria in which strategies adaptive to host dependence are generally associated with genome reduction (24), T. whipplei possesses a small genome (<1 Mb). Its analysis reveals striking features: the biosynthetic pathways for 16 amino acids are missing or impaired, genes encoding enzymes of the tricarboxylic acid cycle are missing, and T. whipplei lacks clear homologs for thioredoxin and thioredoxin reductase. In addition, the gene encoding glutaredoxin is missing, and T. whipplei exhibits only a distant homolog to glutathione reductase (101). Hence, T. whipplei represents the first example of bacteria without the essential thioredoxin/glutaredoxin redox pathways. Thioredoxin and glutaredoxin systems are antioxidant molecules which act as electron donors for numerous cellular processes. Interestingly, we found that the killing of T. whipplei by monocytes was associated with thioredoxin- and glutaredoxin-encoding gene up-regulation (19). Upon infection, monocytes released active thioredoxin, whereas both its transcription and release were repressed in macrophages. Adding exogenous thioredoxin to infected macrophages decreased bacterial replication. We also demonstrated that thioredoxin is not toxic for T. whipplei, since adding thioredoxin to axenic culture of T. whipplei did not interfere with bacterial growth (19). Hence, it is likely that thioredoxin activates monocytes. Indeed, thioredoxin has been shown to activate NF-κB (4) and AP-1 transcription (48), and this might be the way by which apoptosis is prevented in monocytes. Thioredoxin, and more generally intracellular redox status, also affects the Th1-Th2 balance (88), since mice overexpressing human thioredoxin exhibit a long-term T-cell polarization toward Th1 profile (87) depending on the enzymatic activity of thioredoxin (10).
Intestinal Macrophages in WD
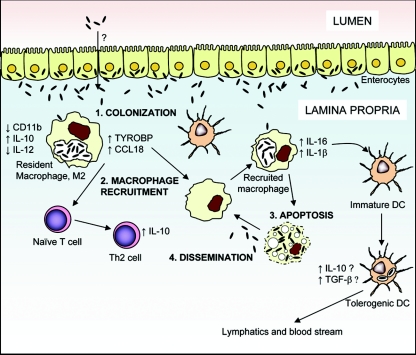
During WD, the small intestinal mucosa of most patients is characterized by a loss of microvilli and the infiltration of large foamy macrophages, which are filled with PAS-positive material. Ultrastructural studies have shown that T. whipplei organisms are present in both intracellular and extracellular locations (111). Recently, we assessed the transcriptional profile of intestinal macrophages from a patient with refractory intestinal WD (18). Histological analyses of duodenal biopsies from this patient showed a massive infiltration of foamy macrophages in the lamina propria which represented more than 80% of the cells. These macrophages contained numerous intracytoplasmic PAS-positive granules. Only 129 genes were differentially modulated in WD lesions. Among them, one of the most up-regulated genes was that encoding CCL18, a chemokine mainly expressed by monocytes/macrophages and dendritic cells. CCL18 expression is induced by Th2-associated cytokines, such as IL-4, IL-13, and IL-10, whereas its expression is repressed by IFN-γ (64, 94). We found that IL-10 was strongly induced in WD lesions and might account for CCL18 expression (18). CCL18 attracts naïve T cells and is produced by tumor-associated macrophages (108). Recruitment of naive T cells in a microenvironment dominated by IL-10, which inhibits dendritic cell maturation, might result in tolerance and immunoregulation. Genes encoding cathepsins, major histocompatibility complex (MHC) class II molecules (HLA-DPβ1 and HLA-DMB), scavenger receptors, CD14, and IL-1 receptor antagonist were also strongly up-regulated in WD lesions. Up-regulation of cathepsin expression may be associated with a defective IFN-γ pathway, since IFN-γ decreases expression of cathepsin B in macrophages (50). These results are in accordance with the successful treatment of a patient with refractory intestinal WD with IFN-γ therapy (107). Interestingly, all these up-regulated genes have been associated with the M2/alternatively activated phenotype of macrophages (39, 73). The M2/alternatively activated transcriptional signature of intestinal macrophages in WD lesions is distinct from that of circulating monocytes and derived macrophages, in which IL-16 seems to be critical (see above) (19). We propose the following model to explain this difference (Fig. 1). Once they have reached the lamina propria by an unknown mechanism, T. whipplei organisms are engulfed by resident intestinal macrophages, which then shift toward the M2/alternatively activated phenotype. They produce high levels of CCL18, IL-10, and TYRO binding protein (DAP12) (18), which may attract other macrophages and naive T cells and orient the local immune response toward a Th2 response. Newly recruited macrophages engulf bacteria and produce IL-16 and IL-1β and undergo apoptosis. T. whipplei infection could then spread gradually. In the intestinal mucosa, IL-16 could promote immature dendritic cell maturation into tolerogenic dendritic cells that orchestrate tolerance of T. whipplei. Apoptosis of infected cells could also play a role by inducing anti-inflammatory cytokine release by macrophages following phagocytosis. Migration of infected dendritic cells or lymphocytes could propagate infection in other lymphoid organs.
FIG. 1.
Model illustrating the pathophysiology of Whipple's disease. DC, dendritic cells.
CONCLUDING REMARKS
It has recently been shown that there is an association between immunosuppressive therapy and the onset of diarrhea in WD, supporting the concept that immunologic factors play a role in disease pathogenesis (72). Macrophages, particularly those of the intestinal mucosa, seem to be of central importance in the development of the disease. The close proximity of bacteria and bacterial products, the local expression of numerous cytokines, and the presence of endogenous mucosal factors probably perpetuate and amplify T. whipplei infection in gastrointestinal tract mucosa. Exploring the underlying immune defects is required to explain why this ubiquitous microorganism causes disease only in certain individuals.
Acknowledgments
This work was supported by a fellowship grant from “Fondation pour la Recherche Médicale” and through the 5th “Programme Cadre de Recherche Technologique” of the European Union (grant no. QRLT-2001-01049).
REFERENCES
- 1.Allison, M. C., S. Cornwall, L. W. Poulter, A. P. Dhillon, and R. E. Pounder. 1988. Macrophage heterogeneity in normal colonic mucosa and in inflammatory bowel disease. Gut 29:1531-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amsler, L., P. Bauernfeind, C. Nigg, R. C. Maibach, R. Steffen, and M. Altwegg. 2003. Prevalence of Tropheryma whipplei DNA in patients with various gastrointestinal diseases and in healthy controls. Infection 31:81-85. [DOI] [PubMed] [Google Scholar]
- 3.Arner, E. S., and A. Holmgren. 2000. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 267:6102-6109. [DOI] [PubMed] [Google Scholar]
- 4.Baeuerle, P. A., and T. Henkel. 1994. Function and activation of NF-kappa B in the immune system. Annu. Rev. Immunol. 12:141-179. [DOI] [PubMed] [Google Scholar]
- 5.Bai, J. C., L. Sen, R. Diez, S. Niveloni, E. C. Maurino, M. E. Estevez, and L. A. Boerr. 1996. Impaired monocyte function in patients successfully treated for Whipple's disease. Acta Gastroenterol. Latinoam. 26:85-89. [PubMed] [Google Scholar]
- 6.Baier, M., N. Bannert, A. Werner, K. Lang, and R. Kurth. 1997. Molecular cloning, sequence, expression, and processing of the interleukin 16 precursor. Proc. Natl. Acad. Sci. USA 94:5273-5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellini, A., H. Yoshimura, E. Vittori, M. Marini, and S. Mattoli. 1993. Bronchial epithelial cells of patients with asthma release chemoattractant factors for T lymphocytes. J. Allergy Clin. Immunol. 92:412-424. [DOI] [PubMed] [Google Scholar]
- 7a.Bentley, S. D., M. Maiwald, L. D. Murphy, M. J. Pallen, C. A. Yeats, L. G. Dover, H. T. Norbertczak, G. S. Besra, M. A. Quail, D. E. Harris, A. von Herbay, A. Goble, S. Rutter, R. Squares, S. Squares, B. G. Barrell, J. Parkhill, and D. A. Relman. 2003. Sequencing and analysis of the genome of the Whipple’s disease bacterium Tropheryma whipplei. Lancet 361:637-644. [DOI] [PubMed] [Google Scholar]
- 8.Berman, J. S., W. W. Cruikshank, D. M. Center, A. C. Theodore, and D. J. Beer. 1985. Chemoattractant lymphokines specific for the helper/inducer T-lymphocyte subset. Cell Immunol. 95:105-112. [DOI] [PubMed] [Google Scholar]
- 9.Bermudez, L. E., A. Parker, and M. Petrofsky. 1999. Apoptosis of Mycobacterium avium-infected macrophages is mediated by both tumour necrosis factor (TNF) and Fas, and involves the activation of caspases. Clin. Exp. Immunol. 116:94-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertini, R., O. M. Howard, H. F. Dong, J. J. Oppenheim, C. Bizzarri, R. Sergi, G. Caselli, S. Pagliei, B. Romines, J. A. Wilshire, M. Mengozzi, H. Nakamura, J. Yodoi, K. Pekkari, R. Gurunath, A. Holmgren, L. A. Herzenberg, and P. Ghezzi. 1999. Thioredoxin, a redox enzyme released in infection and inflammation, is a unique chemoattractant for neutrophils, monocytes, and T cells. J. Exp. Med. 189:1783-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beste, D. J. V., J. Peters, T. Hooper, C. Avignone-Rossa, M. E. Bushell, and J. McFadden. 2005. Compiling a molecular inventory for Mycobacterium bovis BCG at two growth rates: evidence for growth rate-mediated regulation of ribosome biosynthesis and lipid metabolism. J. Bacteriol. 187:1677-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birkemo, G. A., T. Luders, O. Andersen, I. F. Nes, and J. Nissen-Meyer. 2003. Hipposin, a histone-derived antimicrobial peptide in Atlantic halibut (Hippoglossus hippoglossus L.). Biochim. Biophys. Acta 1646:207-215. [DOI] [PubMed] [Google Scholar]
- 13.Bjerknes, R., S. Odegaard, R. Bjerkvig, B. Borkje, and O. D. Laerum. 1988. Whipple's disease. Demonstration of a persisting monocyte and macrophage dysfunction. Scand. J. Gastroenterol. 23:611-619. [DOI] [PubMed] [Google Scholar]
- 14.Burgio, V. L., S. Fais, M. Boirivant, A. Perrone, and F. Pallone. 1995. Peripheral monocyte and naive T-cell recruitment and activation in Crohn's disease. Gastroenterology 109:1029-1038. [DOI] [PubMed] [Google Scholar]
- 15.Byrne, A., and D. J. Reen. 2002. Lipopolysaccharide induces rapid production of IL-10 by monocytes in the presence of apoptotic neutrophils. J. Immunol. 168:1968-1977. [DOI] [PubMed] [Google Scholar]
- 16.Canoso, J. J., M. Saini, and J. A. Hermos. 1978. Whipple's disease and ankylosing spondylitis simultaneous occurrence in HLA-B27 positive male. J. Rheumatol. 5:79-84. [PubMed] [Google Scholar]
- 16a.Chears, W. C., and C. T. Ashworth. 1961. Electron microscopic study of the intestinal mucosa in Whipple’s disease. Demonstration of encapsulated bacilliform bodies in the lesion. Gastroenterology 41:129-138. [PubMed] [Google Scholar]
- 17.Cruikshank, W. W., J. S. Berman, A. C. Theodore, J. Bernardo, and D. M. Center. 1987. Lymphokine activation of T4+ T lymphocytes and monocytes. J. Immunol. 138:3817-3823. [PubMed] [Google Scholar]
- 18.Desnues, B., H. Lepidi, D. Raoult, and J. L. Mege. 2005. Whipple's disease: intestinal infiltrating cells exhibit transcriptional pattern of M2/alternatively activated macrophages. J. Infect. Dis. 192:1642-1646. [DOI] [PubMed] [Google Scholar]
- 19.Desnues, B., D. Raoult, and J. L. Mege. 2005. Interleukin-16 is critical for Tropheryma whipplei replication in Whipple's disease. J. Immunol. 175:4575-4582. [DOI] [PubMed] [Google Scholar]
- 20.Dickman, M. D., A. R. Chappelka, and R. W. Schaedler. 1976. The microbial ecology of the upper small bowel. Am. J. Gastroenterol. 65:57-62. [PubMed] [Google Scholar]
- 21.Dobbins, W. O., III. 1995. The diagnosis of Whipple's disease. N. Engl. J. Med. 332:390-392. [DOI] [PubMed] [Google Scholar]
- 22.Dobbins, W. O., III. 1987. Whipple's disease. Charles C. Thomas, Springfield, Ill.
- 22a.Dobbins, W. O., III, and H. Kawanishi 1981. Bacillary characteristics in Whipple’s disease: an electron microscopic study. Gastroenterology 80:1468-1475. [PubMed] [Google Scholar]
- 23.Donnellan, W. L. 1965. The structure of the colonic mucosa. The epithelium and subepithelial reticulohistiocytic complex. Gastroenterology 49:496-514. [PubMed] [Google Scholar]
- 24.Doolittle, R. F. 2002. Biodiversity: microbial genomes multiply. Nature 416:697-700. [DOI] [PubMed] [Google Scholar]
- 25.do Vale, A., F. Marques, and M. T. Silva. 2003. Apoptosis of sea bass (Dicentrarchus labrax L.) neutrophils and macrophages induced by experimental infection with Photobacterium damselae subsp. piscicida. Fish Shellfish Immunol. 15:129-144. [DOI] [PubMed] [Google Scholar]
- 26.Dutly, F., and M. Altwegg. 2001. Whipple's disease and “Tropheryma whippelii.” Clin. Microbiol. Rev. 14:561-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dutly, F., H. P. Hinrikson, T. Seidel, S. Morgenegg, M. Altwegg, and P. Bauerfeind. 2000. Tropheryma whippelii DNA in saliva of patients without Whipple's disease. Infection 28:219-222. [DOI] [PubMed] [Google Scholar]
- 28.Ehrbar, H. U., P. Bauerfeind, F. Dutly, H. R. Koelz, and M. Altwegg. 1999. PCR-positive tests for Tropheryma whipplei in patients without Whipple's disease. Lancet 353:2214. [DOI] [PubMed] [Google Scholar]
- 29.Elssner, A., A. I. Doseff, M. Duncan, M. Kotur, and M. D. Wewers. 2004. IL-16 is constitutively present in peripheral blood monocytes and spontaneously released during apoptosis. J. Immunol. 172:7721-7725. [DOI] [PubMed] [Google Scholar]
- 30.Fenollar, F., P. E. Fournier, C. Robert, and D. Raoult. 2004. Use of genome selected repeated sequences increases the sensitivity of PCR detection of Tropheryma whipplei. J. Clin. Microbiol. 42:401-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferreri, N. R., W. C. Howland, and H. L. Spiegelberg. 1986. Release of leukotrienes C4 and B4 and prostaglandin E2 from human monocytes stimulated with aggregated IgG, IgA, and IgE. J. Immunol. 136:4188-4193. [PubMed] [Google Scholar]
- 32.Fredricks, D. N., and D. A. Relman. 2001. Localization of Tropheryma whippelii rRNA in tissues from patients with Whipple's disease. J. Infect. Dis. 183:1229-1237. [DOI] [PubMed] [Google Scholar]
- 33.Galdiero, E., C. Romano Carratelli, M. Vitiello, I. Nuzzo, E. Del Vecchio, C. Bentivoglio, G. Perillo, and F. Galdiero. 2000. HSP and apoptosis in leukocytes from infected or vaccinated animals by Brucella abortus. New Microbiol. 23:271. [PubMed] [Google Scholar]
- 34.Galindo, C. L., J. Sha, D. A. Ribardo, A. A. Fadl, L. Pillai, and A. K. Chopra. 2003. Identification of Aeromonas hydrophila cytotoxic enterotoxin-induced genes in macrophages using microarrays. J. Biol. Chem. 278:40198-40212. [DOI] [PubMed] [Google Scholar]
- 35.Ghigo, E., C. Capo, M. Aurouze, C. H. Tung, J. P. Gorvel, D. Raoult, and J. L. Mege. 2002. Survival of Tropheryma whipplei, the agent of Whipple's disease, requires phagosome acidification. Infect. Immun. 70:1501-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glas, J., H. P. Torok, H. Unterhuber, M. Radlmayr, and C. Folwaczny. 2003. The -295T-to-C promoter polymorphism of the IL-16 gene is associated with Crohn's disease. Clin. Immunol. 106:197-200. [DOI] [PubMed] [Google Scholar]
- 37.Gobert, A. P., Y. Cheng, J. Y. Wang, J. L. Boucher, R. K. Iyer, S. D. Cederbaum, R. A. Casero, Jr., J. C. Newton, and K. T. Wilson. 2002. Helicobacter pylori induces macrophage apoptosis by activation of arginase II. J. Immunol. 168:4692-4700. [DOI] [PubMed] [Google Scholar]
- 38.Gorbach, S. L., L. Nahas, P. I. Lerner, and L. Weinstein. 1967. Studies of intestinal microflora. I. Effects of diet, age, and periodic sampling on numbers of fecal microorganisms in man. Gastroenterology 53:845-855. [PubMed] [Google Scholar]
- 39.Gordon, S. 2003. Alternative activation of macrophages. Nat. Rev. Immunol. 3:23-35. [DOI] [PubMed] [Google Scholar]
- 40.Gorter, A., P. S. Hiemstra, P. C. Leijh, M. E. van der Sluys, M. T. van den Barselaar, L. A. van Es, and M. R. Daha. 1987. IgA- and secretory IgA-opsonized S. aureus induce a respiratory burst and phagocytosis by polymorphonuclear leucocytes. Immunology 61:303-309. [PMC free article] [PubMed] [Google Scholar]
- 41.Goth, S. R., and R. S. Stephens. 2001. Rapid, transient phosphatidylserine externalization induced in host cells by infection with Chlamydia spp. Infect. Immun. 69:1109-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greub, G., J. L. Mege, and D. Raoult. 2003. Parachlamydia acanthamoebae enters and multiplies within human macrophages and induces their apoptosis. Infect. Immun. 71:5979-5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grimm, M. C., P. Pavli, E. Van de Pol, and W. F. Doe. 1995. Evidence for a CD14+ population of monocytes in inflammatory bowel disease mucosa-implications for pathogenesis. Clin. Exp. Immunol. 100:291-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han, H., T. Iwanaga, Y. Uchiyama, and T. Fujita. 1993. Aggregation of macrophages in the tip of intestinal villi in guinea pigs: their possible role in the phagocytosis of effete epithelial cells. Cell Tissue Res. 271:407-416. [DOI] [PubMed] [Google Scholar]
- 45.He, S. H. 2004. Key role of mast cells and their major secretory products in inflammatory bowel disease. World J. Gastroenterol. 10:309-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hersh, D., D. M. Monack, M. R. Smith, N. Ghori, S. Falkow, and A. Zychlinsky. 1999. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. USA 96:2396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hiemstra, P. S., M. T. van den Barselaar, M. Roest, P. H. Nibbering, and R. van Furth. 1999. Ubiquicidin, a novel murine microbicidal protein present in the cytosolic fraction of macrophages. J. Leukoc. Biol. 66:423-428. [DOI] [PubMed] [Google Scholar]
- 48.Hirota, K., M. Matsui, S. Iwata, A. Nishiyama, K. Mori, and J. Yodoi. 1997. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc. Natl. Acad. Sci. USA 94:3633-3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hohn, H. P., R. Grummer, S. Bosserhoff, S. Graf-Lingnau, B. Reuss, C. Backer, and H. W. Denker. 1996. The role of matrix contact and of cell-cell interactions in choriocarcinoma cell differentiation. Eur. J. Cell Biol. 69:76-85. [PubMed] [Google Scholar]
- 50.Honey, K., and A. Y. Rudensky. 2003. Lysosomal cysteine proteases regulate antigen presentation. Nat. Rev. Immunol. 3:472-482. [DOI] [PubMed] [Google Scholar]
- 51.Howell, S. J., D. Wilk, S. P. Yadav, and C. L. Bevins. 2003. Antimicrobial polypeptides of the human colonic epithelium. Peptides 24:1763-1770. [DOI] [PubMed] [Google Scholar]
- 52.Hutchison, M. L., I. R. Poxton, and J. R. Govan. 1998. Burkholderia cepacia produces a hemolysin that is capable of inducing apoptosis and degranulation of mammalian phagocytes. Infect. Immun. 66:2033-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Imhof, B. A., and M. Aurrand-Lions. 2004. Adhesion mechanisms regulating the migration of monocytes. Nat. Rev. Immunol. 4:432-444. [DOI] [PubMed] [Google Scholar]
- 54.Iwanaga, T., H. Han, and T. Fujita. 1992. Macrophages possibly involved in the disposal of apoptotic epithelial cells in the monkey small and large intestine. Acta. Med. Biol. 40:105-113. [Google Scholar]
- 55.Kaser, A., S. Dunzendorfer, F. A. Offner, O. Ludwiczek, B. Enrich, R. O. Koch, W. W. Cruikshank, C. J. Wiedermann, and H. Tilg. 2000. B lymphocyte-derived IL-16 attracts dendritic cells and Th cells. J. Immunol. 165:2474-2480. [DOI] [PubMed] [Google Scholar]
- 56.Kaser, A., S. Dunzendorfer, F. A. Offner, T. Ryan, A. Schwabegger, W. W. Cruikshank, C. J. Wiedermann, and H. Tilg. 1999. A role for IL-16 in the cross-talk between dendritic cells and T cells. J. Immunol. 163:3232-3238. [PubMed] [Google Scholar]
- 57.Kashimoto, T., S. Ueno, M. Hanajima, H. Hayashi, Y. Akeda, S. Miyoshi, T. Hongo, T. Honda, and N. Susa. 2003. Vibrio vulnificus induces macrophage apoptosis in vitro and in vivo. Infect. Immun. 71:533-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kato, S., M. Muro, S. Akifusa, N. Hanada, I. Semba, T. Fujii, Y. Kowashi, and T. Nishihara. 1995. Evidence for apoptosis of murine macrophages by Actinobacillus actinomycetemcomitans infection. Infect. Immun. 63:3914-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keane, J., M. K. Balcewicz-Sablinska, H. G. Remold, G. L. Chupp, B. B. Meek, M. J. Fenton, and H. Kornfeld. 1997. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect. Immun. 65:298-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keates, A. C., I. Castagliuolo, W. W. Cruickshank, B. Qiu, K. O. Arseneau, W. Brazer, and C. P. Kelly. 2000. Interleukin 16 is up-regulated in Crohn's disease and participates in TNBS colitis in mice. Gastroenterology 119:972-982. [DOI] [PubMed] [Google Scholar]
- 61.Kennedy, D. W., and J. L. Abkowitz. 1997. Kinetics of central nervous system microglial and macrophage engraftment: analysis using a transgenic bone marrow transplantation model. Blood 90:986-993. [PubMed] [Google Scholar]
- 62.Khelef, N., A. Zychlinsky, and N. Guiso. 1993. Bordetella pertussis induces apoptosis in macrophages: role of adenylate cyclase-hemolysin. Infect. Immun. 61:4064-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kobayashi, K. S., M. Chamaillard, Y. Ogura, O. Henegariu, N. Inohara, G. Nunez, and R. A. Flavell. 2005. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307:731-734. [DOI] [PubMed] [Google Scholar]
- 64.Kodelja, V., C. Muller, O. Politz, N. Hakij, C. E. Orfanos, and S. Goerdt. 1998. Alternative macrophage activation-associated CC-chemokine-1, a novel structural homologue of macrophage inflammatory protein-1 α with a Th2-associated expression pattern. J. Immunol. 160:1411-1418. [PubMed] [Google Scholar]
- 65.Lai, X. H., and A. Sjostedt. 2003. Delineation of the molecular mechanisms of Francisella tularensis-induced apoptosis in murine macrophages. Infect. Immun. 71:4642-4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lai, X. H., J. G. Xu, S. Melgar, and B. E. Uhlin. 1999. An apoptotic response by J774 macrophage cells is common upon infection with diarrheagenic Escherichia coli. FEMS Microbiol. Lett. 172:29-34. [DOI] [PubMed] [Google Scholar]
- 67.Lammas, D. A., E. De Heer, J. D. Edgar, V. Novelli, A. Ben-Smith, R. Baretto, P. Drysdale, J. Binch, C. MacLennan, D. S. Kumararatne, S. Panchalingam, T. H. Ottenhoff, J. L. Casanova, and J. F. Emile. 2002. Heterogeneity in the granulomatous response to mycobacterial infection in patients with defined genetic mutations in the interleukin 12-dependent interferon-gamma production pathway. Int. J. Exp. Pathol. 83:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee, S. H., P. M. Starkey, and S. Gordon. 1985. Quantitative analysis of total macrophage content in adult mouse tissues. Immunochemical studies with monoclonal antibody F4/80. J. Exp. Med. 161:475-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lewis, C. E., and J. O. McGee. 1992. The macrophage. Oxford University Press, Oxford, United Kingdom.
- 70.Lim, K. G., H. C. Wan, M. Resnick, D. T. Wong, W. W. Cruikshank, H. Kornfeld, D. M. Center, and P. F. Weller. 1995. Human eosinophils release the lymphocyte and eosinophil active cytokines, RANTES and lymphocyte chemoattractant factor. Int. Arch. Allergy Immunol. 107:342. [DOI] [PubMed] [Google Scholar]
- 71.Mahida, Y. R., A. Galvin, S. Makh, S. Hyde, L. Sanfilippo, S. P. Borriello, and H. F. Sewell. 1998. Effect of Clostridium difficile toxin A on human colonic lamina propria cells: early loss of macrophages followed by T-cell apoptosis. Infect. Immun. 66:5462-5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mahnel, R., A. Kalt, S. Ring, A. Stallmach, W. Strober, and T. Marth. 2005. Immunosuppressive therapy in Whipple's disease patients is associated with the appearance of gastrointestinal manifestations. Am. J. Gastroenterol. 100:1167-1173. [DOI] [PubMed] [Google Scholar]
- 72a.Maiwald, M., F. Schuhmacher, H. J. Ditton, and A. von Herbay. 1998. Environmental occurrence of the Whipple’s disease bacterium (Tropheryma whippelii). Appl. Environ. Microbiol. 64:760-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mantovani, A., A. Sica, S. Sozzani, P. Allavena, A. Vecchi, and M. Locati. 2004. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25:677-686. [DOI] [PubMed] [Google Scholar]
- 74.Marth, T., D. Fredericks, W. Strober, and D. A. Relman. 1996. Limited role for PCR-based diagnosis of Whipple's disease from peripheral blood mononuclear cells. Lancet 348:66-67. [DOI] [PubMed] [Google Scholar]
- 75.Marth, T., N. Kleen, A. Stallmach, S. Ring, S. Aziz, C. Schmidt, W. Strober, M. Zeitz, and T. Schneider. 2002. Dysregulated peripheral and mucosal Th1/Th2 response in Whipple's disease. Gastroenterology 123:1468-1477. [DOI] [PubMed] [Google Scholar]
- 76.Marth, T., M. Neurath, B. A. Cuccherini, and W. Strober. 1997. Defects of monocyte interleukin 12 production and humoral immunity in Whipple's disease. Gastroenterology 113:442-448. [DOI] [PubMed] [Google Scholar]
- 77.Marth, T., and D. Raoult. 2003. Whipple's disease. Lancet 361:239-246. [DOI] [PubMed] [Google Scholar]
- 78.Marth, T., M. Roux, A. von Herbay, S. C. Meuer, and G. E. Feurle. 1994. Persistent reduction of complement receptor 3 alpha-chain expressing mononuclear blood cells and transient inhibitory serum factors in Whipple's disease. Clin. Immunol. Immunopathol. 72:217-226. [DOI] [PubMed] [Google Scholar]
- 79.Mathy, N. L., W. Scheuer, M. Lanzendorfer, K. Honold, D. Ambrosius, S. Norley, and R. Kurth. 2000. Interleukin-16 stimulates the expression and production of pro-inflammatory cytokines by human monocytes. Immunology 100:63-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meagher, L. C., J. S. Savill, A. Baker, R. W. Fuller, and C. Haslett. 1992. Phagocytosis of apoptotic neutrophils does not induce macrophage release of thromboxane B2. J. Leukoc. Biol. 52:269-273. [PubMed] [Google Scholar]
- 81.Merien, F., G. Baranton, and P. Perolat. 1997. Invasion of Vero cells and induction of apoptosis in macrophages by pathogenic Leptospira interrogans are correlated with virulence. Infect. Immun. 65:729-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Middel, P., K. Reich, F. Polzien, V. Blaschke, B. Hemmerlein, J. Herms, M. Korabiowska, and H. J. Radzun. 2001. Interleukin 16 expression and phenotype of interleukin 16 producing cells in Crohn's disease. Gut 49:795-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mills, S. D., A. Boland, M. P. Sory, P. van der Smissen, C. Kerbourch, B. B. Finlay, and G. R. Cornelis. 1997. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc. Natl. Acad. Sci. USA 94:12638-12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Monack, D. M., J. Mecsas, N. Ghori, and S. Falkow. 1997. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc. Natl. Acad. Sci. USA 94:10385-10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morton, H. C., M. van Egmond, and J. G. van de Winkel. 1996. Structure and function of human IgA Fc receptors (Fc α R). Crit. Rev. Immunol. 16:423-440. [PubMed] [Google Scholar]
- 86.Mostov, K., and C. S. Kaetzel. 1999. Immunoglobulin transport and the polymeric immunoglobulin receptor, p. 181. In P. L. Ogra, J. Mestecky, M. E. Lamm, W. Strober, J. Bienenstock, and J. R. McGhee (ed.), Mucosal immunology. Academic Press, San Diego, Calif.
- 87.Murata, Y., M. Amao, J. Yoneda, and J. Hamuro. 2002. Intracellular thiol redox status of macrophages directs the Th1 skewing in thioredoxin transgenic mice during aging. Mol. Immunol. 38:747-757. [DOI] [PubMed] [Google Scholar]
- 88.Murata, Y., T. Shimamura, and J. Hamuro. 2002. The polarization of Th1/Th2 balance is dependent on the intracellular thiol redox status of macrophages due to the distinctive cytokine production. Int. Immunol. 14:201-212. [DOI] [PubMed] [Google Scholar]
- 89.Nagashima, R., K. Maeda, Y. Imai, and T. Takahashi. 1996. Lamina propria macrophages in the human gastrointestinal mucosa: their distribution, immunohistological phenotype, and function. J. Histochem. Cytochem. 44:721-731. [DOI] [PubMed] [Google Scholar]
- 90.Ng, L. C., O. Forslund, S. Koh, K. Kuoppa, and A. Sjostedt. 2003. The response of murine macrophages to infection with Yersinia pestis as revealed by DNA microarray analysis. Adv. Exp. Med. Biol. 529:155-160. [DOI] [PubMed] [Google Scholar]
- 91.Ojcius, D. M., P. Souque, J. L. Perfettini, and A. Dautry-Varsat. 1998. Apoptosis of epithelial cells and macrophages due to infection with the obligate intracellular pathogen Chlamydia psittaci. J. Immunol. 161:4220-4226. [PubMed] [Google Scholar]
- 92.Pavli, P., and W. F. Doe. 1992. Intestinal macrophages, p. 177-188. In R. P. MacDermott and W. F. Stenson (ed.), Inflammatory bowel disease. Elsevier, New York, N.Y.
- 93.Pavli, P., L. Maxwell, E. Van de Pol, and F. Doe. 1996. Distribution of human colonic dendritic cells and macrophages. Clin. Exp. Immunol. 104:124-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Politz, O., V. Kodelja, P. Guillot, C. E. Orfanos, and S. Goerdt. 2000. Pseudoexons and regulatory elements in the genomic sequence of the beta-chemokine, alternative macrophage activation-associated CC-chemokine (AMAC)-1. Cytokine 12:120-126. [DOI] [PubMed] [Google Scholar]
- 95.Popov, S. G., R. Villasmil, J. Bernardi, E. Grene, J. Cardwell, A. Wu, D. Alibek, C. Bailey, and K. Alibek. 2002. Lethal toxin of Bacillus anthracis causes apoptosis of macrophages. Biochem. Biophys. Res. Commun. 293:349-355. [DOI] [PubMed] [Google Scholar]
- 96.Puite, R. H., and H. Tesluk. 1955. Whipple's disease. Am. J. Med. 19:383-400. [DOI] [PubMed] [Google Scholar]
- 97.Rand, T. H., W. W. Cruikshank, D. M. Center, and P. F. Weller. 1991. CD4-mediated stimulation of human eosinophils: lymphocyte chemoattractant factor and other CD4-binding ligands elicit eosinophil migration. J. Exp. Med. 173:1521-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Raoult, D., M. L. Birg, B. La Scola, P. E. Fournier, M. Enea, H. Lepidi, V. Roux, J. C. Piette, F. Vandenesch, D. Vital-Durand, and T. J. Marrie. 2000. Cultivation of the bacillus of Whipple's disease. N. Engl. J. Med. 342:620-625. [DOI] [PubMed] [Google Scholar]
- 99.Raoult, D., B. La Scola, P. Lecocq, H. Lepidi, and P. E. Fournier. 2001. Culture and immunological detection of Tropheryma whippelii from the duodenum of a patient with Whipple disease. JAMA 285:1039-1043. [DOI] [PubMed] [Google Scholar]
- 100.Raoult, D., H. Lepidi, and J. R. Harle. 2001. Tropheryma whipplei circulating in blood monocytes. N. Engl. J. Med. 345:548. [DOI] [PubMed] [Google Scholar]
- 101.Raoult, D., H. Ogata, S. Audic, C. Robert, K. Suhre, M. Drancourt, and J. M. Claverie. 2003. Tropheryma whipplei twist: a human pathogenic actinobacteria with a reduced genome. Genome Res. 13:1800-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Raqib, R., C. Ekberg, P. Sharkar, P. K. Bardhan, A. Zychlinsky, P. J. Sansonetti, and J. Andersson. 2002. Apoptosis in acute shigellosis is associated with increased production of Fas/Fas ligand, perforin, caspase-1, and caspase-3 but reduced production of Bcl-2 and interleukin-2. Infect. Immun. 70:3199-3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102a.Renesto, P., N. Crapoulet, H. Ogata, B. La Scola, G. Vestris, J. M. Claverie, and D. Raoult. 2003. Genome-based design of a cell-free culture medium for Tropheryma whipplei. Lancet 362:447-449. [DOI] [PubMed] [Google Scholar]
- 103.Rogler, G., M. Hausmann, D. Vogl, E. Aschenbrenner, T. Andus, W. Falk, R. Andreesen, J. Scholmerich, and V. Gross. 1998. Isolation and phenotypic characterization of colonic macrophages. Clin. Exp. Immunol. 112:205-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rosenberger, C. M., R. L. Gallo, and B. B. Finlay. 2004. Interplay between antibacterial effectors: a macrophage antimicrobial peptide impairs intracellular Salmonella replication. Proc. Natl. Acad. Sci. USA 101:2422-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rugtveit, J., P. Brandtzaeg, T. Halstensen, O. Fausa, and H. Scott. 1994. Increased macrophage subset in inflammatory bowel disease: apparent recruitment from peripheral blood monocytes. Gut 35:669-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rumsaeng, V., W. W. Cruikshank, B. Foster, C. Prussin, A. S. Kirshenbaum, T. A. Davis, H. Kornfeld, D. M. Center, and D. D. Metcalfe. 1997. Human mast cells produce the CD4+ T lymphocyte chemoattractant factor, IL-16. J. Immunol. 159:2904-2910. [PubMed] [Google Scholar]
- 107.Schneider, T., A. Stallmach, A. von Herbay, T. Marth, W. Strober, and M. Zeitz. 1998. Treatment of refractory Whipple disease with interferon-gamma. Ann. Intern. Med. 129:875-877. [DOI] [PubMed] [Google Scholar]
- 107a.Schoedon, G., D. Goldenberger, R. Forrer, A. Gunz, F. Dutly, M. Hochli, M. Altwegg, and A. Schaffner. 1997. Deactivation of macrophages with interleukin-4 is the key to the isolation of Tropheryma whippelii. J. Infect. Dis. 176:672-677. [DOI] [PubMed] [Google Scholar]
- 108.Schutyser, E., S. Struyf, P. Proost, G. Opdenakker, G. Laureys, B. Verhasselt, L. Peperstraete, I. Van de Putte, A. Saccani, P. Allavena, A. Mantovani, and J. Van Damme. 2002. Identification of biologically active chemokine isoforms from ascitic fluid and elevated levels of CCL18/pulmonary and activation-regulated chemokine in ovarian carcinoma. J. Biol. Chem. 277:24584-24593. [DOI] [PubMed] [Google Scholar]
- 109.Schwan, W. R., X. Z. Huang, L. Hu, and D. J. Kopecko. 2000. Differential bacterial survival, replication, and apoptosis-inducing ability of Salmonella serovars within human and murine macrophages. Infect. Immun. 68:1005-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Seegert, D., P. Rosenstiel, H. Pfahler, P. Pfefferkorn, S. Nikolaus, and S. Schreiber. 2001. Increased expression of IL-16 in inflammatory bowel disease. Gut 48:326-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Silva, M. T., P. M. Macedo, and J. F. Moura Nunes. 1985. Ultrastructure of bacilli and the bacillary origin of the macrophagic inclusions in Whipple's disease. J. Gen. Microbiol. 131:1001-1013. [DOI] [PubMed] [Google Scholar]
- 112.Smith, P. D., E. N. Janoff, M. Mosteller-Barnum, M. Merger, J. M. Orenstein, J. F. Kearney, and M. F. Graham. 1997. Isolation and purification of CD14-negative mucosal macrophages from normal human small intestine. J. Immunol. Methods 202:1-11. [DOI] [PubMed] [Google Scholar]
- 113.Smith, P. D., L. E. Smythies, M. Mosteller-Barnum, D. A. Sibley, M. W. Russell, M. Merger, M. T. Sellers, J. M. Orenstein, T. Shimada, M. F. Graham, and H. Kubagawa. 2001. Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IgA-mediated activities. J. Immunol. 167:2651-2656. [DOI] [PubMed] [Google Scholar]
- 114.Smythies, L. E., M. Sellers, R. H. Clements, M. Mosteller-Barnum, G. Meng, W. H. Benjamin, J. M. Orenstein, and P. D. Smith. 2005. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J. Clin. Investig. 115:66-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Spöttl, T., M. Hausmann, M. Kreutz, A. Peuker, D. Vogl, J. Scholmerich, W. Falk, R. Andreesen, T. Andus, H. Herfarth, and G. Rogler. 2001. Monocyte differentiation in intestine-like macrophage phenotype induced by epithelial cells. J. Leukoc. Biol. 70:241-251. [PubMed] [Google Scholar]
- 116.Stern, M., J. Savill, and C. Haslett. 1996. Human monocyte-derived macrophage phagocytosis of senescent eosinophils undergoing apoptosis. Mediation by αvβ3/CD36/thrombospondin recognition mechanism and lack of phlogistic response. Am. J. Pathol. 149:911-921. [PMC free article] [PubMed] [Google Scholar]
- 117.Stoll, T., G. Keusch, R. Jost, H. Burger, and O. Oelz. 1993. IgA nephropathy and hypercalcemia in Whipple's disease. Nephron 63:222-225. [DOI] [PubMed] [Google Scholar]
- 118.Takahashi-Iwanaga, H., T. Iwanaga, and H. Isayama. 1999. Porosity of the epithelial basement membrane as an indicator of macrophage-enterocyte interaction in the intestinal mucosa. Arch. Histol. Cytol. 62:471-481. [DOI] [PubMed] [Google Scholar]
- 119.Tani, K., W. J. Murphy, O. Chertov, R. Salcedo, C. Y. Koh, I. Utsunomiya, S. Funakoshi, O. Asai, S. H. Herrmann, J. M. Wang, L. W. Kwak, and J. J. Oppenheim. 2000. Defensins act as potent adjuvants that promote cellular and humoral immune responses in mice to a lymphoma idiotype and carrier antigens. Int. Immunol. 12:691-700. [DOI] [PubMed] [Google Scholar]
- 120.Tollin, M., P. Bergman, T. Svenberg, H. Jornvall, G. H. Gudmundsson, and B. Agerberth. 2003. Antimicrobial peptides in the first line defence of human colon mucosa. Peptides 24:523-530. [DOI] [PubMed] [Google Scholar]
- 121.Van Epps, D. E., D. A. Durant, and J. W. Potter. 1983. Migration of human helper/inducer T cells in response to supernatants from Con A-stimulated suppressor/cytotoxic T cells. J. Immunol. 131:697-700. [PubMed] [Google Scholar]
- 121a.Whipple G. H. 1907. A hitherto undescribed disease characterized anatomically by deposits of fat and fatty acids in the intestinal and mesenteric lymphatic tissues. Bull. Johns Hopkins Hosp. 18:382-391. [Google Scholar]
- 122.Yang, D., A. Biragyn, L. W. Kwak, and J. J. Oppenheim. 2002. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 23:291-296. [DOI] [PubMed] [Google Scholar]
- 123.Zhang, Y., D. M. Center, D. M. Wu, W. W. Cruikshank, J. Yuan, D. W. Andrews, and H. Kornfeld. 1998. Processing and activation of pro-interleukin-16 by caspase-3. J. Biol. Chem. 273:1144-1149. [DOI] [PubMed] [Google Scholar]
- 124.Zinkernagel, A. S., R. Gmur, L. Fenner, A. Schaffner, G. Schoedon, and M. Schneemann. 2003. Marginal and subgingival plaque-a natural habitat of Tropheryma whipplei? Infection 31:86-91. [DOI] [PubMed] [Google Scholar]
- 125.Zychlinsky, A., C. Fitting, J. M. Cavaillon, and P. J. Sansonetti. 1994. Interleukin 1 is released by murine macrophages during apoptosis induced by Shigella flexneri. J. Clin. Investig. 94:1328-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zychlinsky, A., B. Kenny, R. Menard, M. C. Prevost, I. B. Holland, and P. J. Sansonetti. 1994. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol. Microbiol. 11:619-627. [DOI] [PubMed] [Google Scholar]
- 127.Zychlinsky, A., M. C. Prevost, and P. J. Sansonetti. 1992. Shigella flexneri induces apoptosis in infected macrophages. Nature 358:167-169. [DOI] [PubMed] [Google Scholar]