Abstract
To increase testing efficiency, a microsphere-based multianalyte immune detection (MAID) system was developed to measure serum immunoglobulin G (IgG) and IgA recognizing two Bordetella pertussis antigens, pertussis toxin (PT) and filamentous hemagglutinin antigen (FHA). The assay was performed as two separate duplexes. One duplex measured IgG to PT and FHA, and the other measured IgA to PT and FHA. The two duplexes were then combined and analyzed as a tetraplex. The MAID system and an in-house enzyme-linked immunosorbent assay (ELISA) system were used to evaluate 100 sera from blood donors and 220 consecutive sera submitted for B. pertussis antibody testing. For both the MAID and ELISA systems, antibody levels were defined as increased if greater than the blood donor group 95th percentile value. The qualitative concordance rates between MAID and ELISA results for the 220 consecutively submitted sera were as follows: PT IgG, 99%; PT IgA, 94%; FHA IgG, 93%; FHA IgA, 94%. The overall concordance rate was 95% (836 of 880 result sets). For 29 of 44 (66%) discordant result sets, the discordant MAID result was supported by the MAID and ELISA results for other B. pertussis antibodies. The MAID and in-house ELISA systems were also used to evaluate 20 sera previously tested for pertussis antibodies at a pertussis vaccine research laboratory; MAID results for all four analytes did not significantly differ from results obtained by the research laboratory. These findings show that antibodies to B. pertussis antigens can be measured easily and accurately using a tetraplex microsphere system.
Bordetella pertussis infection continues to pose a worldwide health problem for unvaccinated and incompletely immunized children (5). Recent studies have further shown that adolescents and adults serve as the main source of infection for neonates and infants (2-4, 6); surveillance studies estimate that >1,000,000 cases of pertussis occur annually in U.S. residents >15 years old (11). Susceptibility of adolescents and adults to B. pertussis infection coincides with the disappearance of pertussis-specific antibodies induced by vaccination during infancy;such antibodies typically last for only 6 to 10 years postvaccination (8, 11). Thus, adolescents and adults usually have low or undetectable levels of pertussis antibodies and mount a potent memory antibody response to pertussis antigens following exposure/infection (2, 5, 8, 11). Assays for these antibodies therefore serve as useful tools for diagnosing recent B. pertussis infection in persons over 11 years old.
The two major antigens utilized for pertussis antibody assays are pertussis toxin (PT) and filamentous hemagglutinin antigen (FHA). Because PT is produced only by B. pertussis (13), an increased level of PT antibodies is highly specific for B. pertussis infection. FHA antibodies are also typically increased following B. pertussis infection but are less specific than PT antibodies; antibodies cross-reactive with B. pertussis FHA may be produced following infection with other Bordetella species, Mycoplasma pneumoniae, Chlamydia pneumoniae, or nonencapsulated Haemophilus influenzae (3, 10). Detection of immunoglobulin G (IgG) to PT and FHA is more sensitive than detection of IgA, since not all exposed individuals mount a detectable IgA response (9, 13, 15).
We currently measure IgG and IgA recognizing PT and FHA using separate in-house enzyme-linked immunosorbent assays (ELISAs). These assays are labor intensive and require substantial amounts of antigens for coating plates. In the studies presented here, we evaluated an in-house multianalyte immune detection (MAID) system for measuring these antibodies. Central to the MAID system is the use of multiple polystyrene bead sets, each containing a distinctive proportion of red and orange fluorescent dyes and thus exhibiting a signature fluorescent pattern (1, 16). A given antigen is covalently linked to a given bead set, and then different bead sets are mixed together with human serum in a single reaction well. After washing and addition of a fluorescent reporter antibody recognizing a specific human immunoglobulin isotype, the bead mixture is analyzed using a modified flow cytometer. Each bead set is identified by its signature fluorescent pattern and is then analyzed for fluorescence characteristics of the reporter antibody. The reporter fluorescence intensity is directly proportional to the amount of analyte (antigen-specific antibody) bound to a given bead set (1, 16). This system thus enables the measurement of antibodies of a given isotype to many antigens in a single reaction well.
MATERIALS AND METHODS
Specimens.
The specimens used for the evaluation included 100 sera from Los Angeles area blood donors, 220 sera consecutively submitted to Focus Diagnostics Reference Laboratory for testing in a pertussis serology panel, and 20 sera previously tested for pertussis antibodies by the UCLA Center for Vaccine Research (CVR) using a well-characterized ELISA system (11).
MAID system for pertussis antibodies.
PT and FHA (List Biological Laboratories, Campbell, CA) were covalently linked to carboxylated microspheres (Luminex, Austin, TX) at a concentration of 5 μg per 6,250,000 beads of a given bead set, using a well-described two-step carbodiimide reaction (1, 16). PT was linked to bead sets 108 and 112, and FHA (List) was linked to bead sets 104 and 107. PT 108 and FHA 104 were combined to form duplex 1, and PT 112 and FHA 107 were combined to form duplex 2. A standard serum with assigned values (units/ml) traceable to Center for Biologic Evaluation and Research (CBER) standard lot 3 (PT IgG and FHA IgG) or standard lot 5 (PT IgA and FHA IgA) was serially diluted in phosphate-buffered saline (PBS) containing 1% bovine serum albumin; similarly, evaluation samples were diluted 1:50 in the same buffer. Standard and sample dilutions were added to an equal volume of each duplex (containing 2,500 beads of each bead set) in filter-bottom microtiter wells and incubated for 20 min at room temperature (RT). Following three washes with PBS-0.05% Tween 20 using a vacuum manifold, wells containing duplex 1 received phycoerythrin-labeled goat anti-human IgG (Jackson ImmunoResearch, West Grove, PA) diluted in PBS-0.05% Tween 20, whereas wells containing duplex 2 received phycoerythrin-labeled goat anti-human IgA (Jackson). After another 20-min incubation at RT and three washes, each duplex was resuspended in PBS containing 1% bovine serum albumin; the two duplexes for a given sample were then mixed and analyzed as a tetraplex using a Luminex 100 instrument. For each analyte, median fluorescent intensity (MFI) values were converted to units/ml by interpolation from the relevant standard curve.
In-house (Focus) ELISAs.
Separate ELISAs, modified from published procedures (7, 11, 15), were used to measure PT IgG, PT IgA, FHA IgG, and FHA IgA. Microtiter wells were coated with the same antigens used for the MAID system (0.2 μg/well). Diluted secondary standards (traceable to CBER standards) and samples were added to wells and incubated for 2 h at 37°C; after washing, wells received either horseradish peroxidase-labeled goat anti-human IgG or anti-IgA (Jackson) for 1 h at 37°C. After washing, wells received enzyme substrate (tetramethylbenzidine; Moss, Inc., Pasadena, MD) for 10 min at RT and then 1 N sulfuric acid (Ricca, Arlington, TX) to stop the reaction. Absorbance at 450 nM was measured, and sample results were expressed as units/ml based on the standard curves.
Discordance analysis.
The following criteria were used to resolve discordant results between the MAID system and the Focus ELISA system. For a given analyte, a finding of concordant normal results for the other three analytes was interpreted as support for the normal result in the discordant pair; similarly, a finding of concordant increased results for the other three analytes was interpreted as support for the increased result in the discordant pair. If these criteria were not met, additional criteria were applied. In the case of PT IgA discordant results, a finding of concordant (normal or increased) results for PT IgG was interpreted as support for the matching qualitative PT IgA result. Likewise, in the case of FHA IgG discordant results, a finding of concordant (normal or increased) results for PT IgG was interpreted as support for the matching qualitative FHA IgG result. In the case of FHA IgA discordant results, a finding of concordant (normal or increased) results for FHA IgG was interpreted as support for the matching qualitative FHA IgA result.
Comparison of MAID values and Focus ELISA values to CVR ELISA values.
To determine if MAID and Focus ELISA methods gave values significantly different from the CVR ELISA method, results generated by the three methods for the 20 sera supplied by CVR were log transformed and analyzed using a mixed linear model to account for random effects at the subject level; fixed effects were estimated for the compared method. Statistical models were fitted using the analysis package R (17; http://www.r-project.org). Due to the small sample size, significance was defined as a P value of <0.01.
RESULTS
Demonstration that duplex mixing does not lead to false-positive results due to conjugate migration.
Before instituting our plan to mix one duplex for IgG detection and a second duplex for IgA detection for analysis as a tetraplex, it was necessary to demonstrate that conjugate specifically attached to one bead set did not migrate to the comparable bead set after the two duplexes were mixed. We needed to show, for example, that anti-IgG conjugate attached to IgG-coated PT beads in duplex 1 did not detach and bind to the IgG attached to PT beads in duplex 2. To accomplish this task, a specimen known to be positive for all four analytes under investigation was used to treat duplex 1 and duplex 2 separately per the routine procedure; the duplexes were then differentially treated with the relevant conjugates (Table 1), washed, mixed, and analyzed as a tetraplex. As expected, the tetraplex where conjugate was added per the routine procedure (anti-IgG added to duplex 1, anti-IgA added to duplex 2) showed high MFI values for all four bead sets. In contrast, the tetraplex where anti-IgA conjugate was not added to duplex 2 showed high MFI values only for duplex 1 bead sets; in other words, anti-IgG bound to duplex 1 beads did not dissociate from these beads and attach to duplex 2 beads. Similarly, the tetraplex where anti-IgG was omitted from duplex 1 showed high MFI values only for duplex 2 beads, demonstrating that anti-IgA bound to duplex 2 beads did not migrate to duplex 1 beads.
TABLE 1.
Bound conjugate does not migrate during analysis from one bead set to the comparable bead set bearing antibodies of the same isotypea
| Conjugate(s) added (duplex 1, duplex 2) | MFI values forb:
|
|||
|---|---|---|---|---|
| Duplex 1 beads
|
Duplex 2 beads
|
|||
| PT 108 | FHA 104 | PT 112 | FHA 107 | |
| Anti-IgG, anti-IgA | 7,253 | 16,837 | 1,082 | 2,608 |
| Anti-IgG, none | 6,318 | 16,015 | 6 | 8 |
| None, anti-IgA | 4 | 4 | 1,001 | 2,442 |
Duplex 1 beads and duplex 2 beads were treated with a serum specimen known to contain antibodies to all four analytes; after washing, the duplexes were differentially treated with conjugate as indicated in the left column. Following washing, the two duplexes (on the same line in the table) were mixed and analyzed as a tetraplex. High MFI values were associated only with the duplex receiving conjugate, indicating that there was no migration of conjugate from one duplex to the other duplex.
MFI values were determined after duplexes were mixed and analyzed as a tetraplex.
MAID standard curves and assay performance characteristics.
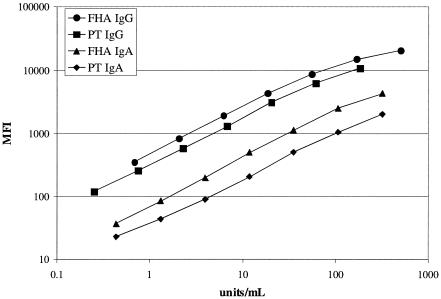
Standard curves for the four analytes from a representative assay run are shown in Fig. 1. All curves showed good linearity from <1 unit/ml to at least 200 units/ml. The MAID assay demonstrated within-run coefficients of variation of ≤10% for all analytes, as assessed using one serum sample containing increased levels of all four analytes and another serum sample containing normal levels of all four analytes (data not shown). Between-run coefficients of variation were ≤16% for the sample with increased antibody levels and ≤23% for the sample with normal antibody levels (data not shown). A comparison of three separate lots of bead sets demonstrated good reproducibility of the procedure for coupling antigens to beads; in pairwise linear regression analysis of quantitative antibody values for 16 specimens tested using each of the three lots of bead sets, R2 values of >0.95 were obtained for all analytes (data not shown).
FIG. 1.
Standard curves from a representative MAID assay. Units/ml represents the assigned quantitative value for the indicated standard point.
Control group 95th percentile values for pertussis antibodies.
One hundred sera from Los Angeles area blood donors were tested for pertussis antibodies by both the MAID and Focus ELISA procedures. For each analyte, a value less than or equal to the 95th percentile value was defined as normal, and higher values were defined as increased. For the MAID assays, the 95th percentile values (units/ml) were as follows: PT IgG, 44; PT IgA, 7; FHA IgG, 80; FHA IgA, 38. The comparable values for the Focus ELISAs were as follows: PT IgG, 22; PT IgA, 4; FHA IgG, 97; FHA IgA, 62.
Concordance between MAID and in-house ELISA results and resolution of discordant result sets.
Pertussis antibodies were measured by both the MAID and Focus ELISA procedures in 220 consecutive sera submitted for pertussis serologic analysis, and the results were defined as normal or increased as described in the previous section. Data for qualitative agreement between MAID and ELISA results are shown in Table 2. Concordance rates for the four analytes ranged from 93% to 99%, with a combined concordance rate of 95%. For the 44 discordant result sets, the other MAID and ELISA pertussis antibody results were examined in an effort to identify support for one of the discordant results. The findings from this analysis are shown in Table 3. Data for the other pertussis antibodies supported the MAID result for 29 discordant result sets (66%) and supported the ELISA result for 10 discordant result sets (23%); the other antibody results did not clearly support either the MAID or ELISA result for 5 result sets (11%).
TABLE 2.
MAID versus Focus ELISA: qualitative reactivity patterns for 220 consecutive sera submitted for B. pertussis antibody testing
| Reactivity pattern | No. of sera exhibiting the indicated reactivity pattern for:
|
Total | |||
|---|---|---|---|---|---|
| PT IgG | PT IgA | FHA IgG | FHA IgA | ||
| Concordant normal | 191 | 195 | 174 | 186 | 746 |
| Concordant increased | 26 | 12 | 31 | 21 | 90 |
| MAID increased, ELISA normal | 0 | 10 | 1 | 11 | 22 |
| MAID normal, ELISA increased | 3 | 3 | 14 | 2 | 22 |
| Total (%) concordant | 217 (99) | 207 (94) | 205 (93) | 207 (94) | 836 (95) |
| Total (%) discordant | 3 (1) | 13 (6) | 15 (7) | 13 (6) | 44 (5) |
TABLE 3.
Resolution of qualitatively discordant MAID versus Focus ELISA results
| Analyte | Discordant pattern
|
Other results support
|
||||
|---|---|---|---|---|---|---|
| MAID result | ELISA result | No. of sera | Method | No. of sera | Explanation | |
| PT IgG | Normal | Increased | 3 | Unclear | 2 | FHA IgG concordant increased, but PT IgA concordant normal or discordant |
| MAID | 1 | All other analytes concordant normal | ||||
| PT IgA | Increased | Normal | 10 | Unclear | 1 | PT IgG discordant |
| MAID | 7 | PT IgG concordant increased | ||||
| ELISA | 2 | All other analytes concordant normal | ||||
| Normal | Increased | 3 | MAID | 2 | All other analytes concordant normal | |
| ELISA | 1 | PT IgG concordant increased | ||||
| FHA IgG | Normal | Increased | 14 | MAID | 11 | All other analytes concordant normal |
| ELISA | 3 | PT IgG concordant increased | ||||
| Increased | Normal | 1 | MAID | 1 | PT IgG concordant increased | |
| FHA IgA | Increased | Normal | 11 | Unclear | 2 | FHA IgG discordant |
| MAID | 6 | FHA IgG concordant increased | ||||
| ELISA | 3 | All other analytes concordant normal | ||||
| Normal | Increased | 2 | MAID | 1 | FHA IgG concordant normal | |
| ELISA | 1 | FHA IgG concordant increased | ||||
Quantitative comparison of MAID and in-house ELISA results with CVR results.
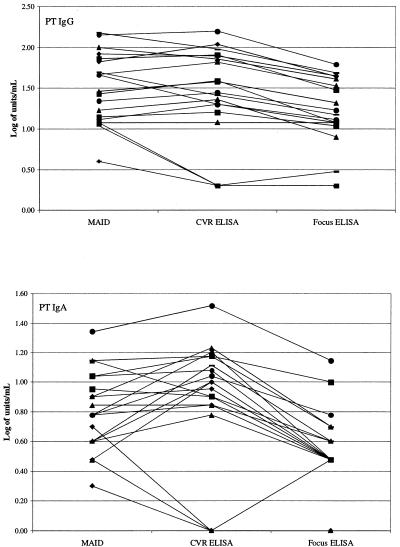
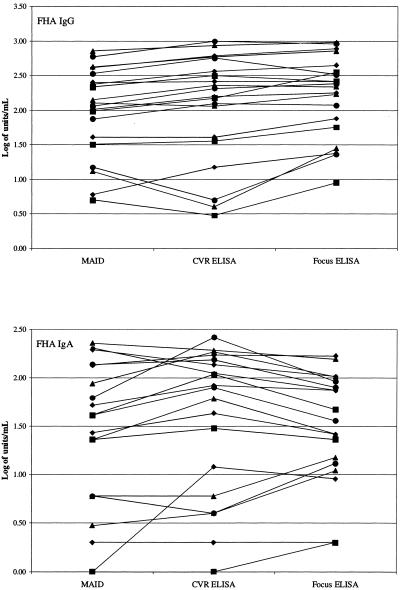
To demonstrate that pertussis antibody results determined by MAID were accurate, the MAID and Focus ELISA procedures were used to measure pertussis antibodies in 20 sera supplied by the UCLA CVR; these sera had previously been tested for pertussis antibodies at CVR using their well-characterized ELISA system (11). PT IgG and IgA results obtained in the three assays are shown in Fig. 2, and FHA IgG and IgA results are shown in Fig. 3. When examining Fig. 2 and 3, it is important to remember that log-transformed values of <0.7 correspond to actual values of <5 units/ml; thus, the differences in low values appear exaggerated (e.g., PT IgA in Fig. 2). Statistical analysis revealed that, for all four analytes, the MAID results did not significantly differ from the CVR ELISA results; in contrast, the Focus ELISA results were significantly lower than the CVR ELISA results for PT IgG and PT IgA.
FIG. 2.
Comparison of PT antibody results obtained by MAID or Focus ELISA to CVR ELISA results. Each line connects the results obtained for a single specimen.
FIG. 3.
Comparison of FHA antibody results obtained by MAID or Focus ELISA to CVR ELISA results. Each line connects the results obtained for a single specimen.
DISCUSSION
Our findings demonstrate that antibodies to B. pertussis antigens can be measured easily and accurately using a multiplex microsphere fluorescent assay. Results obtained using the MAID procedure showed very good qualitative agreement with results obtained using our in-house ELISA system. Further, from a quantitative standpoint, MAID results did not significantly differ from those obtained by another laboratory that routinely measures pertussis antibodies using a well-characterized ELISA system.
The multiplex microsphere instruments currently available are not configured to allow measurement of different antibody isotypes recognizing multiple immobilized antigens in a single reaction well. Thus, we performed two duplex assays concurrently, one for IgG antibodies and one for IgA antibodies, and then mixed the two duplexes together for analysis. Although each duplex could have been analyzed separately, mixing to form a tetraplex allowed analysis in half the time. Initial experiments indicated that the antigen-coated bead-antibody-conjugate complexes formed prior to duplex mixing were stable and that their fluorescent signals were reliably measured in the tetraplex format.
Our primary requirement for an acceptable MAID assay was that it compare favorably to the Focus ELISA system in its power to discriminate between normal and increased antibody levels. We thus used the 95th percentile value for a group of 100 blood donor sera to define the discrimination point between normal and increased antibody levels for each analyte and then qualitatively assessed concordance between these two methods for a large panel of sera submitted for pertussis serology. The concordance rates were ≥93% for all four analytes, indicating good qualitative agreement between MAID and Focus ELISA methods. Other pertussis antibody results supported the MAID result for two-thirds of discordant datasets, suggesting that MAID results more often represented the correct results.
The availability of serum standards has enabled the development of pertussis antibody ELISAs that generate comparable results across laboratories (12, 14). We thus sought to demonstrate that the results produced by our new MAID assay were comparable to those generated by another laboratory. Using a small serum panel supplied by CVR, we found that MAID assay results did not significantly differ from those produced by the CVR ELISA system, a well-characterized group of assays that has been used to measure both infection-induced and vaccine-induced antibodies (11). MAID systems for measuring pertussis antibodies should thus prove to be acceptable alternatives to ELISAs.
In our experience, a MAID system offers several advantages over an ELISA system for measuring pertussis antibodies. These advantages include less specimen volume, shorter and fewer incubation periods, and room temperature incubations. Further, the MAID system required lower amounts of antigens per reportable result; the quantity of PT or FHA required to coat 2,500 beads (the bead number used to measure one antibody isotype for one patient specimen) was 100-fold less than the quantity used to coat an ELISA well. Another benefit of a MAID system is the ease with which it can be modified to increase the number of analytes measured, without increasing the required sample volume or assay time. For example, additional bead sets coated with other proteins contained in the current acellular pertussis vaccines (e.g., pertactin, fimbriae) (3) could be added to the current duplexes, allowing measurement of antibodies to all vaccine components in a single analysis well. Thus, laboratories measuring multiple pertussis antibodies in large numbers of samples should see improved efficiency with the institution of MAID assays.
Acknowledgments
We thank James Cherry and Joel Ward of the UCLA Center for Vaccine Research for supplying specimens and helpful discussions.
REFERENCES
- 1.Biagini, R. E., D. L. Sammons, J. P Smith, B. A. MacKenzie, C. A. F. Striley, V. Semenova, E. Steward-Clark, K. Stamey, A. E. Freeman, C. P. Quinn, and J. E. Snawder. 2004. Comparison of a multiplexed fluorescent covalent microsphere immunoassay and an enzyme-linked immunosorbent assay for measurement of human immunoglobulin G antibodies to anthrax toxin. Clin. Diagn. Lab. Immunol. 11:50-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cherry, J. D. 1998. Pertussis in adults. Ann. Int. Med. 128:64-66. [DOI] [PubMed] [Google Scholar]
- 3.Cherry, J. D., S.-J. Chang, D. Klein, M. Lee, S. Barenkamp, D. Bernstein, R. Edelman, M. D. Decker, D. P. Greenberg, W. Keitel, J. Treanor, and J. I. Ward. 2004. Prevalence of antibody to Bordetella pertussis antigens in serum specimens obtained from 1793 adolescents and adults. Clin. Infect. Dis. 39:1715-1718. [DOI] [PubMed] [Google Scholar]
- 4.Deville, J. G., J. D. Cherry, P. D. Christenson, E. Pineda, C. T. Leach, T. L. Kuhls, and S. Viker. 1995. Frequency of unrecognized Bordetella pertussis infections in adults. Clin. Infect. Dis. 21:639-642. [DOI] [PubMed] [Google Scholar]
- 5.Forsyth, K. D., M. Campins-Marti, J. Caro, J. D. Cherry, D. Greenberg, N. Guiso, U. Heininger, J. Schellekens, C.-H. Wirsing von Konig, and S. Plotkin. 2004. New pertussis vaccination strategies beyond infancy: recommendations by the global pertussis initiative. Clin. Infect. Dis. 39:1802-1809. [DOI] [PubMed] [Google Scholar]
- 6.Guris, D., P. M. Strebel, B. Bardenheier, M. Brennan, R. Tachdjian, E. Finch, M. Wharton, and J. R. Livengood. 1999. Changing epidemiology of pertussis in the United States: increasing reported incidence among adolescents and adults, 1990-1996. Clin. Infect. Dis. 28:1230-1237. [DOI] [PubMed] [Google Scholar]
- 7.Halperin, S. A., R. Bortolussi, and A. J. Wort. 1989. Evaluation of culture, immunofluorescence, and serology for the diagnosis of pertussis. J. Clin. Microbiol. 27:752-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heininger, U., J. D. Cherry, and K. Stehr. 2004. Serologic response and antibody titer decay in adults with pertussis. Clin. Infect. Dis. 38:591-594. [DOI] [PubMed] [Google Scholar]
- 9.Hodder, S. L., J. D. Cherry, E. A. Mortimer, Jr., A. B. Ford, J. Gornbein, and K. Papp. Antibody responses to Bordetella pertussis antigens and clinical correlation in elderly community residents. Clin. Infect. Dis. 31:7-14. [DOI] [PubMed]
- 10.Isacson, J., B. Trollfors, J. Taranger, and T. Lagergard. 1995. Acquisition of IgG serum antibodies against two Bordetella antigens (filamentous hemagglutinin and pertactin) in children with no symptoms of pertussis. Pediatr. Infect. Dis. J. 14:517-521. [DOI] [PubMed] [Google Scholar]
- 11.Le, T., J. D. Cherry, S.-J. Chang, M. D. Knoll, M. L. Lee, S. Barenkamp, D. Bernstein, R. Edelman, K. M. Edwards, D. Greenberg, W. Keitel, J. Treanor, and J. I. Ward. 2004. Immune responses and antibody decay after immunization of adolescents and adults with an acellular pertussis vaccine: the APERT study. J. Infect. Dis. 190:535-544. [DOI] [PubMed] [Google Scholar]
- 12.Lynn, F., G. F. Reed, and B. D. Meade. 1996. Collaborative study for the evaluation of enzyme-linked immunosorbent assays used to measure human antibodies to Bordetella pertussis antigens. Clin. Diagn. Lab. Immunol. 3:689-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchant, C. D., A. M. Loughlin, S. M. Lett, C. W. Todd, L. H. Wetterlow, R. Bicchieri, S. Higham, P. Etkind, E. Silva, and G. R. Siber. 1994. Pertussis in Massachusetts, 1981-1991: incidence, serologic diagnosis, and vaccine effectiveness. J. Infect. Dis. 169:1297-1305. [DOI] [PubMed] [Google Scholar]
- 14.Meade, B. D., A. Deforest, K. M. Edwards, T. A. Romani, F. Lynn, C. H. O'Brien, C. B. Swartz, G. F. Reed, and M. A. Deloria. 1995. Description and evaluation of serologic assays used in a multicenter trial of acellular pertussis vaccines. Pediatrics 96:570-575. [PubMed] [Google Scholar]
- 15.Muller, F.-M. C., J. E. Hoppe, and C.-H. Wirsing von Konig. 1997. Laboratory diagnosis of pertussis: state of the art in 1997. J. Clin. Microbiol. 35:2435-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Opalka, D., C. E. Lachman, S. A. MacMullen, K. U. Jansen, J. F. Smith, N. Chirmule, and M. T. Esser. 2003. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed Luminex assay. Clin. Diagn. Lab. Immunol. 10:108-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.R Development Core Team. 2004. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.