Abstract
Terminal differentiation and cellular senescence display common properties including irreversible growth arrest. To define the molecular and ultimately the biochemical basis of the complex physiological changes associated with terminal differentiation and senescence, an overlapping-pathway screen was used to identify genes displaying coordinated expression as a consequence of both processes. This approach involved screening of a subtracted cDNA library prepared from human melanoma cells induced to terminally differentiate by treatment with fibroblast IFN and mezerein with mRNA derived from senescent human progeria cells. This strategy identified old-35, which encodes an evolutionary conserved gene, human polynucleotide phosphorylase (hPNPaseold-35), that is regulated predominantly by type I IFNs. The hPNPaseOLD-35 protein localizes in the cytoplasm of human cells and induces RNA degradation in vitro, as does its purified bacterial protein homologue. Ectopic expression of hPNPaseold-35 in human melanoma cells reduces colony formation, confirming inhibitory activity of this RNA-degradation enzyme. Identification of hPNPaseold-35, an IFN-inducible 3′-5′ RNA exonuclease, provides additional support for a relationship between IFN action and RNA processing and suggests an important role for this gene in growth control associated with terminal differentiation and cellular senescence.
Keywords: overlapping-pathway screen, terminal cell differentiation, senescent phenotype, interferon-inducible gene, evolutionary conserved gene
Plasticity of the transformed phenotype is suggested by the ability of differentiation-inducing agents to revert the cancerous properties of specific tumors (1–3). This attribute of tumor cells provides the basis for a potentially less toxic form of therapy, “differentiation therapy.” In metastatic human melanoma, a combination of IFN-β and the protein kinase C activator mezerein (MEZ) produces irreversible growth arrest, a loss of tumorigenic competence, and terminal differentiation (1, 4). To define gene-expression changes associated with induction of terminal differentiation, a subtracted cDNA library enriched for genes associated with terminal differentiation was constructed (5). This construction was accomplished by subtracting control HO-1 human melanoma mRNAs from IFN-β + MEZ-treated HO-1 mRNAs, which were temporally collected over a 24-h period (5). This subtracted cDNA library then was screened by random isolation of phage colonies and Northern blotting, high-density cDNA microarray analysis, and reverse Northern screening followed by Northern blotting (5–7). These approaches have identified both unknown and known genes associated with tumor and normal growth control, cell-cycle regulation, IFN response, differentiation, and apoptosis (5–12). Four classes of melanoma differentiation-associated (mda) genes have been identified (5, 10).
Terminal cell differentiation and cellular senescence are characterized by changes in cell morphology, lack of responsiveness to mitogenic stimulation, and irreversible growth arrest (1, 4, 13–18). Normal cells cultured in vitro lose their proliferative potential after a finite number of doublings in a process described as cellular senescence (13). Experiments in human diploid fibroblasts and additional cell types document an inverse correlation between replicative senescence and donor age and a direct relationship between replicative senescence and donor-species life span (13, 19, 20). In agreement with this relationship, cells from patients with premature aging syndromes such as Werner's syndrome and progeria achieve a quiescent state more rapidly than normal human fibroblasts (21). Although senescence is a time-dependent process, terminal differentiation can be induced in a variety of cell types by appropriate treatment (2, 3, 16). Growth of HO-1 cells in IFN-β and MEZ results in irreversible growth arrest, altered cellular morphology, modifications in antigenic phenotype, and an increase in melanogenesis (1, 4, 8, 22).
Induction of terminal differentiation in melanocytes by cAMP results in similar and distinct changes in gene expression in comparison with senescent melanocytes (17). Although both pathways result in elevated p21 (WAF1, Cip1, and mda-6) expression and an inability to phosphorylate ERK2, only the differentiated cells display elevated levels of p27 and the melanocyte-specific transcription factor MITF (17, 23). Terminal differentiation and senescence also involve additional overlapping gene changes in melanoma and other cell types including enhanced expression of various interleukins (IL-1, IL-15, and mda-7/IL-24), cell cycle-regulatory genes [CDK inhibitor, p21 (WAF1, Cip1, and mda-6), and DNA damage-inducible genes (GADD153 and GADD34] (6, 7, 24). Based on these findings, a comparison of senescence and terminal differentiation provides a unique opportunity to identify genes that may govern the growth-suppressive changes underlying both processes.
We have exploited the overlapping-pathway hypothesis as a means of identifying genes coordinately up-regulated during terminal differentiation and cellular senescence. To achieve this goal, a differentiation inducer-treated subtracted HO-1 melanoma cDNA library constructed from temporally spaced poly(A) RNAs from untreated and IFN-β + MEZ-treated HO-1 cells (5) was screened with mRNA from progeria fibroblasts, an accelerated-aging syndrome. This screening stratagem referred to as the overlapping-pathway screen, OPS (Fig. 1), permitted the identification and cloning of old-35, human polynucleotide phosphorylase (hPNPaseold-35), a gene that displays high homology and similar properties with bacterial PNPase, an enzyme involved in RNA degradation (25, 26). The present report describes the cloning, expression profile, and biological activity of hPNPaseold-35, a type I mda gene displaying elevated expression in diverse cell types, after treatment with IFN-β and IFN-β + MEZ and exhibiting growth-inhibitory effects in colony-formation assays. In these contexts, hPNPaseold-35 may provide a link between IFN action and growth cessation, which is a defining parameter of terminal differentiation and senescence.
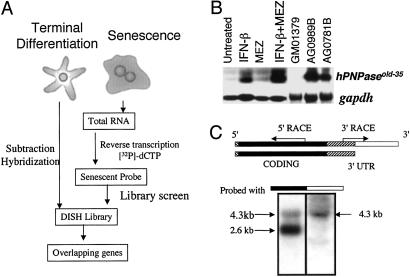
Fig 1.
(A) Schematic representation of the OPS approach. In this application, a differentiation-induction subtraction-hybridization (DISH) library was constructed by subtraction hybridization from terminally differentiated HO-1 human melanoma cells (5). This library was screened with a senescent probe derived from progeria cells. This screening protocol permits the identification of genes displaying parallel (overlapping) changes in expression during terminal differentiation and senescence. (B) Expression of hPNPaseold-35 during terminal differentiation and senescence. Northern blot of hPNPaseold-35 in HO-1 melanoma cells treated for 24 h with IFN-β (2,000 units/ml), MEZ (10 ng/ml) or IFN-β + MEZ (2,000 units/ml + 10 ng/ml), early-passage GM01379 fibroblasts (normal fibroblasts), and two senescent progeroid fibroblasts (AG0781B and AG0989B). gapdh was used as a loading control. (C) Representation of two hPNPaseold-35 transcripts visualized in HO-1 cells on Northern blots. The 5′ UTR (13 bp) in the 5′ region is indicated as a hatched box, the protein-coding region is indicated in black, the 3′ UTR common to both hPNPaseold-35 variants is indicated as a hatched box adjacent to the protein-coding region, and the 3′ UTR present in the longer hPNPaseold-35 variant is indicated in white. The region between the arrows indicates the EST that was identified by using the OPS approach, and the arrows indicate the directions in which the remainder of the gene was cloned. Northern blot analysis of mRNA from HO-1 cells after IFN-β treatment (2,000 units/ml, 7 h) was performed to determine sizes of the hPNPaseold-35 variants. Two membranes containing the same RNA extracts were probed with either the coding region (black, Left) or the 3′ UTR of the longer variant (white, Right).
Materials and Methods
Cell Culture.
Human melanoma, breast carcinoma, and osteosarcoma cells were obtained from the American Type Culture Collection (ATCC) or as reported and were cultured as described (9, 22, 27, 28). Early-passage human fetal lung fibroblasts (GM01379) and human progeria fibroblasts (AG0989B and AG0781B) (Corriel Repositories, Camden, NJ) were grown in DMEM supplemented with 10% FBS and penicillin/streptomycin (100 units/100 μg/ml) at 37°C in a 5% CO2/95% air humidified incubator. Sf9 insect cells were cultured in TNM-FH medium (Mediatech Laboratories, Cody, NY) supplemented with 10% FBS and penicillin/streptomycin (100 units/100 μg/ml) at 27°C in a humidified incubator.
Library Screening.
AG0989B progeria fibroblast cells were cultured until they became senescent and stained positive for senescence-associated β-galactosidase activity (29). One microgram of poly(A) RNA from AG0989B cells was reverse-transcribed into radiolabeled cDNA as described (6). A subtracted cDNA library enriched for genes modified during terminal differentiation in human melanoma cells was screened with the senescent cDNA probe as described (5).
RNA Extraction and Northern Blotting.
Total RNA was purified from cells by using the RNeasy kit (Qiagen, Valencia, CA). Poly(A) RNA for senescent-probe preparation was extracted by using a Poly(A) Pure kit (Ambion, Austin, TX). For Northern blotting, 10 μg of total RNA was resolved in 1% agarose gels with 2% formaldehyde and transferred to nylon membranes (Hybond-N). The XhoI fragment of hPNPaseold-35 cDNA (1.5 kb) and 0.7-kb fragment of gapdh were labeled with [α-32P]dCTP (Roche, Basel). The membrane was hybridized with 32P-labeled hPNPaseold-35. The blots were stripped and reprobed with a 32P-labeled gapdh probe and exposed for autoradiography.
Cloning of hPNPaseold-35 and Expression Vector Construction.
The full-length hPNPaseold-35 cDNA was cloned from IFN-β-treated HO-1 cell RNA by using C-ORF and 3′ RACE in the 5′ and the 3′ directions, respectively (12). The hPNPaseold-35-specific primers used in C-ORF and 3′ RACE were P1 (5′-TTTTGCTCGTTTTGATAATG-3′), P2 (5′-TAATGGGAGAACCTATTTCA-3′), and P3 (5′-CTAATTCTCAGTGATTTTTT-3′). The full-length hPNPaseold-35 cDNA was obtained by RT-PCR from IFN-β-treated HO-1 cell RNA using primers P4 (5′-CTAATTCTCAGTGATTTTTT-3′) and P5 (5′-ATTAAACAAATATGGGTTAC-3′). The ≈4.3-kb hPNPaseold-35 variant was identified by analysis of the dbEST database and confirmed by acquiring and sequencing ATCC cDNA clone no. 213524. An hPNPaseold-35 expression vector was constructed by cloning an RT-PCR product (5′-GGATCCGCGGCCTGCAGGTACTGC-3′ and 5′-GGGCGCCGCTCACTGAGAAATTAGAT-3′) into BamHI- and NotI-digested pEF1/His B (Invitrogen). A baculovirus transfer vector, pAcGHLT-hPNPaseold-35, was constructed by cloning a product amplified by RT-PCR (5′-CGCGGCCCGCGGCCTGCAGGTACTGC-3′ and 5′-GGGCGCCGCTCACTGAGAAATTAGAT-3′) into pAcGHLT-B (PharMingen) at the NotI site. hPNPaseold-35 RT-PCRed with 5′-GAGCTCAGGATCCGCGGCCTGCAGGTACTGC-3′ and 5′-GGATATCACTGAGAATTAGATTGATGA-3′ was cloned into the SacI and SmaI site of pEGFP-C2 (CLONTECH) to generate GFP-hPNPaseold-35.
Western Blot Analysis and Fluorescence Microscopy.
Ten micrograms of supercoiled plasmid DNA (pEGFP-C2 and pEGFP-C2-hPNPaseold-35) were transfected into ≈70% confluent HO-1 cells with Superfect (Qiagen) per manufacturer protocol. Two days after transfection, cells were harvested, and protein-sample preparation and Western blotting were performed as described (12). Expression of GFP-hPNPaseold-35 was detected with anti-GFP (CLONTECH) antibody followed by anti-mouse-horseradish peroxidase and ECL (Amersham Pharmacia). For intracellular localization, pEGFP-C2 and pEGFP-C2-hPNPaseold-35 were transfected as described (12) and observed by fluorescent confocal microscopy (×400).
Colony-Forming Assays.
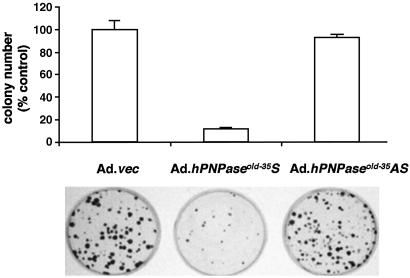
Colony-forming assays using HO-1 melanoma cells after transfection with pEF1/His B and pEF1/His B-hPNPaseold-35 were performed as described (12). For adenoviral studies, HO-1 cells were plated at a density of 1 × 105 cells per 6-cm dish, and after 24 h the cells were infected with Ad.vec, Ad.hPNPaseold-35S, or Ad.hPNPaseold-35AS at a multiplicity of infection of 100 plaque-forming units per cell as described (27). Six hours after infection the cells were trypsinized and plated at a density of 103 cells per 6-cm dish. Colonies ≥50 cells were scored 3 weeks later. The recombinant replication-incompetent adenoviruses were constructed, grown, and assayed as described (30).
Purification of GST-hPNPaseOLD-35 Fusion Protein and Degradation Assays.
GST-hPNPaseOLD-35 purification was performed by glutathione-Sepharose affinity chromatography as described (12). Purified fusion protein was digested with thrombin (50 units/mg fusion protein) for 2 h at room temperature. After digestion was completed, hPNPaseOLD-35 protein was separated further on a Sephacryl S-200 column (1.5 × 50 cm) at a constant flow rate of 1.5 ml/min. For enzyme assays, substrate RNA GEM-A0 was prepared as described (31). RNA was diluted 10 times, and 1 μl was used for each degradation-assay reaction. Each reaction was performed in 20 μl of degradation buffer containing 20 mM Tris⋅HCl (pH 7.5), 1.5 mM DTT, 1 mM MgCl2, and 20 mM KCl with or without 10 mM Na3PO4 for various times as described (32). The reaction products were treated with proteinase K (10 mg/ml) for 30 min at room temperature, and 5 μl of each reaction was spotted on polyethyleneimine cellulose TLC plates (Aldrich) and resolved in 1 M formic acid.
Results
OPS.
The OPS approach was used to identify genes mediating altered physiological changes commonly associated with terminal cell differentiation and senescence. Because normal cells senesce slowly in culture, fibroblasts from patients with the accelerated-aging syndrome progeria, which become senescent more rapidly than normal cells, were chosen for this study (33). Progeria cells were subcultured until they exhibited profound morphological changes and senescence-associated β-galactosidase activity, a marker of the senescent state (29). To define genes with expression that changes as a function of induction of terminal differentiation, a subtracted cDNA (differentiation-induction subtraction hybridization) library was prepared from mRNAs isolated over a 24-h period from HO-1 human melanoma cells treated with IFN-β + MEZ (5). This differentiation-induction subtraction-hybridization library was screened with a probe derived from the RNA of senescent progeria fibroblasts (AG0989B) (Fig. 1A). Seventy-five ESTs were identified initially in the OPS screen and designated as old-1 to old-75 (data not shown). Among the old genes, old-35 demonstrated an elevated expression pattern associated with both induction of terminal differentiation and senescence and was chosen for further evaluation (Fig. 1B). Expression of old-35 increased in IFN-β- and IFN-β + MEZ-treated HO-1 cells in comparison with untreated HO-1 controls (Fig. 1B) and in senescent progeroid fibroblasts (AG0781B and AG0989B) relative to early-passage fetal fibroblasts (GM01379) (Fig. 1B). An overlapping expression pattern of old-35 in senescent fibroblasts and IFN-β + MEZ-induced terminally differentiated human melanoma cells suggests that old-35 might correlate with or contribute to the cellular changes that characterize both processes.
Cloning of the old-35 cDNA.
The original old-35 clone obtained from library screening (600-bp) contained an internal region of the old-35 cDNA and lacked 3′- and 5′-flanking sequences. The 5′ and 3′ regions of old-35 were cloned from IFN-β-treated HO-1 cells by using a modified RACE protocol (c-ORF), which resulted in the cloning of a 2,629-bp old-35 cDNA (ref. 12; Fig. 1C). Northern blotting analysis demonstrated that the old-35 EST hybridized to two mRNA species of ≈4.0 and 2.6 kb in RNA isolated from IFN-β-treated HO-1 cells (Fig. 1C). An ≈4-kb old-35 variant was identified by a BLAST search of the dbEST database, purchased from ATCC (no. 213524), and sequenced. Comparison of the sequences of the two old-35 cDNA clones (2,629 and 4,331 bp, respectively) indicated identical ORFs that extended from 53 to 2,404 bp, encoding a protein of 783 amino acids with a predicted molecular mass of 86 kDa and a pI of 7.87. The ORF of old-35 starts at the first AUG codon. Although A−3 in the Kozak consensus sequence (AXXaugG) is not conserved, G+4 is conserved (34). Sequence analysis of old-35 revealed that this cDNA (≈2.6 kb) contains a less frequently used polyadenylation site (AUUAAA, found in only ≈10% of cDNAs) (35). In addition, a canonical polyadenylation site was not detected in the ≈4.3-kb variant (Fig. 1A). However, the 4.3-kb clone contained a longer 3′ UTR, possibly because of differential polyadenylation. To confirm this possibility, a Northern blot containing total RNA from IFN-β-treated HO-1 cells was probed with either the coding region of the old-35 gene or the 3′ UTR of the longer ≈4.3-kb variant (Fig. 1C). Although the ≈2.6- and ≈4.3-kb bands were detected with a coding-region probe, only the upper ≈4.3-kb band was identified with the 3′-UTR probe. Sequence analysis and Northern blot results indicate that the ≈4.3-kb mRNA is a variant of old-35, which may result from alternative polyadenylation.
old-35 Is hPNPase.
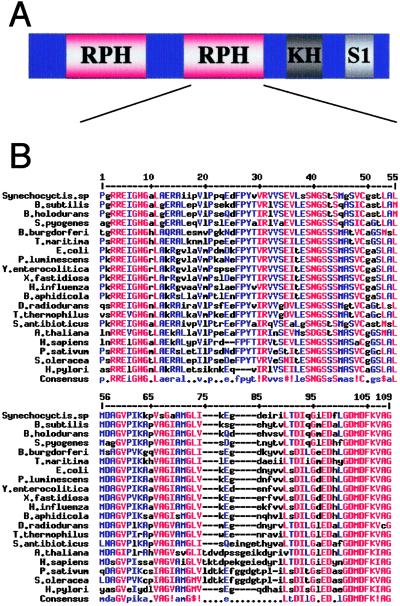
A BLAST search of the translated sequence of the old-35 ORF suggested that old-35 encodes a phosphate-dependent 3′–5′ RNA exonuclease–PNPase, previously recognized only in bacteria and plants (36, 37). Further sequence analysis with PROSITE, PFAM, and PRODOM identified four PNPase-specific domains present in the OLD-35 protein sequence (Fig. 2A). Similar to other PNPases, the OLD-35 protein contains two RNase PH domains, one KH domain, and one S1 domain (Fig. 2A). Alignment of the PNPase sequences from a number of different species indicates high conservation in the critical catalytic regions of PNPase, especially in the RNase PH domains (Fig. 2B).
Fig 2.
(A) Structure of PNPase proteins. PNPases have two RNase PH domains, one KH domain, and one S1 domain. (B) Alignment of 20 members of the PNPase family using MultAlin (http://prodes.toulouse.inra.fr/multalin/multalin.html, ref. 51). A residue that is highly conserved appears in high-consensus color (90%, red) and as an uppercase letter in the consensus line. A residue that is weakly conserved appears in low-consensus color (50%, blue) and as a lowercase letter in the consensus line. Other residues appear in black. A position with no conserved residue is represented by a dot in the consensus line. !, IV; $, LM; %, FY; #, NDQE.
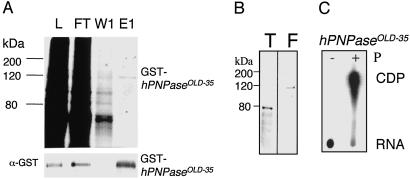
To determine whether sequence conservation translates into conservation of PNPase function (phosphate-dependent degradation of RNA substrates), GST-OLD-35 was expressed by using a baculovirus system and purified to homogeneity by using glutathione-affinity chromatography. After purification with glutathione beads, protein purity was assessed by using SDS/PAGE with Coomassie staining and a Western blot with an anti-GST antibody (Fig. 3A). Purified GST-OLD-35 protein migrated as a single 120-kDa band (Fig. 3A). The GST tag was removed by thrombin digestion followed by gel filtration to facilitate functional studies (Fig. 3B). PNPase is a phosphate-dependent 3′–5′ RNA exonuclease that produces nucleotide diphosphates (NDPs) instead of nucleotide monophosphates (NMPs) during the degradation of the RNA substrate (31). Thus, PNPase activity was assayed with or without phosphate with radiolabeled RNA, and undigested radiolabeled RNA and its degradation product ([32P]CDP) were resolved by polyethyleneimine TLC in 1 M formic acid (32). In the absence of phosphate no cytosine diphosphate (CDP) formation was detected, whereas CDP accumulation was observed in the presence of phosphate (Fig. 3C). These data illustrate that OLD-35 protein degraded the RNA substrate in a phosphate-dependent manner. The confirmed phosphate-dependent degradative activity of OLD-35 implies that old-35 encodes human PNPase as is suggested by sequence homology and indicates that old-35 is hPNPaseold-35.
Fig 3.
Purification of GST-hPNPaseOLD-35. (A) Coomassie staining of a 10% SDS/PAGE gel. L, lysate; FT, flow through; W1, wash 1; E1, eluate 1. (Lower) The Western blot was probed with anti-GST antibody. (B) Coomassie staining of a 10% SDS/PAGE gel containing thrombin-digested hPNPaseOLD-35 (T) and undigested GST-hPNPaseOLD-35 (F). (C) Degradation of RNA by hPNPaseOLD-35. The reaction was performed as described in Materials and Methods in the absence (−) or presence (+) of phosphate. The degradation products then were resolved on TLC in 1 M formic acid. E. coli PNPase (Sigma) was used as a positive control (data not shown).
hPNPaseold-35Is a Type I IFN-Inducible Gene.
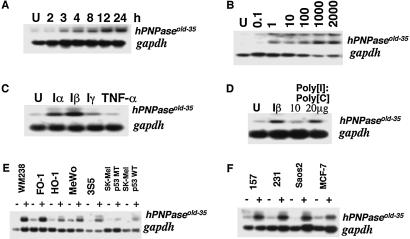
Northern blot hybridizations were performed to define the induction profile of hPNPaseold-35 after treatment with different IFNs and in different cell backgrounds. hPNPaseold-35 was induced within 3 h by IFN-β (2,000 units/ml) and accumulated until 24 h (Fig. 4A) followed by a gradual decrease in steady-state message level (data not shown). Because IFN-β induces growth suppression in HO-1 cells at 2,000 units/ml, it was important to establish whether up-regulation of hPNPaseold-35 occurs as a result of IFN-induced growth suppression. hPNPaseold-35 expression was induced in HO-1 cells with as little as 1 unit/ml of IFN-β, which is not growth-inhibitory (Fig. 4B), indicating that induction of hPNPaseold-35 expression by IFN can be dissociated from IFN-induced growth suppression. Treatment of HO-1 cells with leukocyte IFN (IFN-α) also resulted in significant up-regulation of hPNPaseold-35 in HO-1 cells, whereas expression of hPNPaseold-35 was marginally stimulated by IFN-γ, and no detectable or consistent induction occurred with tumor necrosis factor α (Fig. 4C). Double-stranded RNA, poly(I)·poly(C), a known inducer of IFN-α and IFN-β, genes also stimulated hPNPaseold-35 expression (Fig. 4D).
Fig 4.
Induction of hPNPaseold-35 mRNA by IFNs in various cell types. (A) Time-course treatment of HO-1 cells with IFN-β (2,000 units/ml). (B) IFN-β dose response in HO-1 cells. Cells were untreated (U) or treated with 0.1–2,000 units/ml for 7 h. (C) Treatment of HO-1 cells with different cytokines for 17 h. U, untreated; Iα, IFN-α (1,000 units/ml); Iβ, IFN-β (1,000 units/ml); Iγ, IFN-γ (1,000 units/ml); TNF-α, tumor necrosis factor α (10 ng/ml). (D) Effect of IFN-β and poly(I)·poly(C) treatment on hPNPaseold-35 and gapdh expression in HO-1 cells. U, untreated or treated for 24 h with IFN-β (2,000 units/ml), poly(I)·poly(C), 1× (10 μg/ml), or 2× (20 μg/ml). (E) Analysis of hPNPaseold-35 and gapdh expression in various human melanomas without (−) or with (+) IFN-β (2,000 units/ml) treatment for 18 h. (F) Analysis of hPNPaseold-35 and gapdh expression in human breast carcinoma (MDA-MB-157, MDA-MB-231, and MCF-7) or human osteosarcoma (Saos2) cells without (−) or with (+) IFN-β (2,000 units/ml) treatment for 18 h. A lower quantity of RNA is present in the untreated versus the treated WM238 sample.
Because hPNPaseold-35 was cloned from HO-1 cells, a metastatic human melanoma cell line, its expression was examined in additional melanoma cell lines. The steady-state de novo expression of hPNPaseold-35 was comparable in FO-1, HO-1, MeWo, and 3S5 (a nonmetastatic variant of MeWo) human melanomas with reduced de novo expression in the WM238, SK-MEL 110 (mutant p53), and SK-MEL 470 (WT p53) melanoma cell lines (Fig. 4E). However, when treated with IFN-β, expression of hPNPaseold-35 was elevated to variable extents in all the melanoma cell lines. To test for up-regulation of hPNPaseold-35 by IFN-β in cancer cells other than melanoma, MDA-MB-157 (p53-null), MDA-MB-231 (mutant p53), and MCF-7 (WT p53) human breast carcinoma cells and Saos-2 human osteosarcoma cells (p53- and Rb-null) were treated with IFN-β for 18 h, and mRNA levels were determined (Fig. 4F). Treatment of null, mutant, or WT p53 breast carcinoma cells and Saos-2 osteosarcoma cells, which are null for both p53 and Rb, with IFN-β resulted in elevated hPNPaseold-35 expression. Similarly, treatment with IFN-β elevated hPNPaseold-35 expression in normal skin fibroblasts and normal immortal melanocytes, indicating that induction of hPNPaseold-35 by IFN-β is not restricted to cancer cells (data not shown). These experiments document differential regulation of hPNPaseold-35 expression by different cytokines, with type I IFNs (IFN-α/β) being the most active cytokines tested in inducing hPNPaseold-35 expression in HO-1 cells. In addition, expression of WT p53 or Rb is not required for induction of hPNPaseold-35 by IFN-β in melanoma or additional human cancer cell lines.
hPNPaseold-35Expression Suppresses HO-1 Melanoma Cell Colony Formation.
Because growth inhibition is a common event associated with terminal differentiation, senescence, and IFN treatment, it is possible that hPNPaseold-35 could mediate growth inhibition during these processes. To test this possibility initially, the inhibitory effect of hPNPaseold-35 was measured by assaying colony-forming ability of HO-1 cells infected with replication-incompetent adenovirus vectors including Ad.vec (an adenovirus lacking the hPNPaseold-35 gene), Ad.hPNPaseold-35S, or Ad.hPNPaseold-35AS (Ad.hPNPaseold-35 antisense). Infection with Ad.hPNPaseold-35S resulted in ≈90% reduction in colony formation in comparison with infection with either Ad.vec or Ad.hPNPaseold-35AS (Fig. 5), confirming that hPNPaseold-35 has profound growth-suppressing effects in HO-1 cells. The colonies formed in the presence of Ad.hPNPaseold-35S were also much smaller than those formed in the presence of either Ad.vec or Ad.hPNPaseold-35AS (Fig. 5). To eliminate the possibility that high-level expression resulting from adenovirus infection was the reason for growth-inhibitory properties of hPNPaseold-35, transfection experiments with an EF-1α promoter-driven hPNPaseold-35 expression vector were performed. This experimental protocol resulted in a significant but decreased reduction in colony formation in comparison with adenoviral infection (≈40%, P < 0.05, data not shown). Both colony-formation assays document that hPNPaseold-35 has growth-inhibitory activity, which is consistent with the hypothesis that this gene may contribute to growth modulation during IFN-associated terminal differentiation and senescence. Further studies are required to determine whether growth inhibition is associated with induction of apoptosis or involves reduced cell proliferation.
Fig 5.
Colony formation after infection of HO-1 cells with Ad.vec, Ad.hPNPaseold-35S, or Ad.hPNPaseold-35AS. HO-1 cells (1 × 105) were infected at 100 plaque-forming units per cell; 6 h later cells were reseeded at 103 per 6-cm plate, and colony formation was determined 3 weeks later. Graphical representation of three independent experiments using triplicate samples for each condition ± SD is shown.
Cellular Localization of the hPNPaseOLD-35 Protein.
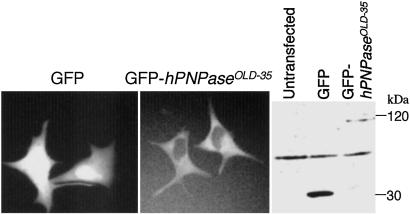
The subcellular localization of hPNPaseOLD-35 was determined by examining localization of GFP-fusion hPNPaseOLD-35 by fluorescent microscopy. A Western blot was probed with an anti-GFP antibody to confirm that the fusion protein was expressed in the transfected cell lines. GFP antibody detected proteins of the expected sizes: 30 kDa (GFP) and 120 kDa (hPNPaseOLD-35-GFP). Fluorescent microscopy of GFP-hPNPaseOLD-35-transfected cells demonstrated that hPNPaseOLD-35 protein localized in the cytoplasm of HO-1 cells, as anticipated for a degradative enzyme (Fig. 6).
Fig 6.
Cellular localization of hPNPaseOLD-35. Cellular localization of hPNPaseOLD-35 was assessed by using a GFP-hPNPaseOLD-35 expression plasmid. (Left) HO-1 cells were transiently transfected with a GFP-hPNPaseOLD-35 construct and observed by fluorescent microscope (×400). (Right) Western blot of GFP and GFP-hPNPaseOLD-35.
Discussion
A phenotype-driven differential gene-cloning method, OPS, has identified hPNPaseold-35, which encodes the human homologue of bacterial and plant PNPase. The complete hPNPaseOLD-35 protein exhibits 37% identity and 54% similarity to the prokaryotic members of this gene family. However, conservation of protein sequence is much higher at specific domain sites where it approaches 70% (Fig. 2B). This sequence conservation also translates into functional conservation. Similar to other PNPases, hPNPaseold-35 exhibits phosphate-dependent exonuclease activity (Fig. 3C). Although polymerization activity associated with bacterial PNPase has not been demonstrated, both sequence analysis and phosphate-dependent exonuclease activity argue that old-35 is a PNPase, and this article reports the existence of PNPase in the animal kingdom. BLAST searches also indicate the presence of PNPase in Drosophila melanogaster and putative PNPase ESTs in Gallus gallus, confirming the presence of PNPase in animal cells (data not shown).
PNPase is a component of a multiprotein complex called a degradosome. The bacterial degradosome consists of an endonuclease (RNase E), ATP-dependent helicase (RhlB), an exonuclease (PNPase), and an enolase, a glycolytic enzyme (26, 38). In Escherichia coli, decay of mRNAs is initiated by RNase E followed by exonucleolytic degradation at the new 3′ ends by PNPase and an additional 3′–5′ RNA exonuclease, RNase II (26). Growth of E. coli under normal growth conditions is unaffected by deletion of pnp (PNPase), but pnp mutants exhibit a cold-sensitive growth phenotype preventing growth at temperatures below 30°C (39, 40). In contrast, deletion of both exonucleases, pnp and rnb (RNase II), is lethal (40, 41). In plants PNPase functions during chloroplast differentiation, most likely as a homohexamer (42, 43), whereas PNPase has not been identified in yeast. However, a multiprotein complex called the exosome functions in yeast in pre-mRNA and mRNA degradation and in rRNA processing (44). The exosome contains endonuclease, 3′–5′ exonuclease (RNase PH), and RNA helicase. A human exosome has been described as polymyositis/scleroderma (PM/Scl) overlap syndrome particle, which is related to the yeast exosome (45). The human exosome is reported to contain human homologues of several yeast exosomal components (Rrp40p, Rrp41p, and Rrp46p) and mediates mRNA degradation of AU-rich elements (46–48). Further studies are required to determine whether PNPase is a component of the human exosome, because the structure of this complex is well conserved in evolution.
hPNPaseold-35 is a type I IFN (α/β)-responsive gene, which is induced as early as 3 h by as little as 1 unit/ml IFN-β in HO-1 melanoma cells (Fig. 4). Moreover, hPNPaseold-35 is induced by IFN-β in normal and additional cancer cells with diverse genetic backgrounds, suggesting that induction of hPNPaseold-35 by IFNs represents a general cellular response to these cytokines. The fact that double-stranded RNA, which mimics viral replication intermediates, stimulates hPNPaseold-35 expression suggests a possible role of hPNPaseold-35 in IFN-mediated antiviral responses. Overexpression of hPNPaseold-35 in human melanoma cells results in growth suppression (Fig. 5). In these contexts, hPNPaseold-35 may play a pivotal role in IFN-mediated antiviral response by modulating cell growth.
It is established that IFNs induce a plethora of genes, many of which function in mRNA stability and translation (12, 49, 50). Two well characterized pathways are translational repression by double-stranded RNA-dependent protein kinase (PKR) and RNA degradation by 2–5 A-dependent RNase L (OAS/RNase L) (49). hPNPaseold-35, mda-5 (a putative RNA helicase with growth-suppressive activity) (12) and RNase II (mda-E-63) (24) were identified as IFN-inducible genes in human melanoma cells, suggesting the existence of alternative IFN-stimulated RNA-decay pathways in mammalian cells. It remains to be determined how IFNs acting through these molecules may modify gene expression by regulating RNA degradation/stability. However, based on induction in response to IFN treatment or viral infection and cytoplasmic localization, it is possible that an exosomal structure consisting of hPNPaseold-35 and mda-5 may be assembled in concert with other proteins that then target growth-related mRNA species for degradation. In future studies it will be essential to determine the specificity of hPNPaseold-35 action, this information will permit elucidation of the mechanism by which hPNPaseold-35 regulates cell growth during IFN treatment, differentiation, and senescence.
A cloning strategy called OPS has been developed to facilitate the identification of genes displaying overlapping expression profiles as a function of induction of complex analogous phenotypic changes in target cells. Presently, OPS was applied to the processes of terminal differentiation and cellular senescence resulting in cloning of the human homologue of PNPase, hPNPaseold-35. As predicted based on the underlying premise of OPS, steady-state levels of hPNPaseold-35 message were higher in senescing cells in comparison with proliferating fibroblasts and were also increased during terminal cell differentiation. Because OPS is a phenotype-driven screening methodology and ectopic expression of hPNPaseold-35 is growth-suppressive, it seems that this gene contributes toward the common attribute of growth arrest that is shared between the processes of terminal differentiation and senescence. Preliminary studies suggest that expression of antisense hPNPaseold-35 in HO-1 human melanoma cells inhibits IFN-β + MEZ-induced terminal differentiation, indicating that hPNPaseold-35 is essential for induction of this process (data not shown). hPNPaseold-35 encodes a putative 3′-5′ RNA exonuclease, suggesting that it might regulate the stability of downstream genes involved in terminal cell differentiation. Although the specific targets of hPNPaseold-35 action are not known currently, they might involve important regulatory proteins involved in signaling such as c-fos, c-myc, or c-jun. Because these molecules have been found to participate in programs of proliferation and differentiation, where they are regulated at the level of mRNA stability, it is likely that they can be modulated by hPNPaseold-35.
As a final cautionary note, although quite robust in identifying numerous interesting and potentially relevant genes involved in growth control, differentiation, and cancer suppression, previous screening approaches did not identify hPNPaseold-35 in the context of differentiating melanoma cells (5–7, 12, 24). This suggests that multiple cloning approaches will be required to define the full spectrum of differentially regulated genes associated with and contributing to growth control and terminal differentiation in cancer cells.
Acknowledgments
We thank Dr. J. Wilusz for GEM-A0 and Dr. D. H. Bechhofer for helpful suggestions. The present studies were supported in part by National Institutes of Health Grant CA35675, an award from the Samuel Waxman Cancer Research Foundation, and the Chernow Endowment. P.B.F. is a Michael and Stella Chernow Urological Cancer Research Scientist.
Abbreviations
MEZ, mezerein
mda, melanoma differentiation-associated
OPS, overlapping-pathway screen
PNPase, polynucleotide phosphorylase
hPNPaseold-35, human PNPase (old-35)
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AY027528).
References
- 1.Fisher P. B., Prignoli, D. R., Hermo, H., Jr., Weinstein, I. B. & Pestka, S. (1985) J. Interferon Res. 5, 11-22. [DOI] [PubMed] [Google Scholar]
- 2.Leszczyniecka M., Roberts, T., Dent, P., Grant, S. & Fisher, P. B. (2001) Pharmacol. Ther. 90, 105-156. [DOI] [PubMed] [Google Scholar]
- 3.Waxman S., (1996) Differentiation Therapy (Ares-Serono Symposia, Rome), pp. 1–531.
- 4.Jiang H., Su, Z.-Z., Boyd, J. & Fisher, P. B. (1993) Mol. Cell. Differ. 1, 41-66. [Google Scholar]
- 5.Jiang H. & Fisher, P. B. (1993) Mol. Cell. Differ. 1, 285-299. [Google Scholar]
- 6.Huang F., Adelman, J., Jiang, H., Goldstein, N. I. & Fisher, P. B. (1999) Gene 236, 125-131. [DOI] [PubMed] [Google Scholar]
- 7.Huang F., Adelman, J., Jiang, H., Goldstein, N. I. & Fisher, P. B. (1999) Oncogene 18, 3546-3552. [DOI] [PubMed] [Google Scholar]
- 8.Jiang H., Lin, J. J., Su, Z. Z., Goldstein, N. I. & Fisher, P. B. (1995) Oncogene 11, 2477-2486. [PubMed] [Google Scholar]
- 9.Jiang H., Lin, J., Su, Z. Z., Herlyn, M., Kerbel, R. S., Weissman, B. E., Welch, D. R. & Fisher, P. B. (1995) Oncogene 10, 1855-1864. [PubMed] [Google Scholar]
- 10.Jiang H., Lin, J. & Fisher, P. B. (1994) Mol. Cell. Differ. 2, 221-239. [Google Scholar]
- 11.Madireddi M. T., Su, Z. Z., Young, C. S., Goldstein, N. I. & Fisher, P. B. (2000) Adv. Exp. Med. Biol. 465, 239-261. [DOI] [PubMed] [Google Scholar]
- 12.Kang D.-C., Gopalkrishnan, R. V., Wu, Q., Jankowsky, E., Pyle, A. M. & Fisher, P. B. (2002) Proc. Natl. Acad. Sci. USA 99, 637-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayflick L. (1976) N. Engl. J. Med. 295, 1302-1308. [DOI] [PubMed] [Google Scholar]
- 14.Delia D., Greaves, M. F., Newman, R. A., Sutherland, D. R., Minowada, J., Kung, P. & Goldstein, G. (1982) Int. J. Cancer 29, 23-31. [DOI] [PubMed] [Google Scholar]
- 15.Sparks R. L., Seibel-Ross, E. I., Wier, M. L. & Scott, R. E. (1986) Cancer Res. 46, 5312-5319. [PubMed] [Google Scholar]
- 16.Tortora G., Tagliaferri, P., Clair, T., Colamonici, O., Neckers, L. M., Robins, R. K. & Cho-Chung, Y. S. (1988) Blood 71, 230-233. [PubMed] [Google Scholar]
- 17.Medrano E. E., Yang, F., Boissy, R., Farooqui, J., Shah, V., Matsumoto, K., Nordlund, J. J. & Park, H. Y. (1994) Mol. Biol. Cell 5, 497-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campisi J., Dimri, G. P., Nehlin, J. O., Testori, A. & Yoshimoto, K. (1996) Exp. Gerontol. 31, 7-12. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein S. (1990) Annu. Rev. Gerontol. Geriatr. 10, 97-116. [DOI] [PubMed] [Google Scholar]
- 20.Murano S., Thweatt, R., Shmookler Reis, R. J., Jones, R. A., Moerman, E. J. & Goldstein, S. (1991) Mol. Cell. Biol. 11, 3905-3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Little J. B. (1976) Gerontology 22, 28-55. [DOI] [PubMed] [Google Scholar]
- 22.Graham G. M., Guarini, L., Moulton, T. A., Datta, S., Ferrone, S., Giacomini, P., Kerbel, R. S. & Fisher, P. B. (1991) Cancer Immunol. Immunother. 32, 382-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith J. R. & Pereira-Smith, O. M. (1996) Science 273, 63-67. [DOI] [PubMed] [Google Scholar]
- 24.Jiang H., Kang, D. C., Alexandre, D. & Fisher, P. B. (2000) Proc. Natl. Acad. Sci. USA 97, 12684-12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey R. A. & Grunberg-Manago, M. (1966) Biochem. Biophys. Res. Commun. 23, 448-452. [DOI] [PubMed] [Google Scholar]
- 26.Carpousis A. J. (2002) Biochem. Soc. Trans. 30, 150-155. [PubMed] [Google Scholar]
- 27.Su Z. Z., Madireddi, M. T., Lin, J. J., Young, C. S., Kitada, S., Reed, J. C., Goldstein, N. I. & Fisher, P. B. (1998) Proc. Natl. Acad. Sci. USA 95, 14400-14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lebedeva I. V., Su, Z. Z., Chang, Y., Kitada, S., Reed, J. C. & Fisher, P. B. (2002) Oncogene 21, 708-718. [DOI] [PubMed] [Google Scholar]
- 29.Dimri G. P., Lee, X., Basile, G., Acosta, M., Scott, G., Roskelley, C., Medrano, E. E., Linskens, M., Rubelj, I., Pereira-Smith, O., et al. (1995) Proc. Natl. Acad. Sci. USA 92, 9363-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valerie K. (1999) in Biopharmaceutical Drug Design and Development, eds. Wu-Pong, S. & Rojanasakul, Y. (Humana Press, Totowa, NJ), pp. 69–142.
- 31.Gao M., Wilusz, C. J., Peltz, S. W. & Wilusz, J. (2001) EMBO J. 20, 1134-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carpousis A. J., Van Houwe, G., Ehretsmann, C. & Krisch, H. M. (1994) Cell 76, 889-900. [DOI] [PubMed] [Google Scholar]
- 33.Vincent R. A. & Huang, P. C. (1976) Exp. Cell Res. 102, 31-42. [DOI] [PubMed] [Google Scholar]
- 34.Kozak M. (1996) Mamm. Genome 7, 563-574. [DOI] [PubMed] [Google Scholar]
- 35.Manley J. L. (1988) Biochim. Biophys. Acta 950, 1-12. [DOI] [PubMed] [Google Scholar]
- 36.Reuven N. B. & Deutscher, M. P. (1993) FASEB J. 7, 143-148. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J. R. & Deutscher, M. P. (1988) J. Bacteriol. 170, 522-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grunberg-Manago M. (1999) Annu. Rev. Genet. 33, 193-227. [DOI] [PubMed] [Google Scholar]
- 39.Mathy N., Jarrige, A. C., Robert-Le Meur, M. & Portier, C. (2001) J. Bacteriol. 183, 3848-3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yancey S. D. & Kushner, S. R. (1990) Biochimie 72, 835-843. [DOI] [PubMed] [Google Scholar]
- 41.Donovan W. P. & Kushner, S. R. (1986) Proc. Natl. Acad. Sci. USA 83, 120-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baginsky S., Shteiman-Kotler, A., Liveanu, V., Yehudai-Resheff, S., Bellaoui, M., Settlage, R. E., Shabanowitz, J., Hunt, D. F., Schuster, G. & Gruissem, W. (2001) RNA 7, 1464-1475. [PMC free article] [PubMed] [Google Scholar]
- 43.Hayes R., Kudla, J., Schuster, G., Gabay, L., Maliga, P. & Gruissem, W. (1996) EMBO J. 15, 1132-1141. [PMC free article] [PubMed] [Google Scholar]
- 44.Mitchell P., Petfalski, E., Shevchenko, A., Mann, M. & Tollervey, D. (1997) Cell 91, 457-466. [DOI] [PubMed] [Google Scholar]
- 45.Allmang C., Petfalski, E., Podtelejnikov, A., Mann, M., Tollervey, D. & Mitchell, P. (1999) Genes Dev. 13, 2148-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen C. Y., Gherzi, R., Ong, S. E., Chan, E. L., Raijmakers, R., Pruijn, G. J., Stoecklin, G., Moroni, C., Mann, M. & Karin, M. (2001) Cell 107, 451-464. [DOI] [PubMed] [Google Scholar]
- 47.Brouwer R., Pruijn, G. J. & van Venrooij, W. J. (2001) Arthritis Res. 3, 102-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brouwer R., Allmang, C., Raijmakers, R., van Aarssen, Y., Egberts, W. V., Petfalski, E., van Venrooij, W. J., Tollervey, D. & Pruijn, G. J. (2001) J. Biol. Chem. 276, 6177-6184. [DOI] [PubMed] [Google Scholar]
- 49.Player M. R. & Torrence, P. F. (1998) Pharmacol. Ther. 78, 55-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rebouillat D. & Hovanessian, A. G. (1999) J. Interferon Cytokine Res. 19, 295-308. [DOI] [PubMed] [Google Scholar]
- 51.Corpet F. (1988) Nucleic Acids Res. 16, 10881-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]