Abstract
Anthrax toxin consists of protective antigen (PA) and two toxic components, lethal factor (LF) and edema factor (EF). PA binds to mammalian cellular receptors and delivers the toxic components to the cytoplasm. PA is the primary antigenic component of the current anthrax vaccine. Immunity is due to the generation of antibodies that prevent the PA-mediated internalization of LF and EF. In this study, we characterized sera obtained from vaccinated military personnel. Anthrax vaccine is administered in a series of six injections at 0, 2, and 4 weeks and 6, 12, and 18 months, followed by annual boosters. The vaccination histories of the subjects were highly varied; many subjects had not completed the entire series, and several had not received annual boosters. We developed a simple colorimetric assay using alamarBlue dye to assess the antibody-mediated neutralization of LF-mediated toxicity to the J774A.1 murine macrophage cell line. Recently vaccinated individuals had high antibody levels and neutralizing activity. One individual who had not been boosted for 5 years had low immunoglobulin G antibody levels but a detectable neutralization activity, suggesting that this individual produced low levels of very active antibodies.
A major virulence factor of anthrax is a unique, tripartite toxin (5). The enzymatically active domains, lethal factor (LF) and edema factor (EF), are produced as separate proteins. Alone, they lack toxic activity because they cannot enter mammalian cells. Toxic function requires a third protein, protective antigen (PA), which binds to cell surface receptors (4, 7, 26). Following proteolytic processing, cell-associated PA oligomerizes and binds LF or EF, and the complex is internalized by receptor-mediated endocytosis. Acidification of the endosome induces a conformational change, allowing the complex to insert into the membrane, forming a pore that enables the enzymatic portion (EF or LF) to enter the cytoplasm and seek the molecular target for its toxic activity (for a review, see reference 7). LF is a protease, and EF is a calmodulin-activated adenylate cyclase. Both toxins cause cellular disregulation and are particularly effective at altering the ability to generate a productive immune response. LF targets cells involved in generating the adaptive immune response (1). In contrast, EF targets innate immune defenses, in particular, phagocytosis and clearance by neutrophils (19).
The current human vaccine for anthrax is based on developing toxin-neutralizing antibodies following immunization with PA. The anthrax vaccine used by the U.S. Armed Forces, anthrax vaccine adsorbed, or BioThrax, is formulated from an aluminum hydroxide-adsorbed, cell-free, formalin-treated filtrate culture of strain V770-NP1-R, a toxigenic, noncapsulated, and nonproteolytic strain (27). This strain has been reported to produce high levels of PA, whereas levels of LF and EF are minimal.
The generation of protective immunity requires a lengthy immunization schedule that consists of the initial inoculation; inoculations at 2 weeks, 4 weeks, 6 months, 12 months, and 18 months; and then a yearly booster. While anthrax vaccine adsorbed has been shown to protect animals from both cutaneous- and inhalational-anthrax challenges (14), its effectiveness in humans has been more difficult to ascertain. One study in 1962 (3) examined the incidence of anthrax among workers in the animal industry, comparing vaccinated and unvaccinated individuals over a period of 4 years. In all, 26 cases of anthrax were reported. Only one of those cases occurred in the fully vaccinated group, and four cases occurred in the partially vaccinated group. The remaining 21 cases occurred in the unvaccinated group (27).
Aside from this report, much of what is known regarding the ability of the anthrax vaccine to generate protective immunity in humans has been inferred from in vitro studies that assess the development of neutralizing antibodies. Currently, the most widely used in vitro assay to assess vaccine efficacy measures the presence of serum antibody that neutralizes LF toxicity to a mouse macrophage-like cell line, J774A.1 (9, 11, 15, 18, 22, 23). Cellular viability following toxin treatment is determined by a colorimetric assay based upon the chemical reduction of 3-(4-5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) by living cells. However, to quantify the amount of reduced MTT, the cells must be lysed and the dye must be solubilized before the values can be determined spectrophotometrically.
We developed a variation of this assay that is simpler to perform using alamarBlue as an indicator of cell survival. alamarBlue is a nontoxic, fluorogenic indicator (2). In its oxidized form, alamarBlue is nonfluorescent and blue. As with MTT, metabolically active cells can internalize alamarBlue and convert the oxidized form to the reduced form. The reduced form is fluorescent and pink. Cellular viability, as reflected by the amount of metabolic activity, is assessed by determining the amount of reduced alamarBlue. Unlike with MTT, quantification of the amount of reduced alamarBlue can be done in the presence of viable cells and does not require a cell lysis step. Furthermore, sequential readings can be taken without killing the cells. In this study, the ability of sera from vaccinated individuals to neutralize LF toxicity to J774A.1 cells was assessed using the alamarBlue assay and compared to antibody titers to PA as determined by enzyme-linked immunosorbent assay (ELISA) and Western blotting.
MATERIALS AND METHODS
PA, LF, and anti-PA antibody were obtained from List Biological Laboratories (Campbell, CA). Goat anti-human antibody was obtained from Cappel (Aurora, OH).
Serum sample collection.
Serum samples were obtained from retired military personnel and active personnel stationed at Wright-Patterson Air Force Base near Dayton, OH. Samples were collected in a single 10-ml Vacutainer tube (Becton, Dickinson and Company, Franklin Lakes, NJ) and allowed to clot for 30 min. The tubes were centrifuged at 1,500 × g for 10 min. The sera were collected in a sterile tube and placed on dry ice for transport. Samples were thawed, heat inactivated (56°C for 30 min), and filter sterilized using a 0.22-μm cellulose acetate centrifuge tube filter (Costar, Corning, Inc., Corning, NY). Each sample was aliquoted and stored at −80°C. For a negative control, sera were drawn from five individuals presumed to have had no exposure to anthrax or the vaccine, pooled, heat inactivated, and then filter sterilized as described above.
ELISA analysis of serum.
Serum samples were characterized using an anthrax protective-antigen IgG ELISA (Bio-Quant, Inc., San Diego, CA), which was performed according to the manufacturer's instructions. Samples were prepared by adding 5 μl of serum to 200 μl of sample diluent. One-hundred-microliter samples of the sample, calibrator, positive control, and negative control were added to each of three wells, and 100 μl of the sample diluent was added to the blank well. The plate was incubated at room temperature for 30 min, and then all wells were washed three times with wash buffer using an automated plate washer (ELx405 plate washer; BIO-TEK, Winooski, VT). The plate was read on the plate reader (BIO-TEK ELx800) at 450 nm, and the level of anti-PA antibodies was determined. Antibody index (AI) values, or increases (n-fold) over the cutoff value, were calculated according to the manufacturer's instructions.
Western blot analyses.
PA (5 μg/ml or 20 μg/ml) was mixed with equal volumes of 2× sample buffer plus β-mercaptoethanol (62.5 mM Tris-HCl [pH 6.8], 20% glycerol, 2% sodium dodecyl sulfate, 5% β-mercaptoethanol), boiled for 10 min, and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using 10% Tris-glycine precast gels (Cambrex Bio Science Rockland, Inc., Rockland, ME). The proteins were transferred to polyvinylidene difluoride membranes (Millipore Corp., Bedford, MA) using a semidry transfer device (Fisher Biotech, Fisher Scientific, Pittsburgh, PA). The membranes were incubated in blocking buffer (5 g nonfat dairy milk-500 ml of phosphate-buffered saline blocking buffer [0.08 M Na2HPO4, 0.025 M NaH2PO4, 0.1 M NaCl]) and then washed three times in wash buffer (1.25 g nonfat dairy milk and 2.5 ml Tween 20 in 500 ml of phosphate-buffered saline blocking buffer). Human serum samples were diluted 1:500 in wash buffer, and the positive control, goat anti-PA (List Biological Laboratories), was diluted 1:7,500. The membranes were incubated with the sera for 1 h at room temperature and washed three times with wash buffer. Peroxidase-conjugated goat anti-human immunoglobulin (ICN/Cappel, Aurora, OH) added at a 1:2,000 dilution was used for human samples, and peroxidase-conjugated rabbit anti-goat (Cappel, West Chester, PA) added at a 1:20,000 dilution was used for the control anti-PA. The membranes were incubated for 1 h and washed three times in wash buffer, and the bands were visualized using Western Lightning Chemiluminescence Reagent Plus (PerkinElmer Life Sciences, Boston, MA).
Mammalian cells.
Primary human cells, including neutrophils, monocytes, macrophages, and peripheral blood mononuclear cells, were obtained from human blood donors as previously described (17, 25, 29). J774A.1 cells obtained from the American Type Culture Collection (ATCC) (Manassas, VA) were grown in Dulbecco's modified Eagle medium (high glucose with l-glutamine; Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (10,000 U/ml penicillin G sodium and 10,000 μg/ml streptomycin sulfate) for 3 days. Human umbilical vein endothelial cells (Clonetics, San Diego, CA) were grown in endothelial growth medium (Clonetics) supplemented with bovine brain extract. The human monocytic cell line U934, obtained from the ATCC, and the mouse monocytic cell line WEHI3, kindly provided by John Monaco, University of Cincinnati College of Medicine, were grown in RPMI 1640 medium (Invitrogen) supplemented with 10% FBS and 1% penicillin-streptomycin. Chinese hamster ovary (CHO) cells obtained from the ATCC were grown in Ham's F-12 nutrient mixture (Invitrogen) supplemented with 10% FBS and 1% penicillin-streptomycin.
alamarBlue assay to detect antibody-mediated LeTx neutralization.
J774A.1 cells were harvested, and viable-cell concentration was determined by trypan blue exclusion using a hemacytometer. Cells were brought to a final concentration of 106 cells/ml, and 100 μl was added to a 96-well flat-bottomed tissue culture plate and incubated overnight. For neutralization assays, duplicate twofold dilutions of goat anti-PA antibody or human serum were prepared on a 96-well plate. PA and LF were added to one dilution series to generate lethal toxin (LeTx), and an equivalent volume of Dulbecco's modified Eagle medium was added to the second dilution series to serve as a negative control. The medium was removed from the cells and replaced with 100 μl of the serum dilutions. The final concentration of PA and LF was 0.1 μg/ml. The plates were incubated for 4 h at 37°C in 5% CO2. To serve as a negative control, cells in some wells were lysed by the addition of 100 μl of Triton X-100 prior to the addition of alamarBlue. alamarBlue (80% solution in Hanks' balanced salt solution; Trek Diagnostic Systems, Inc., Westlake, OH) was added at 10% of the well volume, and the cells were incubated for 20 h at 37°C in 5% CO2. Absorbance at 570 and 595 nm was determined using a BIO-TEK Elx800 plate reader, and the percentage of reduced alamarBlue was determined. Data were analyzed using Student's paired t test.
RESULTS
The vaccine histories of the subjects enrolled in this study are summarized in Table 1. Only one subject (sample JHUC14) (Table 1) received all six inoculations, but this subject had not been boosted in 5 years. Twelve of the 16 subjects were receiving their primary immunization series during the study, 10 subjects had received five of the six inoculations, and 1 subject did not provide these data. Three subjects had not received the complete series. JHUC26 and JHUC27 are samples from the same subject drawn 18 months apart. Sera were also drawn from five individuals presumed to have had no exposure to anthrax or the vaccine and pooled to serve as a negative control (pool) (Table 1). The serum samples were examined for immune responses to PA in three ways: by Western blot analysis, by an anti-PA ELISA, and by testing for the ability to neutralize LeTx.
TABLE 1.
Characteristics of the samples used in this studyd
| Sample ID | No. of mos since last shot | No. of inoculations | Western blot resulta | Antibody index | Neutralization titerb |
|---|---|---|---|---|---|
| JHUC17 | 0.17 | NP | + | 2.8 | 1,024 |
| JHUC19 | 0.17 | 5 | + | 2.6 | 1,024 |
| JHUC25 | 0.17 | 5 | + | 1.7 | 256 |
| JHUC13 | 1 | 5 | + | 2.8 | 512 |
| JHUC15 | 1 | 5 | + | 3.0 | 2,048 |
| JHUC16 | 1 | 5 | + | 2.8 | 2,048 |
| JHUC20 | 1 | 5 | + | 2.6 | 2,048 |
| JHUC22 | 1 | 5 | + | 2.4 | 1,024 |
| JHUC18 | 2 | 5 | + | 1.7 | 256 |
| JHUC24 | 3 | 5 | + | 2.0 | 1,024 |
| JHUC23 | 6 | 4 | + | 2.1 | 512 |
| JHUC12 | 9 | 5 | + | 3.0 | 1,024 |
| JHUC26c | 19 | 3 | +/− | 1.6 | <4 |
| JHUC27c | 37 | 3 | − | 1.6 | <4 |
| JHUC21 | 45 | 5 | + | 1.2 | 128 |
| JHUC14 | 60 | 6 | +/− | 1.2 | 64 |
| JHUC28 | 96 | 2 | − | 1.1 | <4 |
| Pool | NA | 0 | − | 1.3 | <4 |
| ELISA control | 0.37 |
+, strong reactivity; +/−, weak reactivity observed at high PA concentrations; −, no reactivity detected.
A neutralization titer of 4 was determined to be the limit of detection.
Specimens JHUC26 and JHUC27 were drawn from the same donor, approximately 18 months apart.
NP, no data provided; NA, not applicable; ID, identification.
Western blot analysis.
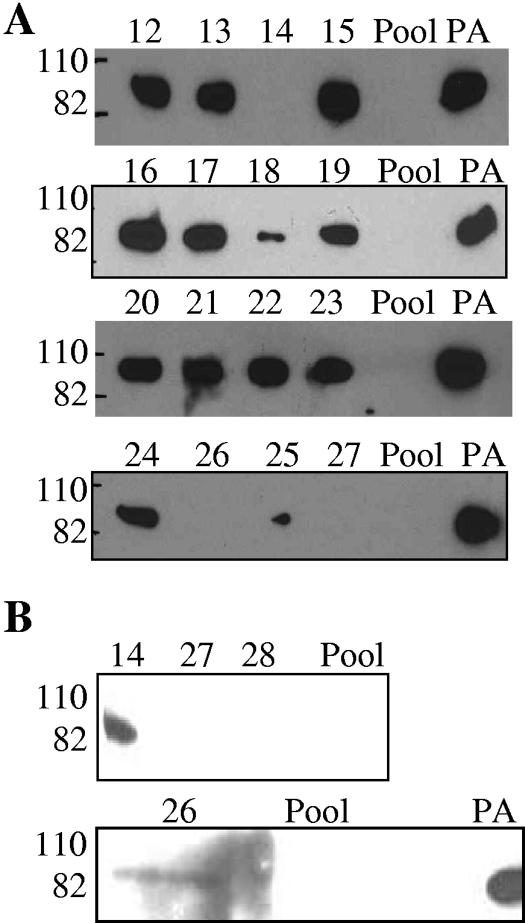
Western blot analyses were performed on the samples (Fig. 1). In initial experiments, reactivity to PA was evident for 13 of the 18 serum samples (Fig. 1A). Negative results were obtained for the pooled negative-control serum, the individuals who had received the fewest inoculations (samples JHUC26, JHUC27, and JHUC28), and an individual who received the complete series of inoculations but who had not received a booster for 5 years (sample JHUC14). The Western blot analysis was repeated using PA at a higher concentration (Fig. 1B), and weak bands were detected with samples JHUC14 and JHUC26 but not with JHUC27 and JHUC28.
FIG. 1.
Western blot analyses of serum samples. Numbers 12 to 28 refer to serum samples JHUC12 through JHUC28, respectively. The relative migrations of molecular mass standards, 110 kDa and 82 kDa, are indicated on the left. A, PA used at 5 μg/ml; B, PA used at 20 μg/ml. Pool, negative-control sera from five individuals presumed to have had no exposure to anthrax or the vaccine.
Determination of antibody index.
Antibody index values for IgG antibodies to PA, or increase (n-fold) over the cutoff value, were determined by using a commercially available ELISA. Each sample was tested three times in triplicate (Table 1). The anti-PA IgG levels varied considerably, although all of the values, including those of the negative-control serum, were greater than or equal to the manufacturer's cutoff of 1.1 as a positive value. The lowest values (1.1 to 1.6) were seen for the pooled control serum and the samples from the individuals with the fewest inoculations or the longest interval since a booster.
In vitro cell culture assays to assess neutralizing antibodies.
We examined LeTx toxicity to primary human cells and human and animal cell lines incubated with PA at 10 μg/ml and LF at 10 μg/ml. Neutrophils, monocytes, macrophages, and peripheral blood mononuclear cells isolated from human blood donors were treated for up to 3 days, and no loss of viability was observed. The susceptibility of the human monocytic cell line U934 was also evaluated. It was not susceptible to LeTx in the undifferentiated state. Addition of phorbol myristate acetate causes U934 to become macrophage-like. U934 cells were treated for 3 to 5 days with phorbol myristate acetate at 0.01 to 10 μg/ml and then incubated with LeTx, and no loss of viability was observed. Human umbilical vein endothelial cells were also reported to display some susceptibility to LeTx (12), but we were unable to confirm these results when PA and LF were added at 10 μg/ml for up to 3 days (data not shown).
We also tested a mouse monocytic cell line, WEHI3, and unlike the J774A.1 cells, this line was totally resistant to PA at 10 μg/ml and LF at 10 μg/ml. CHO cells, which are highly susceptible to edema toxin-mediated elevation of cyclic AMP, did not display sensitivity to LeTx (data not shown). These studies suggest that lethal factor is not universally toxic to cells in culture and that the response of J774A.1 cells is unique. Subsequent studies were performed with the J774A.1 cell line.
Assessment of LeTx toxicity to J774A.1 cells using alamarBlue.
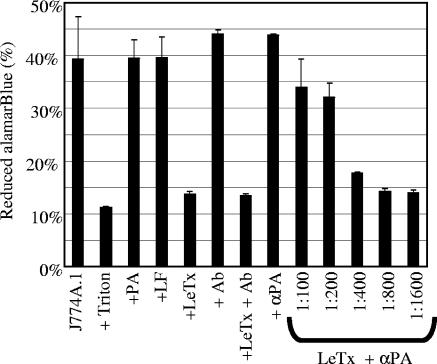
The standard assay to assess antibody neutralization of LeTx to mouse macrophage cell lines uses MTT as an indicator of cellular viability. We developed a simpler method using alamarBlue. AlamarBlue is not toxic to cells, and cellular viability can be determined by simply incubating cells in the presence of alamarBlue. J774A.1 cells were plated at 105 cells/well and incubated with or without LeTx. alamarBlue was added, and the amount of reduced alamarBlue was determined after 20 h. J774A.1 cells reduced about 40% of the alamarBlue, while significantly less reduced alamarBlue was observed (P < 0.03) when the cells were lysed with the detergent Triton X-100 prior to the addition of alamarBlue (Fig. 2). Cells grown in the presence of only PA or LF (0.1 μg/ml) reduced as much alamarBlue as the control cells. In contrast, cells treated with LeTx (PA and LF added at 0.1 μg/ml each) reduced significantly less (P < 0.03) alamarBlue than the control cells.
FIG. 2.
Determination of the protective capability of goat anti-PA antibody. J774A.1 cells were plated at 105 cells/well. The cells were incubated with antibody and toxin components. alamarBlue was added to the wells, and viability was assessed 20 h later. Data are expressed as the means ± standard deviations from one trial, performed in triplicate. J774A.1, control cells incubated in media alone; + Triton, cells lysed with detergent; +PA, cells incubated with 0.1 μg/ml PA; +LF, cells incubated with 0.1 μg/ml LF; +LeTx, cells incubated with 0.1 μg/ml of PA and LF; + Ab, cells incubated with goat anti-human IgG antibody; + αPA, cells incubated with goat anti-PA antibody; LeTx + αPA, cells incubated with 0.1 μg/ml of a PA-LF mixture which had been preincubated with goat anti-PA antibody added at the indicated dilution.
Interestingly, alamarBlue added at the same time as LeTx protected the cells from death (data not shown). Reduced alamarBlue is a weak base (20), and weak bases have been shown to protect cells from several toxins, including LeTx, by preventing acidification of the endosome and subsequent entry of the toxic component into the cytoplasm (8).
Antibody neutralization of LeTx toxicity to J774A.1 cells.
In preliminary studies, we examined the ability of goat anti-PA to neutralize LeTx and protect the cells from death. LeTx was incubated with serial dilutions of a goat anti-PA antibody prior to being added to the cells. In the absence of LeTx, cells incubated with goat anti-human IgG, an irrelevant goat antibody (Fig. 2, bar labeled + Ab), or goat anti-PA (Fig. 2, bar labeled + αPA) reduced as much alamarBlue as control cells. Preincubation of LeTx with the irrelevant antibody did not protect J774A.1 cells from LeTx-mediated killing (Fig. 2, bar labeled +LeTx + Ab). In contrast, preincubation of LeTx with anti-PA antibody diluted 1:100 or 1:200 protected the cells from LeTx-mediated killing, and this protective activity was lost when the antibody was diluted 1:400 or more. These results suggest that monitoring of levels of reduced alamarBlue can be used to assess protection from anthrax toxin. Subsequent studies with human sera were performed essentially the same way.
Neutralization of LeTx by human serum.
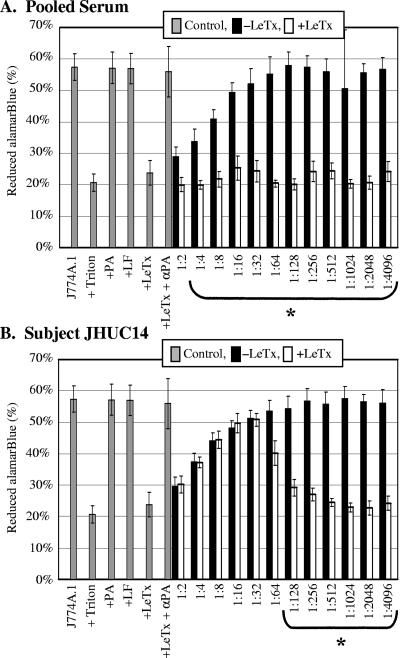
The ability of human serum to protect J774A.1 cells from LeTx treatment was examined (Fig. 3). Control assays performed in parallel with each serum sample reproduced the results seen in Fig. 2. J774A.1 cells with or without PA or LF alone generated significantly more reduced alamarBlue than cells incubated with Triton X-100 or LeTx. Furthermore, goat anti-PA prevented LeTx-mediated death. However, we observed that the addition of high concentrations of human serum (>1:32) inhibited the metabolic activity of the J774A.1 cells compared to that of control cells incubated without human serum (Fig. 3, black bars). We examined the serum-treated cells for viability by trypan blue exclusion. The cells incubated with high concentrations of human serum were viable, as evidenced by trypan blue exclusion. In contrast, cells incubated with LeTx were not viable, as evidenced by the uptake of trypan blue. These results suggest that at high concentrations, some factor in human serum affects the metabolic activity of J774A.1 cells without affecting viability. To account for the influence of human serum on metabolic activity in neutralization assays, we compared the metabolic activity of cells incubated with LeTx in the presence human serum (Fig. 3, white bars) to the metabolic activity of cells incubated with the same amount of human serum but lacking LeTx (Fig. 3, black bars).
FIG. 3.
Neutralization of LeTx by human serum. J774A.1 cells were incubated with twofold serial dilutions of sera in the presence (white bars) or absence (black bars) of LeTx. alamarBlue was added, and percent reduction was determined 20 h later. Controls are shown in gray bars, and abbreviations are defined in the legend for Fig. 2. A, pooled negative-control serum; B, serum sample JHUC14. Data are expressed as the means ± standard deviations from three trials, performed in triplicate. Values for sera with LeTx that are statistically different (P < 0.05) from serum control values are indicated by brackets marked with an asterisk.
Cells incubated with human serum from the pooled negative-control group displayed no evidence of LeTx neutralization (Fig. 3A), since the amount of reduced alamarBlue generated by cells incubated in the absence of LeTx was significantly greater than that generated by cells incubated in the presence of LeTx for all serum dilutions except 1:2 (Fig. 3A, black bars compared to white bars). In contrast, sera from most of the vaccinated individuals displayed evidence of toxin neutralization. For one serum sample, JHUC14, equivalent metabolic activities were observed in the presence and absence of LeTx for all serum concentrations greater than 1:32 (Fig. 3B), suggesting that toxin neutralization had occurred. In contrast, when the serum was diluted 1:128 or more, the amount of metabolic activity was significantly greater in the absence of LeTx than in the presence of LeTx, suggesting that neutralizing antibody was limiting for these dilutions.
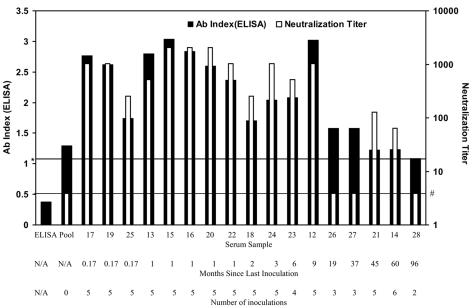
These results suggest that the alamarBlue assay can be used to assess antibody neutralization. We defined the neutralizing titer as the largest serum dilution where the metabolic activity of the toxin-treated cells was greater than or equal to 50% of that of the control cells at the same serum dilution. One hundred percent metabolic activity at each serum dilution was calculated by determining the average amount of reduced alamarBlue generated in the absence of LeTx minus the amount of reduced alamarBlue for Triton X-100-treated cells. In addition, we required that at the neutralizing serum dilution, the amount of reduced alamarBlue generated in the absence of LeTx must be significantly greater (P < 0.05) than that in the LeTx control. Due to the lack of metabolic activity when cells were incubated with a 1:2 dilution of serum (Fig. 3), the limit of detection for neutralization was defined to be a serum dilution of 1:4. All of the serum samples were examined for neutralization as described in the legend of Fig. 3, and the complete data set is available elsewhere (10). Neutralization titers calculated using this formula are reported in Table 1 and are graphically displayed in comparison to antibody indexes as determined by ELISA (Fig. 4), where antibody index is plotted on a linear scale (indicated on the left axis) and neutralization titer is plotted on a log scale (as indicated on the right axis).
FIG. 4.
Comparison of antibody index values and LeTx neutralization. Antibody index values as determined by ELISA are indicated on the left axis, and neutralization titers are indicated on the right axis. Numbers 12 to 28 refer to serum samples JHUC12 through JHUC28, respectively, and the vaccination histories for the subjects who provided these samples are summarized on the next two lines. “ELISA” indicates the value obtained from a no-serum control. The line marked with an asterisk indicates an antibody index value of 1.1, which, according to the manufacturer, is indicative of vaccination or exposure to anthrax. The line marked with a number sign indicates a neutralization titer of 4, the limit of detection for neutralization. Ab, antibody; N/A, not applicable.
Nearly all of the vaccine recipients who received four or five inoculations within the last 9 months possessed moderate to high antibody levels and neutralization titers compared to those of the pooled negative-control sample. A different picture emerges for subjects with fewer shots and whose last inoculation occurred more than 9 months ago. AI as determined by ELISA did not correlate well with neutralization titer. Three samples (JHUC26, JHUC27, and JHUC28) and the pooled control lacked neutralizing activity, although the ELISA suggested that these samples had IgG antibodies to PA. Furthermore, antibody index did not correlate well with the Western blot analysis results (Table 1). JHUC27 (AI, 1.6) and JHUC28 (AI, 1.1) were negative by Western blot analysis, while JHUC14 (AI, 1.2) and JHUC26 (AI, 1.6) had weak activity by Western blot analysis. Interestingly, for the two sera with weak Western blot activity, JHUC26 lacked detectible neutralizing activity, while JHUC14 possessed neutralizing activity when diluted 1:64. Remarkably, the individual who supplied this sample had not been vaccinated within the last 5 years, while the individual who provided sample JHUC26 had been vaccinated less than 2 years ago. The major difference was that the subject who provided sample JHUC14 had received the full six-inoculation series, while that who provided JHUC26 had received only three inoculations. These results suggest that JHUC14 had very low levels of highly effective neutralizing antibodies.
DISCUSSION
In this study, three different assays were used to assess serum antibody levels in anthrax vaccine recipients. Two assays were quantitative, while Western blot analyses indicated only the presence or absence of antibodies. The quantitative assays included a commercially available ELISA that measures IgG antibodies to PA and a new assay that we developed that measures antibody-mediated neutralizing of LeTx toxicity to the J774A.1 murine macrophage cell line using alamarBlue as an indicator of cellular viability.
The crystal structure of PA has been determined (21), and the binding domains of neutralizing monoclonal antibodies to PA have been characterized (6, 16, 24, 28, 30). Neutralizing monoclonal antibodies have been shown to bind to domain 4, which prevents PA binding to the receptor, and to domain 2, which blocks proteolytic cleavage of PA to the active form. It will be interesting to determine whether individuals who possess low levels of highly effective neutralizing antibodies, such as the subject who provided JHUC14, generated a high proportion of antibodies to these regions.
In a disturbing report, it was found that several monoclonal antibodies to PA enhanced toxicity rather than neutralizing toxicity (18). While antibodies of all isotypes were found to be capable of enhancing toxicity, IgG2a antibodies were especially likely to enhance toxicity (18). Mouse IgG2a antibodies are equivalent to human IgG3 antibodies. This class of antibody binds most avidly to the Fc antibody receptor expressed on phagocytic cells, such as macrophages and neutrophils. Interactions of certain monoclonal antibodies with the Fc receptor may play a role in enhancing toxin entry into the cells, thus increasing toxicity rather than neutralizing toxicity. The standard method to assess the presence of neutralizing antibodies to anthrax toxin examines the ability of antibody to protect mouse macrophage cell lines from LeTx-mediated death. However, levels of antibody neutralization of bacterial toxins can differ for cells that express different toxin receptors (17). Ideally, neutralization of anthrax toxin should be examined using human cells that are known to be targeted in diseases. We were unable to detect LeTx toxicity to human cells. Edema factor causes a rise in intracellular cyclic AMP levels in many types of cells, including macrophages (13). Studies to assess the ability of antibody to neutralize edema factor-mediated elevated cyclic AMP levels are in progress.
The 18-month period needed to complete the anthrax immunization series and the need for yearly boosters makes it unlikely that even the armed forces could achieve a fully immunized population using the current anthrax vaccine. Our studies suggest that some individuals generate a more potent neutralizing antibody response than others. It may be possible to develop an anthrax vaccine that maximizes the development of neutralizing antibodies.
Acknowledgments
This work was sponsored by the NIH/NIAID Regional Center of Excellence for Biodefense and Emerging Infectious Disease Research (RCE) Program. We acknowledge membership within and support from the Region V (Great Lakes) RCE (NIH award 1-U54-AI-057153). S.C.T. was supported by NIH/NIAID training grant T32-AI055406 (Training in Biologic Threat Agents) and a University Research Council Summer Fellowship.
REFERENCES
- 1.Agrawal, A., J. Lingappa, S. H. Leppla, S. Agrawal, A. Jabbar, C. Quinn, and B. Pulendran. 2003. Impairment of dendritic cells and adaptive immunity by anthrax lethal toxin. Nature 424:329-334. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed, S. A., R. M. Gogal, Jr., and J. E. Walsh. 1994. A new rapid and simple nonradioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 170:211-224. [DOI] [PubMed] [Google Scholar]
- 3.Brachman, P. S., H. Gold, S. A. Plotkin, F. R. Fekety, M. Werrin, and N. R. Ingraham. 1962. Field evaluation of a human anthrax vaccine. Am. J. Public Health 52:632-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley, K. A., J. Mogridge, M. Mourez, R. J. Collier, and J. A. Young. 2001. Identification of the cellular receptor for anthrax toxin. Nature 414:225-229. [DOI] [PubMed] [Google Scholar]
- 5.Brossier, F., and M. Mock. 2001. Toxins of Bacillus anthracis. Toxicon 39:1747-1755. [DOI] [PubMed] [Google Scholar]
- 6.Cirino, N. M., D. Sblattero, D. Allen, S. R. Peterson, J. D. Marks, P. J. Jackson, A. Bradbury, and B. E. Lehnert. 1999. Disruption of anthrax toxin binding with the use of human antibodies and competitive inhibitors. Infect. Immun. 67:2957-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collier, R. J., and J. A. Young. 2003. Anthrax toxin. Annu. Rev. Cell Dev. Biol. 19:45-70. [DOI] [PubMed] [Google Scholar]
- 8.Gordon, V. M., S. H. Leppla, and E. L. Hewlett. 1988. Inhibitors of receptor-mediated endocytosis block the entry of Bacillus anthracis adenylate cyclase toxin but not that of Bordetella pertussis adenylate cyclase toxin. Infect. Immun. 56:1066-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanna, P. C., S. Kochi, and R. J. Collier. 1992. Biochemical and physiological changes induced by anthrax lethal toxin in J774 macrophage-like cells. Mol. Biol. Cell 3:1269-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanson, J. F. 2004. Characterization of neutralizing responses to anthrax toxin and isolation and characterization of the Shiga-toxin encoding phage from Escherichia coli O157:H7. M.S. thesis. University of Cincinnati, Cincinnati, Ohio.
- 11.Ivins, B. E., M. L. Pitt, P. F. Fellows, J. W. Farchaus, G. E. Benner, D. M. Waag, S. F. Little, G. W. Anderson, Jr., P. H. Gibbs, and A. M. Friedlander. 1998. Comparative efficacy of experimental anthrax vaccine candidates against inhalation anthrax in rhesus macaques. Vaccine 16:1141-1148. [DOI] [PubMed] [Google Scholar]
- 12.Kirby, J. E. 2004. Anthrax lethal toxin induces human endothelial cell apop-tosis. Infect. Immun. 72:430-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar, P., N. Ahuja, and R. Bhatnagar. 2002. Anthrax edema toxin requires influx of calcium for inducing cyclic AMP toxicity in target cells. Infect. Immun. 70:4997-5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leppla, S. H., J. B. Robbins, R. Schneerson, and J. Shiloach. 2002. Development of an improved vaccine for anthrax. J. Clin. Investig. 110:141-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Little, S. F., B. E. Ivins, P. F. Fellows, and A. M. Friedlander. 1997. Passive protection by polyclonal antibodies against Bacillus anthracis infection in guinea pigs. Infect. Immun. 65:5171-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maynard, J. A., C. B. Maassen, S. H. Leppla, K. Brasky, J. L. Patterson, B. L. Iverson, and G. Georgiou. 2002. Protection against anthrax toxin by recombinant antibody fragments correlates with antigen affinity. Nat. Biotechnol. 20:597-601. [DOI] [PubMed] [Google Scholar]
- 17.Millen, S. H., D. I. Bernstein, B. Connelly, J. I. Ward, S.-J. Chang, and A. A. Weiss. 2004. Antibody-mediated neutralization of pertussis toxin-induced mitogenicity of human peripheral blood mononuclear cells. Infect. Immun. 72:615-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohamed, N., J. Li, C. S. Ferreira, S. F. Little, A. M. Friedlander, G. L. Spitalny, and L. S. Casey. 2004. Enhancement of anthrax lethal toxin cytotoxicity: a subset of monoclonal antibodies against protective antigen increases lethal toxin-mediated killing of murine macrophages. Infect. Immun. 72:3276-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Brien, J., A. Friedlander, T. Dreier, J. Ezzell, and S. Leppla. 1985. Effects of anthrax toxin components on human neutrophils. Infect. Immun. 47:306-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Brien, J., I. Wilson, T. Orton, and F. Pognan. 2000. Investigation of the Alamar blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 267:5421-5426. [DOI] [PubMed] [Google Scholar]
- 21.Petosa, C., R. J. Collier, K. R. Klimpel, S. H. Leppla, and R. C. Liddington. 1997. Crystal structure of the anthrax toxin protective antigen. Nature 385:833-838. [DOI] [PubMed] [Google Scholar]
- 22.Pitt, M. L., S. Little, B. E. Ivins, P. Fellows, J. Boles, J. Barth, J. Hewetson, and A. M. Friedlander. 1999. In vitro correlate of immunity in an animal model of inhalational anthrax. J. Appl. Microbiol. 87:304. [DOI] [PubMed] [Google Scholar]
- 23.Pitt, M. L., S. F. Little, B. E. Ivins, P. Fellows, J. Barth, J. Hewetson, P. Gibbs, M. Dertzbaugh, and A. M. Friedlander. 2001. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine 19:4768-4773. [DOI] [PubMed] [Google Scholar]
- 24.Sawada-Hirai, R., I. Jiang, F. Wang, S. M. Sun, R. Nedellec, P. Ruther, A. Alvarez, D. Millis, P. R. Morrow, and A. S. Kang. 2004. Human antianthrax protective antigen neutralizing monoclonal antibodies derived from donors vaccinated with anthrax vaccine adsorbed. J. Immune Based Ther. Vaccines 2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaeffer, L. M., and A. A. Weiss. 2001. Pertussis toxin and lipopolysaccharide influence phagocytosis of Bordetella pertussis by human monocytes. Infect. Immun. 69:7635-7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scobie, H. M., G. J. Rainey, K. A. Bradley, and J. A. Young. 2003. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc. Natl. Acad. Sci. USA 100:5170-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turnbull, P. C. 1991. Anthrax vaccines: past, present and future. Vaccine 9:533-539. [DOI] [PubMed] [Google Scholar]
- 28.Wang, F., P. Ruther, I. Jiang, R. Sawada-Hirai, S. M. Sun, R. Nedellec, P. R. Morrow, and A. S. Kang. 2004. Human monoclonal antibodies that neutralize anthrax toxin by inhibiting heptamer assembly. Hum. Antib. 13:105-110. [PubMed] [Google Scholar]
- 29.Weingart, C. L., G. Broitman-Maduro, G. Dean, S. Newman, M. Peppler, and A. A. Weiss. 1999. Fluorescent labels influence phagocytosis of Bordetella pertussis by human neutrophils. Infect. Immun. 67:4264-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wild, M. A., H. Xin, T. Maruyama, M. J. Nolan, P. M. Calveley, J. D. Malone, M. R. Wallace, and K. S. Bowdish. 2003. Human antibodies from immunized donors are protective against anthrax toxin in vivo. Nat. Biotechnol. 21:1305-1306. [DOI] [PubMed] [Google Scholar]