Abstract
Commercially available pokeweed mitogen (PWM) has been reported to activate macrophages, leading to production of proinflammatory cytokines and nitric oxide (NO). However, we found that polymyxin B (PMB), a specific inhibitor of endotoxin activity, inhibited the PWM-induced expression of proinflammatory cytokines and NO and the activation of Toll-like receptor 4 (TLR4). A kinetic-turbidimetric Limulus amebocyte lysate assay demonstrated that commercial PWM contained substantial endotoxin, over 104 endotoxin units/mg of the PWM. A PWM repurified by PMB-coupled beads no longer induced the expression of proinflammatory cytokines, TLR4 activation, or dendritic cell maturation. However, the repurified PWM remained able to induce proliferation of human lymphocytes, which is a representative characteristic of PWM. These results suggest that commercial PWM might be contaminated with a large amount of endotoxin, resulting in the attribution of misleading immunological properties to PWM.
Pokeweed mitogen (PWM), a lectin with mitogenic activities, is obtained as a crude extract from roots of pokeweed, Phytolacca americana. For several decades, PWM has been investigated as a therapeutic agent for cancer treatment in animal models, most commonly in rodents, cats, and dogs (33). In addition to the therapeutic application, PWM is widely used in various in vitro assays related to lymphocyte proliferation along with concanavalin A (ConA) and phytohemagglutinin (PHA). In contrast to ConA and PHA, with typical T-cell mitogenicity, PWM can trigger nonspecific mitosis of both T and B lymphocytes in humans and rodents (10, 12, 32). Moreover, PWM has the ability to induce polyclonal immunoglobulin production in human lymphocytes, while lipopolysaccharide (LPS) is mitogenic to murine (but not human) B lymphocytes (2). For this reason, PWM occupies an important spot in basic research and has been used in a number of clinical studies to examine functional activation and proliferation of human lymphocytes.
Besides mitogenic activities on B and T lymphocytes, PWM activates innate immunity, which influences the subsequent development of adaptive immunity. Macrophage-depleted human peripheral blood mononuclear cells (PBMC) were unresponsive to PWM (24). In murine macrophages, PWM induced expression of proinflammatory cytokines, including interleukin-1 (IL-1), IL-6, and tumor necrosis factor alpha (TNF-α) (15). It also promoted the expression of inducible nitric oxide synthase (iNOS) and nitric oxide (NO) production (14). The expression of proinflammatory cytokines and iNOS is likely due to the activation of cytosolic NF-κB/Rel, which mainly mediates LPS signals (13, 34). Interestingly, all of the aforementioned biological activities seem to be shared with those of the bacterial endotoxin LPS.
In the present study, we examined whether the previous results showing the activation of murine macrophages could be due to endotoxin contamination. Our results demonstrate that commercially available PWM contains a significant amount of endotoxin, which induces the activation of murine macrophages and the maturation of human dendritic cells (DC).
MATERIALS AND METHODS
Reagents and chemicals.
PWMs were obtained from two different manufacturers, Sigma-Aldrich (St. Louis, Missouri) and Biochrom AG (Berlin, Germany). LPS, PHA, and proteinase K were obtained from Sigma-Aldrich. Antibodies used for flow-cytometric analysis were purchased from BD Biosciences (San Diego, California) unless otherwise indicated.
Culture of RAW 264.7 cell line.
A mouse macrophage cell line, RAW 264.7 (TIB-71), was purchased from the American Type Culture Collection (Manassas, Virginia). The cells were cultured in Dulbecco's modified Eagle's medium (Cellgro Mediatech, Herndon, Virginia) supplemented with 10% fetal bovine serum (HyClone, Logan, Utah), 100 U/ml of penicillin, and 100 μg/ml streptomycin at 37°C in 5% CO2 in a humidified incubator.
Determination of TNF-α and IL-6.
The amounts of TNF-α and IL-6 in the culture supernatant were determined with enzyme-linked immunosorbent assay (ELISA) kits (BD OptEIA ELISA; BD Biosciences), according to the manufacturer's protocol.
Analysis of human DC maturation.
Immature DC (iDC) were prepared as described previously (30). The iDC were stimulated with PWM or LPS for 48 h in the presence of IL-4 (500 U/ml) and granulocyte-macrophage colony-stimulating factor (800 U/ml), and DC were harvested and stained with phycoerythrin-conjugated anti-human CD80 (clone L307.4), fluorescein isothiocyanate-conjugated anti-human CD86 (clone 2331), and allophycocyanin (APC)-conjugated anti-human CD83 (clone HB15e) for 20 min. DC maturation was examined by flow cytometry (FACSCalibur; BD Biosciences), and all flow-cytometric data were analyzed using FlowJo software (Tree Star, San Carlos, California).
Measurement of TLR4 activation.
An NF-κB reporter cell line, CHO/CD14/TLR4, coexpressing CD14 and Toll-like receptor 4 (TLR4) was cultured and used for analyzing the ability of PWM to activate TLR4 as described previously (9). The cell line has the gene encoding membrane CD25 with the human E-selectin promoter, which has NF-κB binding sites.
LAL assay.
Two types of Limulus amebocyte lysate (LAL) tests were used for measuring endotoxins: a chromogenic end point quantitation and a kinetic-turbidimetric quantitation. The chromogenic end point assay was performed with commercial test kit QCL-1000 (Cambrex Bio Science Walkersville Inc., Walkersville, Maryland) according to the manufacturer's instructions. LAL-reactive material (LAL-RM) including β-d-glucan was false positive in this assay. For more-specific determination of endotoxin content in the PWM, a kinetic-turbidimetric test was carried out in the presence or absence of LAL-RM blocker as previously described (29). Turbidity change was measured by a ELx808IU microplate reader (Bio-Tek Instruments Inc., Winooski, Vermont) at 340 nm. The LPS contents were analyzed using endotoxin-measuring software (EndoScan-V; Charles River Endosafe).
Repurification of PWM.
Polymyxin B (PMB)-coupled beads (Detoxi-Gel endotoxin-removing gel; Pierce, Rockford, IL) were prepared by washing five times with 1% sodium deoxycholate and with 0.5 M NaCl to remove nonspecific ionic interaction and then finally by equilibrating with phosphate-buffered saline. We mixed an equal volume of the PMB-coupled beads with 1 mg/ml of the Sigma-Aldrich PWM dissolved in pyrogen-free water and gently shook the solution for 1 h. Then the beads were precipitated by centrifugation at 16,000 × g for 3 min. The supernatant was transferred to a new tube, and the same procedures were repeated six more times. At the end, endotoxin was undetectable in the repurified PWM (RPWM) by the kinetic-turbidimetric LAL test, indicating that endotoxin resides at less than 0.001 endotoxin units (EU)/mg of RPWM.
Analysis of human T-cell division.
Human PBMC were labeled with 2 μM carboxyfluoroscein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene Oregon) for 10 min at room temperature. Labeling was quenched by addition of ice-cold phosphate-buffered saline. Cells were washed and resuspended in complete RPMI 1640 medium at 1 × 106 cells/ml. CFSE-labeled cells were cultured in a 96-well plate with appropriate mitogenic stimuli. After 6 days, cells were harvested and stained with APC-conjugated anti-human CD3 antibody in ice for 30 min and then washed and analyzed by flow cytometry.
Proteinase K treatment.
PWM or repurified PWM was incubated with proteinase K (100 μg/ml) at 37°C for 60 min in a buffer containing 10 mM Tris-HCl, 5 mM EDTA, and 50 mM NaCl (pH 8.0). At the end of incubation, this enzyme was inactivated by heating at 75°C for 20 min.
Statistical analysis.
The mean ± standard deviation was determined for each treatment group; values were compared (with appropriate controls) to find significant differences (P < 0.05) using a two-tailed t test.
RESULTS
The ability of PWM to induce proinflammatory cytokines was abrogated by PMB.
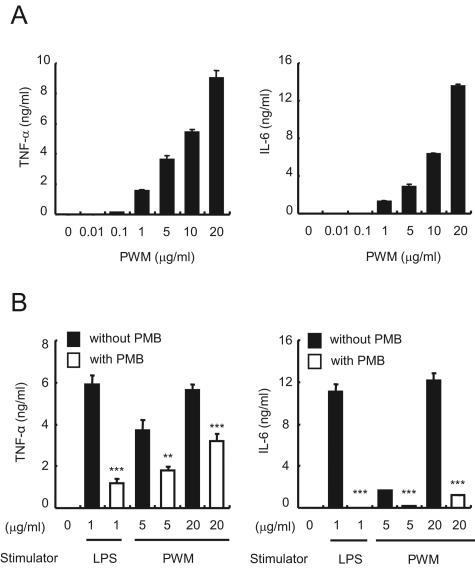
As previously reported, PWM induced the production of TNF-α and IL-6 by RAW 264.7 cells at 1- to 20-μg/ml concentrations in a dose-dependent manner (Fig. 1A). Other inflammatory molecules such as IL-1β and NO were also produced under the same condition (data not shown). Surprisingly, 50 μg/ml of PMB, a specific inhibitor of endotoxin, inhibited both TNF-α and IL-6 expression induced by 5 or 20 μg/ml of PWM (Fig. 1B). Such an inhibitory effect was also observed in the production of IL-1β and NO (data not shown). The results suggest that the inflammatory activity of commercial PWM might be due to endotoxin contamination.
FIG. 1.
Induction of proinflammatory cytokines TNF-α and IL-6 by PWM-activated RAW 264.7 cells is inhibited by PMB treatment. (A) RAW 264.7 cells were stimulated with the indicated concentrations of PWM for 15 h, and supernatants were collected to analyze TNF-α and IL-6 concentrations. (B) RAW 264.7 cells were treated with 50 μg/ml PMB for 1 h and then further incubated with 5 or 20 μg/ml PWM for 15 h to quantitatively examine the concentrations of TNF-α and IL-6. LPS was used as a control to confirm the inhibitory effect of PMB. Error bars indicate standard deviations.
PWM-activated TLR4 was completely inhibited by PMB.
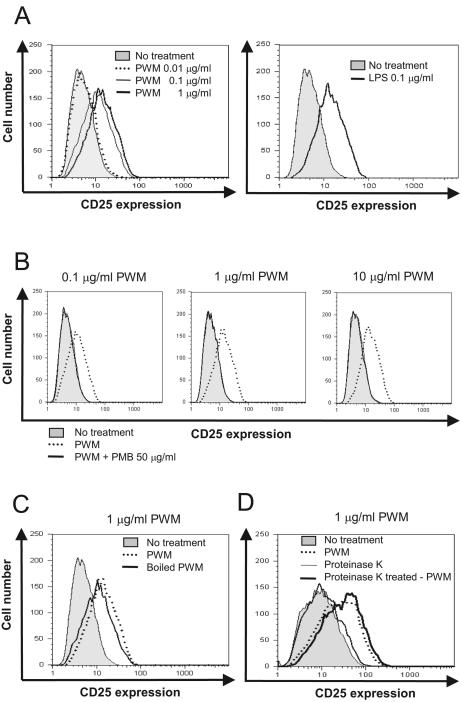
TLR4 is a representative receptor for bacterial endotoxins (11, 23). Next, we assessed whether PWM is able to activate TLR4 as LPS does using the CHO/CD14/TLR4 cell line, which is an NF-κB-dependent CD25 reporter cell line constitutively expressing CD14 and TLR4. Prior to the exposure to stimulants, the geometric mean fluorescence intensity (MFI) of CD25 was 3.0. Upon exposure to 0.01, 0.1, and 1 μg/ml of the PWM for 16 h, the geometric MFI rose to 5.6, 10.5, and 13.7, respectively (Fig. 2A). However, 50 μg/ml of PMB completely abrogated the induction of CD25 expression by 0.1, 1, or even 10 μg/ml of PWM (Fig. 2B).
FIG. 2.
The mode of action of PWM mimics LPS. CHO/CD14/TLR4 cells were incubated for 16 h with (A) PWM (0, 0.01, 0.1, or 1 μg/ml), (B) 0, 0.1, 1 or 10 μg/ml PWM in the presence of 50 μg/ml PMB, (C) PWM or heat-treated PWM (1 μg/ml), and (D) PWM or proteinase K-treated PWM (1 μg/ml). TLR4-dependent NF-κB activation was determined by flow-cytometric analysis of CD25.
LPS is a heat-stable molecule, whereas proteins are susceptible to heat treatment, leading to denaturation (6, 20, 21). To test the possibility that proteinaceous components in the PWM preparation induced TLR4 activation, we boiled PWM for 10 min prior to stimulation of CHO/CD14/TLR4. In contrast to PMB treatment, heat treatment did not alter the ability of PWM to induce TLR4 activation (Fig. 2C). Furthermore, proteinase K treatment did not reduce the ability of the PWM to induce TLR4 activation (Fig. 2D). Collectively, these results suggest that significant amounts of endotoxin might be contaminated in the commercial PWM preparation, leading to TLR4 and, further, to NF-κB activation.
Commercial PWM contains significant amounts of endotoxin.
To obtain direct evidence, we quantified the amounts of endotoxin in the PWM by LAL assay. Two different lots of PWM from Sigma-Aldrich (catalogue number L9379, lot numbers 112K7513 and 044K7602) were examined by chromogenic end point LAL test, and it was observed that the PWM contained large amounts of endotoxins (approximately 7 × 104 EU/mg of PWM). To more accurately determine endotoxin content, a kinetic-turbidimetric LAL assay was performed with PWMs from two unrelated manufacturers, Sigma-Aldrich (catalogue number L9379, lot number 112K7513) and Biochrom AG (catalogue number M 5060, lot number 0123 H). Both lots of PWM contained considerable quantities of endotoxins, 7.1 × 104 and 1.6 × 104 EU/mg of PWM, respectively (Table 1). Notably, 100 pg/ml (equivalent to approximately 2 EU/ml of the endotoxins used in this assay) is the minimum concentration of LPS that sufficiently activates macrophages (7). Although the LAL assay is relatively specific for detection of endotoxins, nonendotoxin molecules, so-called LAL-RM, such as β-d-glucan cause false-positive results in the assay. To determine whether LAL-positive data resulted from the existence of endotoxins or LAL-RM, we carried out the kinetic-turbidimetric LAL assay with LAL-RM blocker (i.e., endotoxin-specific [ES] buffer). The amounts of endotoxins determined by the LAL assay were similar with or without ES buffer (Table 1). These results indicate that commercial PWM has endotoxin contaminants that are capable of significantly influencing the biological activities of PWM.
TABLE 1.
Quantification of endotoxin in commercially available PWM by a kinetic-turbidimetric LAL analysisa
| Assay condition | Amt of endotoxin (EU/mg of PWM) with PWM from:
|
|
|---|---|---|
| Sigma-Aldrich (L9379, 112K7513) | Biochrom AG (M 5060, 0123 H) | |
| Without LAL-RM blockers | 7.1 × 104 | 1.6 × 104 |
| With LAL-RM blockers | 6.7 × 104 | 1.6 × 104 |
PWMs from Sigma-Aldrich and Biochrom AG were dissolved in endotoxin-free water and mixed with Limulus amebocyte lysate dissolved in endotoxin-free water or endotoxin-specific buffer containing LAL-RM blockers. Turbidity change was measured at 340 nm, with incubation of the reaction mixture at 37°C. An LPS from E. coli O55:B5 was used as a reference endotoxin standard.
RPWM was unable to induce proinflammatory cytokines or TLR4 activation.
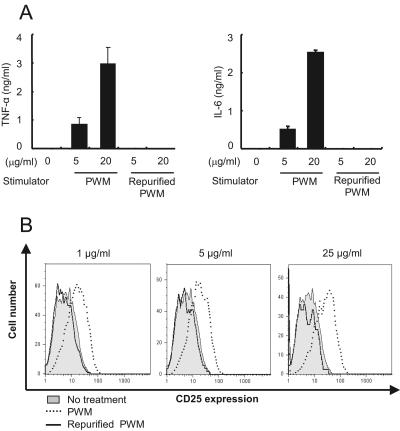
To further test whether inflammatory activity and TLR4 activation were solely due to the LPS contaminants, we repurified the PWM by removal of endotoxin using repeated application of PMB-coupled beads. After repurification, LPS was undetectable in the RPWM by LAL test. The RPWM at 5- and 20-μg/ml concentrations lost the ability to stimulate RAW 264.7 cells for the induction of TNF-α and IL-6 expression (Fig. 3A). Likewise, neither IL-1 nor NO was expressed by the RPWM (data not shown). Concomitantly with the abrogated expression of proinflammatory cytokines, the RPWM was incapable of inducing TLR4 activation (Fig. 3B). These results suggest that LPS might be a major biologically active contaminant in commercial PWM, resulting in the induction of proinflammatory cytokines and TLR4 activation.
FIG. 3.
RPWM induces neither the expression of proinflammatory cytokines TNF-α and IL-6 nor TLR4 activation. RPWM was prepared by eliminating endotoxin with repeated application of polymyxin B-coupled beads. (A) RAW 264.7 cells were stimulated with 0, 5, or 20 μg/ml of PWM or RPWM for 15 h, and supernatants were analyzed for TNF-α and IL-6. (B) CHO/CD14/TLR4 cells were stimulated with 0, 1, 5, or 25 μg/ml of PWM or RPWM for 16 h. TLR4-dependent NF-κB activation was determined by flow-cytometric analysis of CD25.
Maturation of human DC was induced by commercial PWM but not by RPWM.
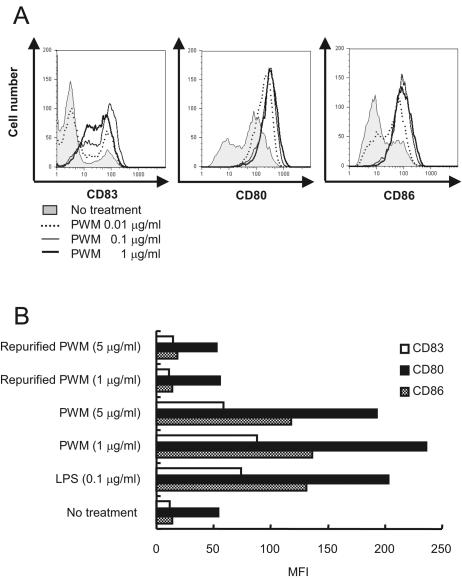
Next, we examined whether the LPS contaminant in PWM causes LPS-mediated biological properties such as the induction of human DC maturation. Flow cytometry showed that 0.01 to 1 μg/ml of commercial PWM induced the expression of CD80, CD83, and CD86, all of which are phenotypic markers representing DC maturation, in a dose-dependent manner (Fig. 4A). However, RPWM could not induce their expression, even at a 5-μg/ml concentration (Fig. 4B). These results imply that endotoxin impurity, but not PWM itself, is able to induce human DC maturation.
FIG. 4.
PWM, but not RPWM, induces human DC maturation. Immature DC derived from CD14+ monocytes of human PBMC were incubated with the indicated concentrations of (A) PWM or (B) RPWM for 48 h. Then, the expression of CD80, CD83, and CD86 was determined by flow cytometry. Data represent the means ± standard deviations of the geometric mean fluorescence intensity.
RPWM was capable of inducing proliferation of human lymphocytes.
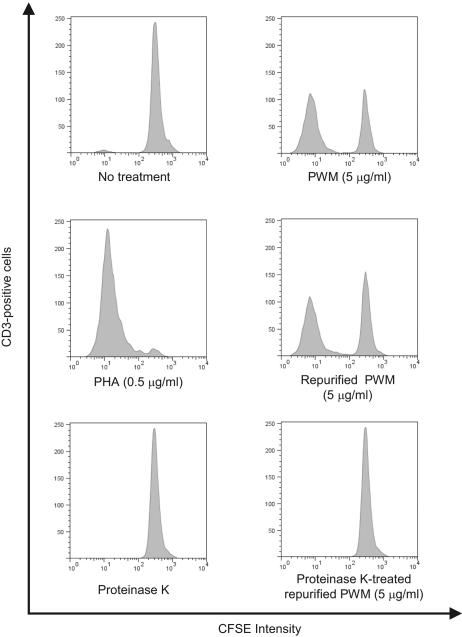
We could not exclude the possibility that RPWM lost its ability to induce LPS-like activities because of loss of biological activity during the repurification process. Since PWM induces proliferation of human lymphocytes, we examined the mitogenic activity of the RPWM to see if it was altered during repurification. The RPWM could successfully induce the proliferation of human CD3-positive cells at 5 μg/ml as the original PWM did (Fig. 5). However, proteinase K treatment eliminated the mitogenic property of the RPWM. These results imply that biologically active components in PWM are proteins which are not removed by PMB-coupled beads.
FIG. 5.
RPWM does not lose the ability to induce proliferation of human lymphocytes. Human PBMC were labeled with 2 μM of CFSE fluorescence dye for 10 min and then stimulated with PWM (5 μg/ml), RPWM (5 μg/ml), proteinase K (control), proteinase K-treated RPWM (5 μg/ml), or PHA (0.5 μg/ml) for 6 days. At the end of incubation, the cells were stained with APC-labeled anti-CD3 antibody and cell division was examined by measuring FL-1 fluorescence intensity of CD3-positive cells with flow-cytometric analysis.
DISCUSSION
A potential for contaminating biological products in purified toxins and mitogens derived from plants has not been much appreciated. Here, we demonstrated that commercially available PWM is contaminated with substantial amounts of endotoxin, which is responsible for the secretion of proinflammatory cytokines and NO production. It is unlikely that molecules other than endotoxin in the PWM preparation contributed to the proinflammatory activities because of the following reasons: (i) PMB is highly specific to lipid A, a key moiety of LPS structure, to the extent that PMB did not antagonize the proinflammatory activity of lipoteichoic acid (LTA), which is structurally analogous to LPS (8, 9, 25); (ii) boiling did not alter the ability of PWM to induce TLR4 activation; (iii) significant quantities of endotoxins were detected in the PWM preparation by the LAL assay, which is unrelated to PMB inhibitory assay (1, 19); (iv) the endotoxin content was not altered in the presence of an inhibitor that blocks the LAL-RM, the only known molecule capable of bringing a positive result in the LAL test (16, 26); (v) endotoxin was detected in the PWMs with three different lot numbers obtained from two unrelated manufacturers.
It would be of interest to speculate on the possible origin of the contaminant, particularly given the fact that PWM is extracted from plants, which do not express endotoxins, as gram-negative bacteria do. One possibility is gram-negative nitrogen-fixing bacteria which have a symbiotic relationship with legume plants such as Phytolacca americana by localizing within the swollen nodules of the roots. On the other hand, endotoxins are frequent contaminants of various biochemical preparations because gram-negative bacteria are ubiquitous and endotoxins are very stable in nature. Commercial preparations of ovalbumin and lactoferrin were found to be highly contaminated with an endotoxin (4, 31), which may not be surprising because both substances have high affinity to endotoxin (5, 22). Furthermore, commercial preparations of LTA also turned out to contain endotoxins (8). This was an unanticipated finding because gram-positive bacteria do not express LPS. Although we were not able to find the source of PWM contaminants in this study, incomplete purification methods may be primarily responsible for endotoxin contamination of the PWM preparation.
Residual impurities often distort results and bring unnecessary debate. For example, LPS, which is widely used in laboratory research, had been believed to activate both TLR2 and TLR4 (3, 11, 17) until it was verified that repurification of LPS using phenol extraction eliminated signaling through TLR2 (27). Furthermore, two lipoproteins known as representative TLR2 activators were identified in the commercial LPS preparation from Escherichia coli K-12 LCD25 (18). More recently, TLR2-dependent bacterial sensing turned out to occur not via peptidoglycan (PGN) recognition but via LTA which was introduced during the PGN purification (28). In the same context, the results of the present study showed that commercially available PWM is contaminated with large amounts of the endotoxins that are associated with false or misleading immunological properties that have been attributed to PWM. Our results and those of others suggest that purification procedures must be considered with great care and sufficient confirmative experiments must be performed to understand genuine biological properties of immunogenic molecules.
Acknowledgments
This work was supported by grants of the Oriental Medicine R&D Project, Ministry of Health and Welfare, Republic of Korea (B050018, to S.H.H.) and the 21C Frontier Microbial Genomics and Application Center Program, Ministry of Science and Technology, Republic of Korea (MG05-0307-2-0, to D.K.C.).
We thank Moon H. Nahm for a careful reading of the manuscript.
We have no financial conflict of interest.
REFERENCES
- 1.Bang, F. B. 1956. A bacterial disease of Limulus polyphemus. Bull. Johns Hopkins Hosp. 98:325-351. [PubMed] [Google Scholar]
- 2.Bessler, W. G., and U. Henning. 1979. Protein I and protein II from the outer membrane of Escherichia coli are mouse B-lymphocyte mitogens. Z. Immunitaetsforsch. Immunobiol. 155:387-398. [PubMed] [Google Scholar]
- 3.Chow, J. C., D. W. Young, D. T. Golenbock, W. J. Christ, and F. Gusovsky. 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274:10689-10692. [DOI] [PubMed] [Google Scholar]
- 4.Cohen, M. S., J. Mao, G. T. Rasmussen, J. S. Serody, and B. E. Britigan. 1992. Interaction of lactoferrin and lipopolysaccharide (LPS): effects on the antioxidant property of lactoferrin and the ability of LPS to prime human neutrophils for enhanced superoxide formation. J. Infect. Dis. 166:1375-1378. [DOI] [PubMed] [Google Scholar]
- 5.Coyne, C. P., J. T. Moritz, and V. C. Langston. 1994. Semi-synthesis of polymyxin-B conjugated ovalbumin: evaluation of lipopolysaccharide binding avidity and neutralization of induced tnf-alpha synthesis. Biotherapy 8:69-83. [DOI] [PubMed] [Google Scholar]
- 6.Dorner, F. 1975. Escherichia coli enterotoxin. Purification and partial characterization. J. Biol. Chem. 250:8712-8719. [PubMed] [Google Scholar]
- 7.Dziarski, R., and D. Gupta. 2005. Staphylococcus aureus peptidoglycan is a Toll-like receptor 2 activator: a reevaluation. Infect. Immun. 73:5212-5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao, J. J., Q. Xue, E. G. Zuvanich, K. R. Haghi, and D. C. Morrison. 2001. Commercial preparations of lipoteichoic acid contain endotoxin that contributes to activation of mouse macrophages in vitro. Infect. Immun. 69:751-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han, S. H., J. H. Kim, M. Martin, S. M. Michalek, and M. H. Nahm. 2003. Pneumococcal lipoteichoic acid (LTA) is not as potent as staphylococcal LTA in stimulating Toll-like receptor 2. Infect. Immun. 71:5541-5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartnett, B. J., R. L. Somberg, S. Krakowka, H. D. Ochs, H. HogenEsch, P. F. Moore, K. I. Weinberg, and P. J. Felsburg. 2000. B-cell function in canine X-linked severe combined immunodeficiency. Vet. Immunol. Immunopathol. 75:121-134. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 12.Ichiki, T., W. Takahashi, and T. Watanabe. 1992. The effect of cytokines and mitogens on the induction of C epsilon germline transcripts in a human Burkitt lymphoma B cell line. Int. Immunol. 4:747-754. [DOI] [PubMed] [Google Scholar]
- 13.Jeon, Y. J., S. H. Han, Y. W. Lee, S. S. Yea, and K. H. Yang. 1998. Inhibition of NF-κB/Rel nuclear translocation by dexamethasone: mechanism for the inhibition of iNOS gene expression. Biochem. Mol. Biol. Int. 45:435-441. [DOI] [PubMed] [Google Scholar]
- 14.Jeon, Y. J., J. S. Lee, and H. G. Jeong. 1999. Induction of inducible nitric oxide synthase gene expression by pokeweed mitogen. Chem. Biol. Interact. 118:113-125. [DOI] [PubMed] [Google Scholar]
- 15.Jeong, H. G. 2001. Cytokine-mediated suppression of cytochrome P450 1A1 in Hepa-1c1c7 cells by pokeweed mitogen. Toxicol. Lett. 119:125-132. [DOI] [PubMed] [Google Scholar]
- 16.Kakinuma, A., T. Asano, H. Torii, and Y. Sugino. 1981. Gelation of Limulus amoebocyte lysate by an antitumor (1 leads to 3)-beta-D-glucan. Biochem. Biophys. Res. Commun. 101:434-439. [DOI] [PubMed] [Google Scholar]
- 17.Kirschning, C. J., H. Wesche, T. Merrill Ayres, and M. Rothe. 1998. Human Toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J. Exp. Med. 188:2091-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, H. K., J. Lee, and P. S. Tobias. 2002. Two lipoproteins extracted from Escherichia coli K-12 LCD25 lipopolysaccharide are the major components responsible for Toll-like receptor 2-mediated signaling. J. Immunol. 168:4012-4017. [DOI] [PubMed] [Google Scholar]
- 19.Levin, J., and F. B. Bang. 1968. Clottable protein in Limulus; its localization and kinetics of its coagulation by endotoxin. Thromb. Diath. Haemorrh. 19:186-197. [PubMed] [Google Scholar]
- 20.Maeno, N., K. Yoshiie, S. Matayoshi, T. Fujimura, S. Mao, M. R. Wahid, and H. Oda. 2002. A heat-stable component of Bartonella henselae upregulates intercellular adhesion molecule-1 expression on vascular endothelial cells. Scand. J. Immunol. 55:366-372. [DOI] [PubMed] [Google Scholar]
- 21.Mao, S., N. Maeno, K. Yoshiie, S. Matayoshi, T. Fujimura, and H. Oda. 2002. CD14-mediated induction of interleukin-8 and monocyte chemoattractant protein-1 by a heat-resistant constituent of Porphyromonas gingivalis in endothelial cells. Scand. J. Immunol. 56:484-491. [DOI] [PubMed] [Google Scholar]
- 22.Na, Y. J., S. B. Han, J. S. Kang, Y. D. Yoon, S. K. Park, H. M. Kim, K. H. Yang, and C. O. Joe. 2004. Lactoferrin works as a new LPS-binding protein in inflammatory activation of macrophages. Int. Immunopharmacol. 4:1187-1199. [DOI] [PubMed] [Google Scholar]
- 23.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg, S. A., and P. E. Lipsky. 1981. The role of monocytes in pokeweed mitogen-stimulated human B cell activation: separate requirements for intact monocytes and a soluble monocyte factor. J. Immunol. 126:1341-1345. [PubMed] [Google Scholar]
- 25.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J. Biol. Chem. 274:17406-17409. [DOI] [PubMed] [Google Scholar]
- 26.Soderhall, K. 1981. Fungal cell wall beta-1,3-glucans induce clotting and phenoloxidase attachment to foreign surfaces of crayfish hemocyte lysate. Dev. Comp. Immunol. 5:565-573. [DOI] [PubMed] [Google Scholar]
- 27.Tapping, R. I., S. Akashi, K. Miyake, P. J. Godowski, and P. S. Tobias. 2000. Toll-like receptor 4, but not Toll-like receptor 2, is a signaling receptor for Escherichia and Salmonella lipopolysaccharides. J. Immunol. 165:5780-5787. [DOI] [PubMed] [Google Scholar]
- 28.Travassos, L. H., S. E. Girardin, D. J. Philpott, D. Blanot, M. A. Nahori, C. Werts, and I. G. Boneca. 2004. Toll-like receptor 2-dependent bacterial sensing does not occur via peptidoglycan recognition. EMBO Rep. 5:1000-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuchiya, M., A. Takaoka, N. Tokioka, and S. Matsuura. 1990. Development of an endotoxin-specific Limulus amebocyte lysate test blocking beta-glucan-mediated pathway by carboxymethylated curdlan and its application. Nippon Saikingaku Zasshi 45:903-911. [DOI] [PubMed] [Google Scholar]
- 30.Tsuji, S., M. Matsumoto, O. Takeuchi, S. Akira, I. Azuma, A. Hayashi, K. Toyoshima, and T. Seya. 2000. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guerin: involvement of Toll-like receptors. Infect. Immun. 68:6883-6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe, J., Y. Miyazaki, G. A. Zimmerman, K. H. Albertine, and T. M. McIntyre. 2003. Endotoxin contamination of ovalbumin suppresses murine immunologic responses and development of airway hyper-reactivity. J. Biol. Chem. 278:42361-42368. [DOI] [PubMed] [Google Scholar]
- 32.Waxdal, M. J. 1974. Isolation, characterization, and biological activities of five mitogens from pokeweed. Biochemistry 13:3671-3677. [DOI] [PubMed] [Google Scholar]
- 33.Wimer, B. M., and P. L. Mann. 2002. Mitogen information summaries. Cancer Biother. Radiopharm. 17:569-597. [DOI] [PubMed] [Google Scholar]
- 34.Xie, Q. W., Y. Kashiwabara, and C. Nathan. 1994. Role of transcription factor NF-κB/Rel in induction of nitric oxide synthase. J. Biol. Chem. 269:4705-4708. [PubMed] [Google Scholar]