Abstract
We showed previously that interleukin-17 (IL-17) plays a significant role in the induction of arthritis associated with Borrelia vaccination and challenge. Little information, however, is available about the chain of immunologic events that leads to the release of IL-17. The production of IL-17 has been linked to stimulation of memory cells by IL-15. Therefore, we hypothesized that IL-15 is involved in the induction of arthritis associated with Borrelia vaccination and infection of mice. Here we present evidence that treatment of Borrelia-vaccinated and -infected mice with anti-IL-15 antibody prevents swelling of the hind paws. More importantly, both anti-IL-15 antibody- and recombinant IL-15 receptor alpha-treated Borrelia-vaccinated and -infected mice were free of major histopathologic indications of arthritis, including hyperplasia, hypertrophy, and vilus formation of the synovium. Similarly, the synovial space and perisynovium were free of inflammatory cells. By contrast, the synovium of nontreated Borrelia-vaccinated and -infected mice had overt hyperplasia, hypertrophy, and vilus formation. Moreover, the synovial space and perisynovium were infiltrated with neutrophils, macrophages, and lymphocytes. Finally, we show that recombinant IL-15 stimulates the release of IL-17 from lymph node cells obtained near the arthritic site. These results suggest that IL-15 plays a major role in orchestrating IL-17 induction of arthritis associated with Borrelia-vaccinated and -infected mice.
Arthritis is a major clinical presentation in humans infected with Borrelia burgdorferi (39). Arthritis is also detected in humans following vaccination with Borrelia outer surface protein A (34) and animals following Borrelia infection (5, 6) or Borrelia vaccination and infection (10, 27). The immune mechanisms responsible for this arthritis are poorly understood. We showed previously that treatment of Borrelia-vaccinated and -infected mice with anti-interleukin-17 (anti-IL-17) or anti-IL-17 receptor antibodies prevented the induction of arthritis (8). This finding is important because it defines a major contributor to the pathogenesis of arthritis. Little is known, however, about the immunologic events that lead to the expression of IL-17 and the development of arthritis. Other precursor cytokines may trigger the release of IL-17.
It is known that production of the proinflammatory cytokine IL-17, especially from memory T cells (43), is stimulated by IL-15 (13, 24, 43). IL-15 is a recently discovered cytokine (16) that is produced by synoviocytes (26), monocytes (31), neutrophils (19), and bone marrow stromal cells (33) but not primary T cells (12, 16, 17, 20). In addition, IL-15 is produced by dendritic cells and epithelial cells (36) and influences the local infiltration, activation, and proliferation of antigen-driven T cells at the site of infection or inflammation (28, 42). Clinically, abnormalities of IL-15 expression have been reported for several diseases (1, 19, 25), including rheumatoid arthritis (28). Blocking endogenous IL-15 has been effective in reducing pathology (35). Thus, IL-15 may act as a chemotactic and proliferation factor for the attraction and activation of cells, especially memory T cells capable of releasing IL-17.
In this report, we show that treatment of Borrelia-vaccinated and -challenged mice with anti-IL-15 or its soluble receptor prevents the development of arthritis. In addition, we show that recombinant IL-15 (rIL-15) can stimulate the release of IL-17 from lymph node cells obtained near the arthritic site.
MATERIALS AND METHODS
Mice.
Gamma interferon gene-deficient mice were obtained from W. P. Weidanz (University of Wisconsin) with permission from Genentech (South San Francisco, CA) and housed at the University of Wisconsin Animal Facility. Eight- to 12-week-old male mice weighing 20 to 30 grams were given food and acidified water ad libitum during a light-and-dark cycle of 12 h for the duration of the study. Experimental protocols were approved by the Animal Care and Use Committee of the University of Wisconsin Medical School, Madison.
Organisms.
Low-passage-number (<10) Borrelia burgdorferi strain 297 (from human spinal fluid) and “Borrelia bissettii” (formerly strain C-1-11, from Microtus pennsylvanicus) were grown at 32°C in modified Barbour-Stoenner-Kelly (BSK) medium until they reached a concentration of approximately 107 spirochetes/ml. Aliquots (500 μl) were then dispensed into 1.5-ml screw-cap tubes (Sarstedt, Newton, NC) containing 500 μl of BSK medium supplemented with 10% glycerol (Sigma Chemical Co., St. Louis, MO). The tubes were sealed and stored at −70°C. When necessary, a frozen suspension of spirochetes was thawed and used to inoculate fresh BSK medium. Spirochetes were viewed and enumerated by using dark-field microscopy.
Vaccine preparation.
B. burgdorferi strain 297 organisms were grown in 1 liter of BSK medium for 6 days before being pelleted by centrifugation (10,000 × g, 15°C, 10 min) and washed three times with phosphate-buffered saline (PBS; pH 7.4). The washed pellet was resuspended in a 1% formalin solution, incubated at 32°C with periodic mixing for 40 min, and then washed three times by centrifugation with PBS (10,000 × g, 15°C, 10 min). Subsequently, the washed pellet was resuspended in 1 ml PBS, dispensed in aliquots, and frozen at −70°C.
On the day of vaccination, an aliquot was thawed and mixed. The spirochetes were enumerated by using dark-field microscopy and a volume was added to 3% aluminum hydroxide (alum; Reheis, Berkeley Heights, NJ) to yield 5 × 106 spirochetes/ml.
Vaccination of mice.
Ninety mice were anesthetized with ether contained in a nose-and-mouth cup and injected subcutaneously in the inguinal region with 250 μl of the formalin-inactivated whole-cell B. burgdorferi 297 vaccine. Thirty sham-vaccinated mice were also injected with 3% alum. The use of whole cells of B. burgdorferi for vaccination is not recommended for humans because of concerns associated with whole-cell vaccines (22). However, the ability of whole cells to consistently induce arthritis in our murine model (8, 10) allowed for the evaluation of the immunological mechanisms that induce arthritis.
Infection of mice.
Twenty-one days after vaccination, mice were anesthetized with ether contained in a nose-and-mouth cup and injected subcutaneously in the right hind paw with 50 μl of BSK medium containing 106 viable B. bissettii organisms. Swelling of the hind paws consistently develops 4 to 6 days after B. bissettii infection and peaks on day 8 to 12 (8, 10). Swelling of the hind paws can also be induced by infection with the homologous B. burgdorferi strain 297. However, B. burgdorferi strain 297-vaccinated mice must be challenged before protective antibodies develop (approximately day 7) or after their decline. Swelling of the hind paws of homologous vaccinated and challenged mice is variable. Therefore, we challenged B. burgdorferi strain 297-vaccinated mice with B. bissettii to obtain consistent swelling of the hind paws. Vaccination of mice with B. bissettii and challenge with B. burgdorferi strain 297 also yields consistent swelling of the hind paws, as does challenge with other infectious isolates of B. burgdorferi (11, 27, 37). Controls included vaccinated mice injected with alum or BSK medium alone.
Administration of anti-IL-15 antibody and rIL-15 receptor alpha.
Lyophilized goat anti-mouse immunoglobulin G polyclonal IL-15 antibody (200 μg), normal goat immunoglobulin G (100 μg), and mouse rIL-15 receptor alpha (100 μg) were obtained from R&D Systems (Minneapolis, MN). The antibodies and rIL-15 receptor were resuspended in filter-sterilized (0.2-μm-pore-size filter) (Acrodisk; Gilman Sciences, Ann Arbor, MI) PBS (pH 7.2) or PBS containing 0.1% bovine serum albumin (Fisher Scientific, Pittsburgh, PA), respectively, to yield concentrations of 50 μg/ml. Twenty-one days after vaccination, three groups of eight mice each were infected with 106 B. bissettii organisms in the right hind paw. Less than 1 h after infection, the mice were injected subcutaneously in the right hind paw with 50 μl of the anti-IL-15 antibody or rIL-15 receptor alpha preparation. Anti-IL-15 antibody or rIL-15 receptor alpha was injected daily for 6 or 8 days, respectively. In other experiments, anti-IL-15 antibody was injected on day 7 after infection and daily thereafter for 6 days. Control groups received injections with the normal goat isotype antibody or with BSK medium.
Measurement of IL-17 produced by immune lymph node cells.
Twenty-one days after vaccination, six mice were euthanized with ether contained in a nose-and-mouth cup and the inguinal lymph nodes were removed. The nodes were teased apart with a forceps, and single-cell suspensions were obtained by passing the cells through a sterile Falcon 100-μm nylon cell strainer (Fisher Scientific) into cold RPMI medium containing 10% fetal calf serum (Sigma, St. Louis, MO) with penicillin and streptomycin (Fisher Scientific). The cells were counted by using a hemacytometer and dispensed at a concentration of 5 × 106 cells per well into a 24-well microtiter plate (Fisher Scientific) in 1 ml of supplemented RPMI medium. Mouse rIL-15 (<1.0 endotoxin unit of endotoxin per 1 μg cytokine; R&D Systems) was reconstituted in PBS containing 0.1% bovine serum albumin and added to wells at a concentration of 50, 100, 200, or 500 ng/well. Wells not receiving rIL-15 were treated with PBS containing 0.1% bovine serum albumin. Viable B. bissettii organisms (5 × 106) were added to some wells. Microtiter plates were incubated at 37°C in 5% CO2 for 48 h before supernatants were removed and analyzed for production of IL-17 using an enzyme-linked immunosorbent assay kit (R&D Systems) according to the manufacturer's instructions.
Assessment of arthritis.
Hind-paw swelling was used to determine the level of the inflammatory response in the study mice. Prior to vaccination, random age-matched mice were chosen and their right hind paws were measured to determine the baseline of paw size. Paws were measured 48 h after infection and every other day thereafter for 14 days by using a Vernifer caliper with a digital readout to 0.01 mm. To obtain the measurements, mice were anesthetized with ether and the widths and thicknesses of the right hind tibiotarsal joints were carefully measured. All caliper values within a group were summed and divided by the number of measurements taken in the group to obtain the daily mean value.
Preparation of tissues for histologic examination.
At 9 and 14 days after infection, mice were euthanized with ether and their hind paws were amputated at mid-femur. Paws were fixed in 10% neutral buffered zinc formalin for 24 h. Subsequently, the paws were placed in decalcifying solution (Lerner Laboratories, Pittsburgh, Pa.) for 24 h, followed by the addition of fresh decalcifying solution for an additional 48 h. Following decalcification, the legs were placed in tissue-embedding cassettes (Fisher Scientific), embedded in paraffin, and cut into 6-μm-thick sections. These sections were cryptically encoded and placed on glass slides for staining with hematoxylin and eosin. Unbiased histopathologic examinations were performed by a board-certified pathologist (T. F. Warner).
Statistical analysis.
All results were tested by analysis of variance. The Fisher least significant test was used to examine pairs of means when a significant F ratio indicated reliable mean differences (38). The alpha level was set at 0.05 before the experiments were started.
RESULTS
Effects of anti-IL-15 antibody treatment on development and progression of hind-paw swelling.
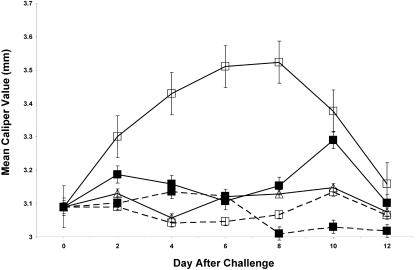
Two groups of eight vaccinated mice each were infected with 106 viable B. bissettii organisms 21 days after vaccination. Concomitantly, one of the two groups of vaccinated and infected mice was treated with anti-IL-15 antibody on the day of the challenge and daily thereafter for 6 days. Significant (P < 0.05) swelling of the right hind paw was detected in vaccinated and infected mice 4 days after infection. The swelling peaked on day 8 and rapidly decreased (Fig. 1). In contrast, vaccinated and challenged mice treated with anti-IL-15 antibody had a delayed onset of swelling of the hind paw and considerably less severity of swelling. When treatment with anti-IL-15 was discontinued on day 6 after infection, swelling of the hind paw occurred rapidly (day 10). Thereafter, the swelling gradually decreased. No swelling of the hind paw occurred in vaccinated, nonchallenged mice treated with anti-IL-15 antibody or in untreated vaccinated mice. Finally, nonvaccinated mice challenged with B. bissettii also failed to develop swelling of the hind paw. Similar results were obtained when these experiments were repeated with four mice per group.
FIG. 1.
Development of swelling of the hind paws of vaccinated mice with (solid lines) or without (broken lines) challenge with B. bissettii and with (▪) or without (□) treatment with anti-IL-15 antibody. The remaining nonvaccinated challenge group (▵) did not receive treatment with anti-IL-15 antibody. Data are the means ± standard errors for the experiment.
Histopathologic confirmation that anti-IL-15 antibody treatment inhibited development of arthritis.
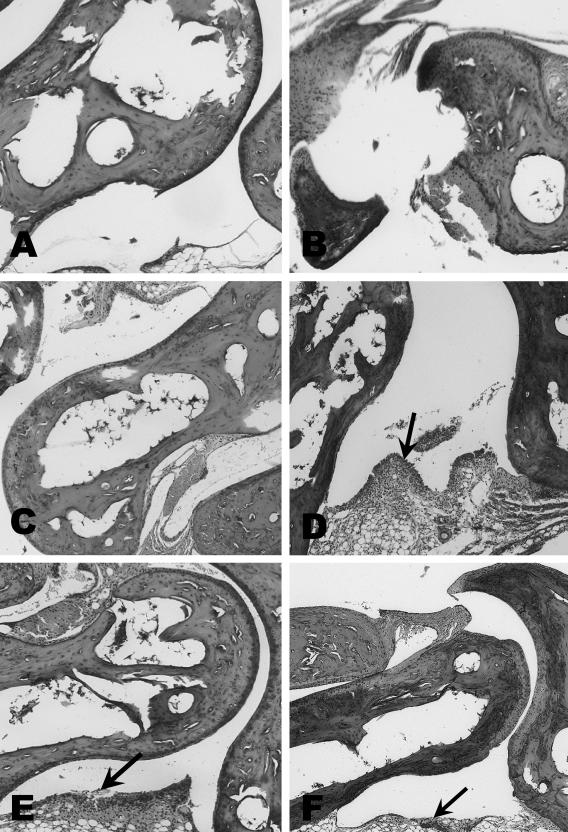
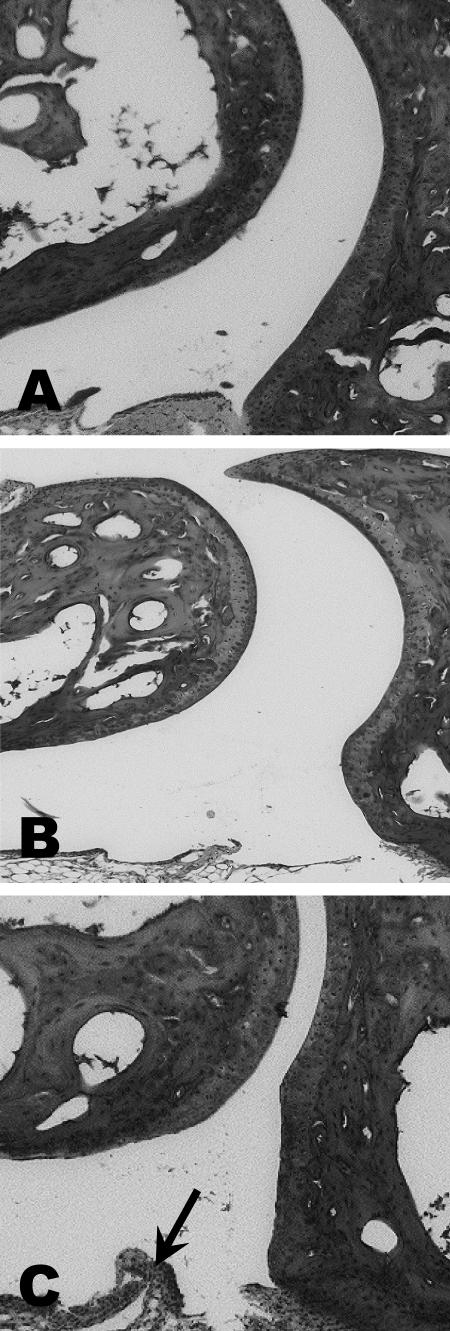
Vaccinated mice challenged with B. bissettii showed severe inflammation and edematous changes throughout the paw, including the synovial space, synovium, perisynovium, and the small bones of the paw 9 days after infection (Fig. 2). Moreover, the synovium had significant hyperplasia and hypertrophy along with vilus formation. However, no evidence of histopathologic changes, except edema and minor infiltration of neutrophils, macrophages, and lymphocytes in the synovium, was detected in vaccinated mice infected with B. bissettii and treated with anti-IL-15 antibody (Fig. 2). The controls, including vaccinated mice and vaccinated mice treated with anti-IL-15 antibody, were also free of significant histopathology (Fig. 3). Nonvaccinated but infected mice developed only marginal pathology of the synovial space, synovium, and perisynovium (Fig. 3).
FIG. 2.
Histopathology of the tibiotarsal joints of three Borrelia-vaccinated and -infected mice (day 9 after infection) with (panels A, B, and C) and without (panels D, E, and F) treatment with anti-IL-15 antibody. No histopathology was found in the three Borrelia-vaccinated and -infected mice (panels A, B, and C) treated with anti-IL-15 antibody. In the absence of anti-IL-15 antibody treatment, the three vaccinated and challenged mice (panels D, E, and F) showed substantial inflammation at the tibiotarsal joint. Arrows indicate inflammation. Magnification, ×40.
FIG. 3.
Histopathology of the tibiotarsal joints of vaccinated mice (A), vaccinated mice treated with anti-IL-15 antibody (B), and nonvaccinated mice infected with B. bissettii (C). No histopathologic changes were detected in the vaccinated mice or vaccinated mice treated with anti-IL-15 antibody. Only minor inflammation (day 9) was observed in nonvaccinated infected mice. The arrow indicates an area of inflammation. Magnification, ×40.
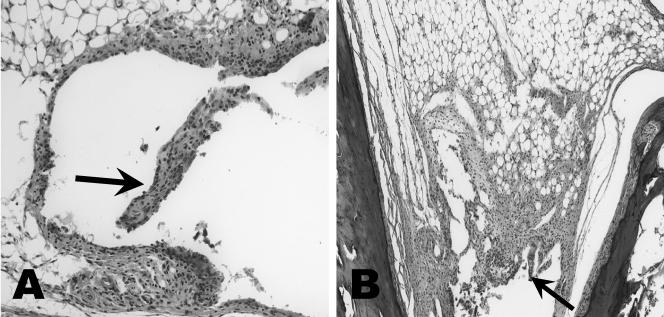
Fourteen days after challenge, considerable infiltration of neutrophils, macrophages, and lymphocytes was observed in the synovial space, synovium, and perisynovium of Borrelia-vaccinated and -infected mice with or without prior treatment with anti-IL-15 antibody (Fig. 4). Hyperplasia and vilus formation were also detected in the synovium of Borrelia-vaccinated and -infected mice treated with anti-IL-15. Treatment with anti-IL-15 antibody, however, was terminated in this group of mice 8 days previously. No significant histopathologic changes were detected in the remaining controls, including vaccinated mice with or without treatment with anti-IL-15 antibody, or in mice challenged with B. bissettii alone.
FIG. 4.
Histopathology of the tibiotarsal joint of Borrelia-vaccinated and -infected mice (day 14 after infection) with (A) and without (B) treatment with anti-IL-15 antibody. (A) Anti-IL-15 antibody treatment was discontinued 8 days previously or 6 days after infection. Inflammation of the tibiotarsal joint is present in both mice. Arrows indicate areas of inflammation. Magnification, ×28 (original magnification, ×40).
In a separate set of experiments, Borrelia-vaccinated and -infected mice were administered anti-IL-15 antibody 7 days after infection and daily thereafter for 6 days. Significant pathology was detected in the tibiotarsal joints of these mice. In fact, no differences in the swelling of the hind paw or histopathologic changes, compared to non-anti-IL-15 antibody-treated vaccinated and infected mice, were detected.
Effects of rIL-15 receptor alpha on arthritis.
Two groups of five vaccinated mice each were infected with 106 B. bissettii organisms. One of these two groups was administered rIL-15 receptor alpha at the time of infection and daily thereafter for 8 days. Controls included vaccinated mice injected with rIL-15 receptor alpha. Hind-paw swelling was detected in vaccinated mice challenged with B. bissettii on day 4 after infection, peaked on day 9, and gradually decreased. Treatment of vaccinated and challenged mice with rIL-15 receptor alpha delayed the onset of swelling of the hind paw and significantly (P < 0.05) decreased its severity at all intervals until day 8 after infection. No swelling of the hind paw was detected in vaccinated, but not infected, mice treated with rIL-15 receptor alpha. Histopathologic examination of the paws from each group of five mice confirmed that rIL-15 receptor alpha prevented severe inflammation of the hind paw.
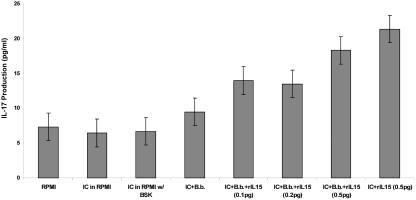
rIL-15 induces IL-17 production from mouse immune cells.
Inguinal lymph nodes were obtained from Borrelia-vaccinated mice at day 21 after vaccination. The lymph node cells were cocultured with or without 5 × 106 viable B. bissettii organisms for 48 h in the presence or absence of rIL-15. Figure 5 shows that immune lymph node cells incubated with 0.5 pg/ml of rIL-15 in the presence or absence of B. bissettii induced the production of 18 or more pg/ml of IL-17. Likewise, lower concentrations, 0.1 and 0.2 pg/ml, of rIL-15 in the presence of spirochetes also stimulated immune lymph node cells to release 14 and 13 pg/ml of IL-17, respectively. Immune lymph node cells cultured in RPMI medium alone or RPMI medium with BSK medium released less than 7 pg/ml of IL-17 (similar to background levels in RPMI medium alone), as did cells cultured with PBS containing 0.1% bovine serum albumin. When these experiments were repeated, comparable findings were obtained. Similar experiments conducted using the lymph nodes of nonvaccinated mice yielded no detectable IL-17 (data not shown).
FIG. 5.
The effects of rIL-15 (0.1, 0.2, or 0.5 pg) on production of IL-17 from lymph node cells obtained from Borrelia-vaccinated mice in the presence or absence of 4 × 106 viable B. bissettii organisms (B.b.). Control groups included supernatants from wells containing only RPMI medium or wells in which immune cells (IC) were cultured with RPMI medium, RPMI medium with BSK medium, or RPMI medium with BSK medium containing 4 × 106 viable B. bissettii organisms. Data are the means ± standard errors for the experiment.
DISCUSSION
In the present study, we hypothesized that IL-15 is a primary mediator involved in the induction of arthritis associated with Borrelia vaccination and infection of mice. In support of this hypothesis, we showed that treatment of Borrelia-vaccinated and -challenged mice with anti-IL-15 antibody prevented swelling of the hind paws. In addition, treatment of Borrelia-vaccinated and -infected mice with rIL-15 receptor alpha also prevented hind-paw swelling. More importantly, both anti-IL-15 antibody and rIL-15 receptor alpha-treated Borrelia-vaccinated and -challenged mice were free of major histopathologic indications of arthritis, including hyperplasia, hypertrophy, and vilus formation of the synovium. Similarly, the synovial space and perisynovium were free of inflammatory cells. By contrast, the synovium of non-anti-IL-15-treated Borrelia-vaccinated and -infected mice had overt hyperplasia, hypertrophy, and vilus formation. Moreover, the synovial space and perisynovium were infiltrated with neutrophils, macrophages, and lymphocytes. These results suggest that IL-15 plays a major role in the induction of arthritis associated with Borrelia-vaccinated and -infected mice.
Although IL-15 is involved in the induction of arthritis associated with Borrelia vaccination and infection, we showed previously that IL-17 is also a major participant in the induction of this arthritis (8). When Borrelia-vaccinated and -infected mice were administered anti-IL-17 antibody, development of arthritis was prevented (8, 32). Likewise, treatment of Borrelia-vaccinated and -infected mice with anti-IL-17 receptor antibody prevented arthritis, including cartilage and bone destruction (8). Taken together, these results show that both IL-15 and IL-17 play a significant role in arthritis induced by Borrelia vaccination and infection of mice.
What is the mechanism by which IL-15 and IL-17 drive the arthritis? Presumably, at the time of infection, some spirochetes are processed by neutrophils (14, 30, 41) and macrophages (15, 29), leading to the release of IL-15 from these cells (19, 31). Since IL-15 is rarely detected in culture supernatants or tissues (4, 31), it probably binds rapidly to the IL-15 receptor alpha, the IL-2 receptor beta, and the common gamma-chain located especially on memory T cells (4, 16, 17, 20). The memory cells, or Borrelia-vaccinated T lymphocytes, then release IL-17 that triggers the production of other proinflammatory cytokines, such as IL-1, IL-6, and IL-8 (3, 9, 21), that contribute to the development of arthritis. In support of this theory, we showed that rIL-15 caused the release of IL-17 in ex vivo cultures of immune lymph node cells isolated from a site adjacent to the hind paws. Ziolkowska et al. (43) and Kim et al. (24) also showed that IL-15 triggers the release of IL-17. Our results, as well as those of Ziolkowska et al. (43) and Kim et al. (24), show a definite connection between IL-15 and IL-17. Additional studies are needed to determine if IL-15 directly causes IL-17 production. This linkage is important for the potential therapy of Borrelia-induced arthritis as well as other inflammatory diseases (1, 9, 25). Clearly, therapy with anti-IL-15 or anti-IL-17 (8) antibody benefits vaccinated mice when it is started at the time of challenge with Borrelia organisms.
The arthritic mechanism, however, may be more complex than the simple linear stimulation of IL-17 production by IL-15. Histopathologic examination showed that treatment of Borrelia-vaccinated and -infected mice with anti-IL-15 antibody or rIL-15 receptor alpha dramatically decreased the infiltration of inflammatory cells at the tibiotarsal joint, especially neutrophils. In contrast, neutrophils dominated the cell infiltrate in non-anti-IL-15-treated Borrelia-vaccinated and -infected mice. It is known that resting neutrophils, along with infiltrating monocytes, express IL-2 receptor beta and IL-2 receptor gamma, which can readily bind high concentrations of IL-15 (43). We speculate that initially resting neutrophils exposed to the infectious challenge release IL-15. Subsequently, IL-15 binds to these IL-2 receptors as well as the high-affinity IL-15 receptor alpha (23). Interactions between IL-15 and its receptor complex lead to increased signaling and activation of neutrophils (19). Further activation of these neutrophils by IL-15 also increases their phagocytic ability (19). Therefore, IL-15 may have a dual role in the response to infection. It promotes the proinflammatory cytokine cascade via IL-17 production and may oversee the elimination of the initial spirochete challenge. In support of this theory, termination of anti-IL-15 treatment 6 days after challenge was followed immediately by an increase in hind-paw swelling in Borrelia-vaccinated and -challenged mice. Histological samples from these animals taken 14 days after challenge (8 days after treatment cessation) revealed dramatic infiltration of immune cells as well as vilus formation and destruction of the synovial lining. In addition, others (18) have shown that IL-15 can affect the migration and phagocytosis of neutrophils in patients with Lyme disease.
Although we showed that treatment of Borrelia-vaccinated mice at the time of challenge with anti-IL-15 antibody prevented arthritis, treatment with anti-IL-15 antibody failed to ameliorate the arthritis once it was established. Specifically, no resolution of arthritis was detected when Borrelia-vaccinated and -challenged mice were administered anti-IL-15 antibody 7 days after infection, as opposed to the case for controls. This was disappointing, and it suggests that anti-IL-15 therapy is not suitable for the treatment of established arthritis associated with Borrelia vaccination and infection. In contrast, Baslund et al. (7) have recently shown that treatment of humans with an antibody targeting IL-15 benefited patients with longstanding rheumatoid arthritis. This discrepancy may be related to differences in immune responses that maintain chronic arthritis in rheumatoid arthritis and arthritis induced by Borrelia burgdorferi. Additional studies are under way to determine which mediators could be neutralized to hasten the resolution of Lyme arthritis.
The failure of anti-IL-15 antibody to ameliorate established arthritis does not lessen the cytokine's clinical importance. A major concern with development of a Lyme disease vaccine is the induction of adverse effects, such as arthritis. Once a Lyme disease vaccine has been shown to be protective, it could also be tested for its ability to cause the release of IL-15 or other proinflammatory cytokines from cells (peripheral lymphocytes) obtained from humans or animals exposed to B. burgdorferi. If proinflammatory cytokines are detected, the vaccine could be modified to remove epitopes that promote adverse effects but do not influence the ability of the vaccine to induce protection.
Finally, several investigators (2, 31, 40) have shown that gamma interferon can up-regulate the production of IL-15. This suggests that gamma interferon may precede IL-15 in the cytokine cascade for the modulation of arthritis associated with Borrelia vaccination and challenge. However, our studies were performed using gamma interferon-deficient mice. Additional studies are needed to define specific cytokines, Borrelia antigens, or other immune factors that initiate the production of IL-15. Some of these approaches may provide a new perspective for the treatment of Borrelia-associated arthritis.
In conclusion, we show that IL-15 plays a major role in the induction of Lyme arthritis. Although rIL-15 treatment of immune cells led to increased production of IL-17 from these cells, additional studies are needed to determine if IL-15 solely promoted this production. Other studies are also needed to determine mediators that could be neutralized to enhance the resolution of established arthritis.
Acknowledgments
This study was supported by the Wisconsin State Laboratory of Hygiene, the public health laboratory for the state of Wisconsin, Madison, WI, and the Gundersen Medical Foundation, La Crosse, WI.
REFERENCES
- 1.Agostini, C., L. Trentin, M. Facco, R. Sancetta, A. Cerutti, C. Tassinari, L. Cimarosto, F. Adami, A. Cipriani, R. Zambello, and G. Semenzato. 1996. Role of IL-15, Il-2, and their receptors in the development of T cell alveolitis in pulmonary sarcoidosis. J. Immunol. 157:910-918. [PubMed] [Google Scholar]
- 2.Ajuwon, K. M., S. K. Jacobi, J. L. Kuske, and M. E. Spurlock. 2004. Interleukin-6 and interleukin-15 are selectively regulated by lipopolysaccharide and interferon-gamma in primary pig adipocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286:R547-R553. [DOI] [PubMed] [Google Scholar]
- 3.Albanesi, C., C. Scarponi, A. Cavani, M. Federici, F. Nasorri, and G. Girolomoni. 2000. Interleukin-17 is produced by both Th1 and Th2 lymphocytes, and modulates interferon-gamma- and interleukin-4-induced activation of human keratinocytes. J. Investig. Dermatol. 115:81-87. [DOI] [PubMed] [Google Scholar]
- 4.Bamford, R. N., A. P. Battiata, J. D. Burton, H. Sharma, and T. A. Waldmann. 1996. Interleukin (IL) 15/IL-T production by the adult T-cell leukemia cell line HuT-102 is associated with a human T-cell lymphotrophic virus type I region /IL-15 fusion message that lacks many upstream AUGs that normally attenuates IL-15 mRNA translation. Proc. Natl. Acad. Sci. USA 93:2897-2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barthold, S. W., D. S. Beck, G. M. Hansen, G. A. Terwilliger, and K. D. Moody. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162:133-138. [DOI] [PubMed] [Google Scholar]
- 6.Barthold, S. W., K. D. Moody, G. A. Terwilliger, P. H. Duray, R. O. Jacoby, and A. C. Steere. 1988. Experimental Lyme arthritis in rats infected with Borrelia burgdorferi. J. Infect. Dis. 157:842-846. [DOI] [PubMed] [Google Scholar]
- 7.Baslund, B., N. Tvede, B. Danneskiold-Samsoe, P. Larsson, G. Panayi, J. Petersen, L. J. Petersen, F. J. M. Beurskens, J. Schuurman, J. G. J. van de Winkel, P. W. H. I. Parren, J. A. Gracie, S. Jongbloed, F. Y. Liew, and I. B. McInnes. 2005. Targeting interleukin-15 in patients with rheumatoid arthritis. Arthritis Rheum. 52:2686-2692. [DOI] [PubMed] [Google Scholar]
- 8.Burchill, M. A., D. T. Nardelli, D. M. England, D. J. DeCoster, J. A. Christopherson, S. M. Callister, and R. F. Schell. 2003. Inhibition of interleukin-17 prevents the development of arthritis in vaccinated mice challenged with Borrelia burgdorferi. Infect. Immun. 71:3437-3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chabaud, M., F. Fossiez, J. L. Taupin, and P. Miossec. 1998. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation of Th2 cytokines. J. Immunol. 161:409-414. [PubMed] [Google Scholar]
- 10.Christopherson, J. A., E. L. Munson, D. M. England, C. L. Croke, M. C. Remington, M. L. Molitor, D. J. DeCoster, S. M. Callister, and R. F. Schell. 2003. Destructive arthritis in vaccinated interferon gamma-deficient mice challenged with Borrelia burgdorferi: modulation by tumor necrosis factor alpha. Clin. Diagn. Lab. Immunol. 10:44-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croke, C. L., E. L. Munson, S. D. Lovrich, J. A. Christopherson, M. C. Remington, D. M. England, S. M. Callister, and R. F. Schell. 2000. Occurrence of severe destructive Lyme arthritis in hamsters vaccinated with outer surface protein A and challenged with Borrelia burgdorferi. Infect. Immun. 68:658-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doherty, T. M., R. A. Seder, and A. Sher. 1996. Induction and regulation of IL-15 expression in murine macrophages. J. Immunol. 156:735-741. [PubMed] [Google Scholar]
- 13.Ferretti, S., O. Bonneau, G. R. Dubois, C. C. Jones, and A. Trifilieff. 2003. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J. Immunol. 170:2106-2112. [DOI] [PubMed] [Google Scholar]
- 14.Garcia, R., L. Gusmani, R. Murgia, C. Guarnaccia, M. Cinco, and G. Rottini. 1998. Elastase is the only human neutrophil granule protein that alone is responsible for in vitro killing of Borrelia burgdorferi. Infect. Immun. 66:1408-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gebbia, J. A., J. L. Coleman, and J. L. Benach. 2004. Selective induction of matrix metalloproteinases by Borrelia burgdorferi via toll-like receptor 2 in monocytes. J. Infect. Dis. 189:113-119. [DOI] [PubMed] [Google Scholar]
- 16.Giri, J. G., S. Kumaki, M. Ahdieh, D. J. Friend, A. Loomis, K. Shanebeck, R. DuBose, D. Cosman, L. S. Park, and D. M. Anderson. 1995. Identification and cloning of a novel IL-15 binding protein that is structurally related to the α chain of the IL-2 receptor. EMBO J. 14:3654-3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grabstein, K. H., J. Eisenman, K. Shanebeck, C. Rauch, S. Srinivasan, V. Fung, C. Beers, J. Richardson, M. A. Schoenborn, M. Ahdieh, et al. 1994. Cloning of a T cell growth factor that interacts with the β chain of the interleukin-2 receptor. Science 264:965-968. [DOI] [PubMed] [Google Scholar]
- 18.Jablonska, E., M. Marcinczyk, A. Izycka, and T. Hermanowska-Szpakowicz. 2003. Effect of interleukin 15 on the PMN activity in Lyme borreliosis. Pol. Merkuriusz Lek. 15:249-252. (In Polish.) [PubMed] [Google Scholar]
- 19.Jablonska, E., M. Marcinczyk, L. Tabarek, S. Pancewicz, T. Hermanowska-Szpakowicz, and J. Jablonski. 2003. IL-15 in the culture supernatants of PMN and PBMC and the serum of patients with Lyme disease. Rocz. Akad. Med. Bialymst. 48:78-81. (In Polish.) [PubMed] [Google Scholar]
- 20.Kakumu, S., A. Okumura, T. Ishikawa, M. Yano, A. Enomoto, H. Nishimura, K. Yoshioka, and Y. Yoshika. 1997. Serum levels of IL-10, IL-15 and soluble tumour necrosis factor-alpha (TNF-alpha) receptors in type C chronic liver disease. Clin. Exp. Immunol. 109:458-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz, Y., O. Nadiv, and Y. Beer. 2001. Interleukin-17 enhances tumor necrosis factor alpha-induced synthesis of interleukin 1, 6, and 8 in skin and synovial fibroblasts: a possible role as a “fine-tuning cytokine” in inflammation processes. Arthritis Rheum. 44:2176-2184. [DOI] [PubMed] [Google Scholar]
- 22.Keitel, W. A. 1999. Cellular and acellular pertussis vaccines in adults. Clin. Infect. Dis. 28(Suppl. 2):S118-S123. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy, M. K., and L. S. Park. 1996. Characterization of interleukin-15 (IL-15) and the IL-15 receptor complex. J. Clin. Immunol. 16:134-143. [DOI] [PubMed] [Google Scholar]
- 24.Kim, K. W., M. L. Cho, M. K. Park, C. H. Yoon, S. H. Park, S. H. Lee, and H. Y. Kim. 2004. Increased interleukin-17 production via a phosphoinositide 3-kinase/Akt and nuclear factor kappaB-dependent pathway in patients with rheumatoid arthritis. Arthritis Res. Ther. 7:R139-R148. [Online.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirman, I., and O. H. Nielsen. 1996. Increased numbers of interleukin-15-expressing cells in active ulcerative colitis. Am. J. Gastroenterol. 91:1789-1794. [PubMed] [Google Scholar]
- 26.Kurowska, M., W. Rudnicka, E. Kontny, I. Janicka, M. Chorazy, J. Kowalczewski, M. Ziolkowska, S. Ferrari-Lacraz, T. B. Strom, and W. Maslinski. 2002. Fibroblast-like synoviocytes from rheumatoid arthritis patients express functional IL-15 receptor complex: endogenous IL-15 in autocrine fashion enhances cell proliferation and expression of Bcl-x(L) and Bcl-2. J. Immunol. 169:1760-1767. [DOI] [PubMed] [Google Scholar]
- 27.Lim, L. C. L., D. M. England, B. K. DuChateau, N. J. Glowacki, J. R. Creson, S. D. Lovrich, S. M. Callister, D. A. Jobe, and R. F. Schell. 1994. Development of destructive arthritis in vaccinated hamsters challenged with Borrelia burgdorferi. Infect. Immun. 62:2825-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McInnes, I. B., J. al-Mughales, M. Field, B. P. Leung, F. P. Huang, R. Dixon, R. D. Sturrock, P. C. Wilkinson, and F. Y. Liew. 1995. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat. Med. 2:175-182. [DOI] [PubMed] [Google Scholar]
- 29.Montgomery, R. R., and S. E. Malawista. 1996. Entry of Borrelia burgdorferi into macrophages is end-on and leads to degradation in lysosomes. Infect. Immun. 64:2867-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrison, T. B., J. H. Weis, and J. J. Weis. 1997. Borrelia burgdorferi outer surface protein A (OspA) activates and primes human neutrophils. J. Immunol. 158:4838-4845. [PubMed] [Google Scholar]
- 31.Musso, T., L. Calosso, M. Zucca, M. Millesimo, D. Ravarino, M. Giovarelli, F. Malavasi, A. N. Ponzi, R. Paus, and S. Bulfone-Paus. 1999. Human monocytes constitutively express membrane-bound, biologically active, and interferon-γ-up-regulated interleukin-15. Blood 93:3531-3539. [PubMed] [Google Scholar]
- 32.Nardelli, D. T., M. A. Burchill, D. M. England, J. Torrealba, S. M. Callister, and R. F. Schell. 2004. Association of CD4+ CD25+ T cells with prevention of severe destructive arthritis in Borrelia burgdorferi-vaccinated and challenged gamma interferon-deficient mice treated with anti-interleukin-17 antibody. Clin. Diagn. Lab. Immunol. 11:1075-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puzanov, I. J., M. Bennett, and V. Kumar. 1996. IL-15 can substitute for marrow microenvironment in the differentiation of natural killer cells. J. Immunol. 157:4282-4285. [PubMed] [Google Scholar]
- 34.Rose, C. D., P. T. Fawcett, and K. M. Gibney. 2001. Arthritis following recombinant outer surface protein A vaccination for Lyme disease. J. Rheumatol. 28:2555-2557. [PubMed] [Google Scholar]
- 35.Ruchatz, H., B. P. Leung, X. Q. Wei, I. B. McInnes, and F. Y. Liew. 1998. Soluble IL-15 receptor alpha-chain administration prevents murine collagen-induced arthritis: a role for IL-15 in development of antigen-induced immunopathology. J. Immunol. 160:5654-5660. [PubMed] [Google Scholar]
- 36.Ruckert, R., K. Brandt, E. Bulanova, F. Mirghomizadeh, R. Paus, and S. Bulfone-Paus. 2003. Dendritic cell-derived IL-15 controls the induction of CD8 T cell immune responses. Eur. J. Immunol. 33:3493-3503. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz, J. L., R. F. Schell, A. Hejka, D. M. England, and L. Konick. 1988. Induction of Lyme arthritis in LSH hamsters. Infect. Immun. 56:2336-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steel, R. G. D., and J. H. Torrie. 1960. Principles and procedures of statistics with special references to the biological sciences, p. 90-160. McGraw-Hill Book Co., New York, N.Y.
- 39.Steere, A. C., R. T. Schoen, and E. Taylor. 1987. The clinical evolution of Lyme arthritis. Ann. Intern. Med. 107:725-731. [DOI] [PubMed] [Google Scholar]
- 40.Stegall, T., and K. A. Krolick. 2001. Myocytes respond in vivo to an antibody reactive with the acetylcholine receptor by upregulating interleukin-15: an interferon-gamma activator with the potential to influence the severity and course of experimental myasthenia gravis. J. Neuroimmunol. 119:377-386. [DOI] [PubMed] [Google Scholar]
- 41.Suhonen, J., K. Hartiala, and M. K. Viljanan. 1998. Tube phagocytosis, a novel way for neutrophils to phagocytize Borrelia burgdorferi. Infect. Immun. 66:3433-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkinson, P. C., and F. Y. Liew. 1995. Chemoattraction of human blood T lymphocytes by interleukin-15. J. Exp. Med. 181:1255-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ziolkowska, M., A. Koc, G. Luszczykiewicz, K. Ksiezopolska-Pietrzak, E. Klimczak, H. Chwalinska-Sadowska, and W. Maslinski. 2000. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporine A-sensitive mechanism. J. Immunol. 164:2832-2838. [DOI] [PubMed] [Google Scholar]