Abstract
Macrophage migration inhibitory factor (MIF) plays a pivotal role in the development of various inflammatory diseases. Here, we found that anti-mouse MIF antibody treatment reduced liver injury and inflammatory cell infiltration into the liver after injection of antigen-specific cytotoxic T lymphocytes into hepatitis B virus transgenic mice.
Macrophage migration inhibitory factor (MIF) was originally identified as a T-cell-derived cytokine that inhibited the migration of guinea pig macrophages in vitro (8). Recently, MIF was reevaluated as a proinflammatory cytokine and pituitary-derived hormone that counterregulates the immunosuppressive effects of steroids (6). Furthermore, anti-MIF antibodies have been shown to effectively suppress mortality due to septic shock (5) and protect against experimental colitis (9), experimental gastritis (20), and tumor growth and tumor-associated angiogenesis (21), suggesting that MIF is involved in not only inflammatory and immune responses but also tumor cell growth. However, its potential as an antiviral cytokine for infectious diseases is unknown.
We previously demonstrated that intrahepatic induction of gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), or IFN-α/β downregulates hepatitis B virus (HBV) replication noncytopathically in the livers of transgenic mice (12). This antiviral effect can be achieved by injecting transgenic mice with HBV-specific CD8, CD4 T cells, α-galactosylceramide, and anti-CD40 (10, 13, 15, 16). Furthermore, we showed that the same antiviral response is initiated by recombinant murine interleukin-12 and interleukin-18 and that the effect is mediated by IFN-γ and IFN-α/β (7, 17). In the present study, we investigated the role of MIF in viral replication using an HBV replicative cell line and transgenic mice, as well as the effect of MIF neutralization on liver injury in a cytotoxic-T-lymphocyte (CTL)-induced acute hepatitis model.
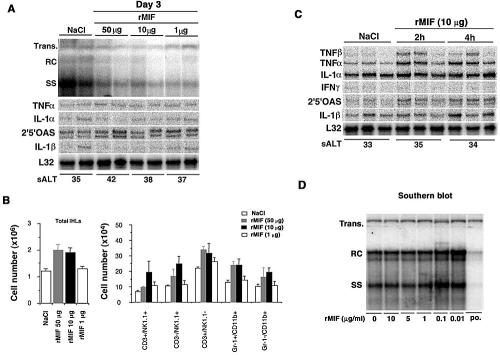
First, to determine whether MIF has antiviral activity against HBV replication in vivo, three age-matched (8 to 10 weeks old), sex-matched (male), and serum HBeAg-matched transgenic mice from lineage 1.3.32 were injected subcutaneously with 1, 10, or 50 μg of recombinant mouse MIF and sacrificed after 3 days. Total hepatic DNA was analyzed for HBV DNA by Southern blot analysis. We found that a dose-dependent antiviral effect was not observed in the liver, although relaxed circular (RC) HBV DNA was faintly reduced, compared to effects with NaCl injection (Fig. 1A). Next, intrahepatic leukocytes (IHLs) were isolated and analyzed for their phenotype by flow cytometry. As shown in Fig. 1B, there was a slight increase in the total number of IHLs recruited to the liver on day 3 after MIF injection. Most of this increase in IHLs was caused by an influx of natural killer (NK) cells (CD3−/NK1.1+), T cells (CD3+/NK1.1−), Gr-1+/CD11b+ neutrophils, and Gr-1−/CD11b+ macrophages. In addition, to determine cytokine mRNA expression in the liver after recombinant MIF injection, we performed an RNase protection assay (RPA) using the same livers. However, we could not find cytokine mRNA induction in the liver at day 3 (Fig. 1A). On the basis of this finding, we analyzed the earlier time point and then we showed that recombinant MIF treatment rapidly induced various cytokine mRNA expressions in the liver. In particular, mRNA expression of TNF-α and 2′5′-oligoadenylate synthetase (an IFN-α/β-inducible gene) were detected from 2 h after the injection (Fig. 1C).
FIG. 1.
Effects of MIF on HBV replication in vivo and vitro. (A) Age-, sex-, and serum HBeAg-matched lineage 1.3.32 HBV transgenic mice were injected subcutaneously with 1, 10, or 50 μg recombinant mouse MIF and sacrificed after 3 days. Total hepatic DNA was analyzed for HBV DNA by Southern blot analysis. All DNA samples were treated with RNase before quantification and gel electrophoresis. The bands corresponding to the integrated transgene (Trans.), RC double-stranded HBV DNA, and single-stranded (SS) linear HBV DNA replicative forms are indicated. The integrated transgene can be used to normalize the amount of DNA bound to the membrane. The filter was hybridized with a 32P-labeled HBV-specific DNA probe. The sALT activities at the time of autopsy are indicated at the bottom and expressed in units per liter. (B) Effects of MIF on IHL population. IHLs from the above-described animals were isolated and analyzed by flow cytometry. The number in each cell subset in the liver was calculated by multiplying the total number of IHLs by the frequency of the subset in the IHL population as evaluated by FACS analysis (BD Biosciences). (C) Total hepatic RNA was analyzed for cytokine transcripts by RPA, as indicated. Note that all cytokine mRNAs were analyzed in the same RPA. The mRNA encoding the ribosomal protein L32 was used to normalize the amount of RNA loaded in each lane of the RPA assay (BD Biosciences Pharmingen). (D) HBV-Met cells were untreated or treated with 0.01- to 10-μg/ml murine MIF or 1,000-U/ml murine IFN-β as a positive control and harvested after 24 h. HBV-α replication was monitored by Southern blot analysis of the HBV RC and SS DNA replicative forms.
Next, to confirm whether MIF has a direct antiviral effect on HBV replication in vitro, we examined HBV DNA expression by using HBV-Met.4, an HBV transgenic immortalized hepatocyte cell clone (22). As described previously, HBV-Met.4 cells were grown to confluence and then kept in complete medium supplemented with 2% dimethyl sulfoxide prior to cytokine treatment. On day 12 of the dimethyl sulfoxide treatment, MIF was added at the indicated concentrations (0 to 10 μg/ml), and the cells were incubated for a further 24 h and then analyzed by Southern blotting. As shown in Fig. 1D, MIF also had no direct antiviral effect in vitro, since HBV replication was not inhibited by MIF treatment. Collectively, these results suggest that MIF has no antiviral effect against HBV replication either in vivo or in vitro.
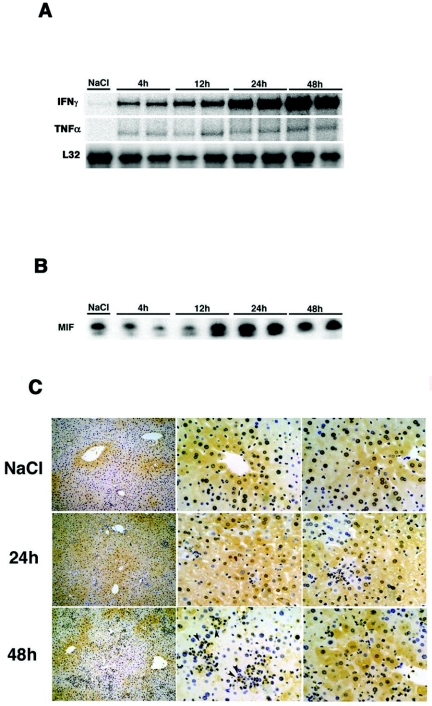
It has been clearly demonstrated that MIF plays an important role in the induction of inflammatory diseases (6). With this knowledge, we analyzed the role of MIF in HBs-specific CTL-induced liver injury using HBV transgenic mice. As previously demonstrated (1), this liver injury model consists of three steps after the CTL injection. The first step depends on antigen recognition on hepatocytes by the transferred CTLs, the second step is due to host-derived inflammatory cells by CTL-produced cytokines and chemokines, and the third step is brought about by host-derived lymph mononuclear cells. First, to determine whether MIF expression increased in the liver of transgenic mice after CTL injection, we injected 4 × 106 HBs-specific CTL clones (6C2) (1) into HBV transgenic mice (lineage 107-5). The mice were sacrificed at various time points, and MIF expression in their livers was analyzed by Western blotting and immunohistochemistry. We confirmed that serum alanine aminotransferase (sALT) activity began to increase at 4 h (sALT: 212 ± 23.1 IU/liter) and reached a peak at 48 h (sALT: 2,653 ± 488.7 IU/liter) in association with inflammatory cytokine production as previously reported (Fig. 2A) (24). In addition, we found that MIF expression was constitutively detected in the liver (2), although it was reduced at 4 h, began to increase at 12 h, and reached a peak at 24 h (Fig. 2B). Immunohistochemical staining revealed the presence of MIF expression in hepatocytes around the central vein and parenchyma under normal conditions (Fig. 2C). After CTL injection, the number of MIF-positive hepatocytes increased at 24 h; inflammatory cells, such as macrophages, also showed MIF expression.
FIG. 2.
MIF expression in the liver after CTL injection. (A) Age- and sex-matched transgenic mice (lineage 107-5) were injected with 4 × 106 CTLs (6C2) and sacrificed at the indicated time points. Total hepatic RNA (10 μg) was isolated from the livers at various time points and analyzed for cytokine expression by RPA. (B) Cell lysis, protein electrophoresis, and Western blotting analyses were performed as described previously (14). The membranes were probed with an anti-MIF antibody and then incubated with a horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (IgG) secondary antibody. Detection was performed using an ECL system. (C) Immunohistochemical staining with a rabbit anti-mouse MIF MAb was performed using an avidin-biotin-peroxidase complex technique. Briefly, tissue slices were incubated with the anti-mouse MIF MAb overnight, followed by treatment with 3% H2O2 in absolute methanol to inhibit endogenous peroxidase activity. Next, the slices were incubated with a biotinylated secondary antibody for 10 min, washed, and incubated with an avidin-biotin-peroxidase complex reagent. Finally, the slides were treated with 0.06% diaminobenzidine and 0.01% H2O2 in 0.05 M Tris-HCl buffer (pH 7.6) for 10 min. The arrowhead indicates macrophages.
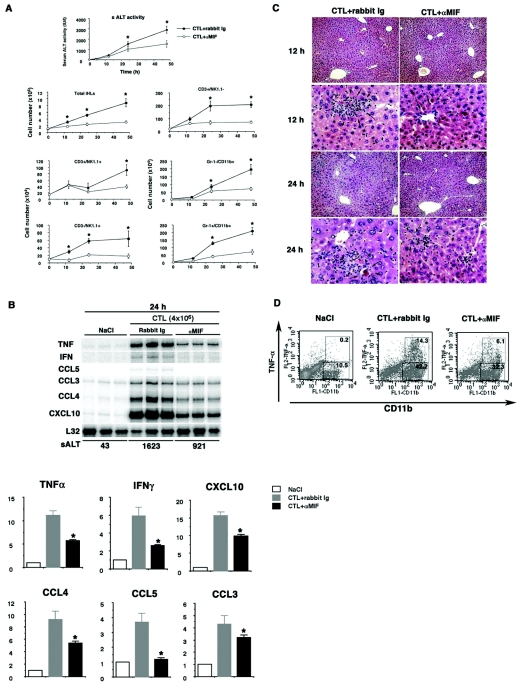
Next, we evaluated the effects of MIF neutralization on liver injury, since MIF expression was observed to increase in the liver after the CTL injection. To achieve this, we injected CTLs (4 × 106) into HBV transgenic mice (lineage 107-5) with or without pretreatment with an anti-mouse MIF monoclonal antibody (MAb; αMIF at 1 mg/mouse), which was administered once a day from 3 days before the CTL injection. As shown in Fig. 3A, αMIF treatment significantly reduced sALT activity at 24 and 48 h after the CTL injection. We also found a reduction in sALT activity with antibody treatment in mice of more CTLs (107) per injection (unpublished data). Consistent with this result, the αMIF treatment decreased inflammatory cell recruitment to the liver. Furthermore, as shown in Fig. 3B for three representative mice per group, αMIF treatment reduced the mRNA expressions of IFN-γ, TNF-α, and inflammatory chemokines at 24 h compared with untreated livers. To quantify the differences in mRNA expression, we evaluated the expression levels by calculating their intensities relative to L32 housekeeping gene expression as shown in Fig. 3B, bottom. Consistent with these findings, histological staining revealed that inflammatory cell infiltration and apoptotic hepatocytes decreased after αMIF treatment compared with control antibody treatment (Fig. 3C). Finally, to access the mechanism of the αMIF suppressive effect, we focused on the role of macrophages, since it has been reported that αMIF protects against serum TNF-α production in inflammatory diseases (5, 9). To clarify this hypothesis, we isolated IHLs from livers at 24 h after CTL injection (16) and analyzed their TNF-α expression by fluorescence-activated cell sorter (FACS) analysis. As shown in Fig. 3D, we found that TNF-α expression in macrophages was reduced after αMIF treatment compared with control antibody treatment, indicating that αMIF protects against inflammatory cytokine production by inflammatory cells in the liver.
FIG. 3.
Effects of anti-MIF antibody (αMIF) treatment on CTL-induced liver injury of transgenic mice. (A) Three lineage 107-5 HBV transgenic mice per group were injected intravenously with 4 × 106 6C2 cells or NaCl in the presence or absence of αMIF. The mean sALT activity measured at the time of autopsy is indicated for each group and expressed in international units per liter (mean ± standard deviation). *, P < 0.05. Next, IHLs were isolated from the animals, and the effects of αMIF treatment were analyzed. The number in each cell subset was calculated by multiplying the total number of IHLs by the frequency of each subset in the IHL population by FACS analysis (mean ± standard deviation). *, P < 0.05. (B) Total hepatic RNA (10 μg) was isolated from the livers at various time points and analyzed for cytokine and chemokine expressions by RPA as described in the legend for Fig. 1. Specific signals were detected using a BAS-2500 Imaging Analyzer (Fuji Film, Japan) and a FLA-3000 phosphorimager (Fuji Film). The mRNA expression levels were calculated as intensities relative to L32 housekeeping gene expression. (Bottom) *, P < 0.05. (C) Liver sections were obtained from mice sacrificed at 12 and 24 h after injection of CTLs and stained with hematoxylin and eosin. Note that in the control rabbit Ig-injected mice, small inflammatory foci containing mostly lymph mononuclear cells are present, and apoptotic hepatocytes are detected (arrows) in the liver. In mice with αMIF treatment, lymph mononuclear cells and apoptotic hepatocytes are reduced in the parenchyma (arrows). (D) The intracellular cytokine expressions by IHLs were examined at 24 h after injection with CTLs. IHLs were stained with anti-CD11b-fluorescein isothiocyanate and anti-TNF-α-phycoerythrin. Representative results of three independent experiments are shown.
In this study, we have demonstrated that, although MIF has no antiviral effect on HBV replication either in vivo or in vitro, it contributes to liver injury in a CTL-induced hepatitis model. To the best of our knowledge, this is the first report to present evidence for whether MIF has an antiviral effect on viral infection. Although we conclude that it has no antiviral effect against HBV replication, our results provide several important findings regarding MIF function. Importantly, we have demonstrated that αMIF treatment provides partial, but not complete, protection against CTL-induced liver injury, in accordance with a reduction in inflammatory cell recruitment. It has already been published that αMIF protects against the induction of inflammatory diseases and the mortality of septic shock (2, 3, 9, 18, 20, 23). Based on our current observations, we speculate that the protective effect of MIF neutralization for liver injury is mediated as follows. (i) αMIF prevents macrophages from triggering an exacerbated inflammatory response, since IFN-γ and TNF-α induce macrophages to express MIF, which in turn induces the expression of nitric oxide and TNF-α (4). (ii) αMIF reduces inflammatory cell recruitment via a reduction in leukocyte adhesion molecules on endothelial cells and inflammatory cells, as previously reported (11, 19). With regard to the last hypothesis, we did not observe any differences in CD44, CD62L, and intercellular adhesion molecule 1 expression on IHLs after αMIF treatment (data not shown), although we are also interested in the role of MIF in the interaction between endothelial cells and inflammatory cells. Further, it is of note that αMIF treatment had no suppressive effect on liver injury when administered after CTL injection (unpublished data), indicating that clinical application required more experiments.
In this study, we demonstrate that MIF has no antiviral effect on HBV replication; however, neutralization of MIF reduces CTLs-induced liver injury. These findings mean that αMIF treatment may be beneficial for therapy of severe hepatitis because MIF is unrelated to inhibition of viral replication.
Acknowledgments
We thank Francis V. Chisari and Stefan Wieland (The Scripps Research Institute) for providing us with HBV transgenic mice and technical advice, Toray Industries for the gift of murine IFN-β, and Shinichi Kakumu and Tetsuya Ishikawa (Aichi Medical University) for scientific advice.
This study was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1.Ando, K., T. Moriyama, L. G. Guidotti, S. Wirth, R. D. Schreiber, H. J. Schlicht, S. Huang, and F. V. Chisari. 1993. Mechanisms of class I restricted immunopathology. A transgenic mouse model of fulminant hepatitis. J. Exp. Med. 178:1541-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacher, M., A. Meinhardt, H. Y. Lan, W. Mu, C. N. Metz, J. A. Chesney, T. Calandra, D. Gemsa, T. Donnelly, R. C. Atkins, and R. Bucala. 1997. Migration inhibitory factor expression in experimentally induced endotoxemia. Am. J. Pathol. 150:235-246. [PMC free article] [PubMed] [Google Scholar]
- 3.Bozza, M., A. R. Satoskar, G. Lin, B. Lu, A. A. Humbles, C. Gerard, and J. R. David. 1999. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J. Exp. Med. 189:341-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calandra, T., J. Bernhagen, R. A. Mitchell, and R. Bucala. 1994. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J. Exp. Med. 179:1895-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calandra, T., B. Echtenacher, D. L. Roy, J. Pugin, C. N. Metz, L. Hultner, D. Heumann, D. Mannel, R. Bucala, and M. P. Glauser. 2000. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat. Med. 6:164-170. [DOI] [PubMed] [Google Scholar]
- 6.Calandra, T., and T. Roger. 2003. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat. Rev. Immunol. 3:791-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavanaugh, V. J., L. G. Guidotti, and F. V. Chisari. 1997. Interleukin-12 inhibits hepatitis B virus replication in transgenic mice. J. Virol. 71:3236-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.David, J. R. 1966. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc. Natl. Acad. Sci. USA 56:72-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jong, Y. P., A. C. Abadia-Molina, A. R. Satoskar, K. Clarke, S. T. Rietdijk, W. A. Faubion, E. Mizoguchi, C. N. Metz, M. Alsahli, T. ten Hove, A. C. Keates, J. B. Lubetsky, R. J. Farrell, P. Michetti, S. J. van Deventer, E. Lolis, J. R. David, A. K. Bhan, and C. Terhorst. 2001. Development of chronic colitis is dependent on the cytokine MIF. Nat. Immunol. 2:1061-1066. [DOI] [PubMed] [Google Scholar]
- 10.Franco, A., L. G. Guidotti, M. V. Hobbs, V. Pasquetto, and F. V. Chisari. 1997. Pathogenetic effector function of CD4-positive T helper 1 cells in hepatitis B virus transgenic mice. J. Immunol. 159:2001-2008. [PubMed] [Google Scholar]
- 11.Gregory, J. L., M. T. Leech, J. R. David, Y. H. Yang, A. Dacumos, and M. J. Hickey. 2004. Reduced leukocyte-endothelial cell interactions in the inflamed microcirculation of macrophage migration inhibitory factor-deficient mice. Arthritis Rheum. 50:3023-3034. [DOI] [PubMed] [Google Scholar]
- 12.Guidotti, L. G., and F. V. Chisari. 2001. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 19:65-91. [DOI] [PubMed] [Google Scholar]
- 13.Guidotti, L. G., T. Ishikawa, M. V. Hobbs, B. Matzke, R. Schreiber, and F. V. Chisari. 1996. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 4:25-36. [DOI] [PubMed] [Google Scholar]
- 14.Imose, M., M. Nagaki, K. Kimura, S. Takai, M. Imao, T. Naiki, Y. Osawa, T. Asano, H. Hayashi, and H. Moriwaki. 2004. Leflunomide protects from T-cell-mediated liver injury in mice through inhibition of nuclear factor kappaB. Hepatology 40:1160-1169. [DOI] [PubMed] [Google Scholar]
- 15.Kakimi, K., L. G. Guidotti, Y. Koezuka, and F. V. Chisari. 2000. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J. Exp. Med. 192:921-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura, K., K. Kakimi, S. Wieland, L. G. Guidotti, and F. V. Chisari. 2002. Activated intrahepatic antigen-presenting cells inhibit hepatitis B virus replication in the liver of transgenic mice. J. Immunol. 169:5188-5195. [DOI] [PubMed] [Google Scholar]
- 17.Kimura, K., K. Kakimi, S. Wieland, L. G. Guidotti, and F. V. Chisari. 2002. Interleukin-18 inhibits hepatitis B virus replication in the livers of transgenic mice. J. Virol. 76:10702-10707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi, S., J. Nishihira, S. Watanabe, and S. Todo. 1999. Prevention of lethal acute hepatic failure by antimacrophage migration inhibitory factor antibody in mice treated with bacille Calmette-Guerin and lipopolysaccharide. Hepatology 29:1752-1759. [DOI] [PubMed] [Google Scholar]
- 19.Lan, H. Y., M. Bacher, N. Yang, W. Mu, D. J. Nikolic-Paterson, C. Metz, A. Meinhardt, R. Bucala, and R. C. Atkins. 1997. The pathogenic role of macrophage migration inhibitory factor in immunologically induced kidney disease in the rat. J. Exp. Med. 185:1455-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maaser, C., L. Eckmann, G. Paesold, H. S. Kim, and M. F. Kagnoff. 2002. Ubiquitous production of macrophage migration inhibitory factor by human gastric and intestinal epithelium. Gastroenterology 122:667-680. [DOI] [PubMed] [Google Scholar]
- 21.Nishihira, J. 1998. Novel pathophysiological aspects of macrophage migration inhibitory factor (review). Int. J. Mol. Med. 2:17-28. [DOI] [PubMed] [Google Scholar]
- 22.Pasquetto, V., S. F. Wieland, S. L. Uprichard, M. Tripodi, and F. V. Chisari. 2002. Cytokine-sensitive replication of hepatitis B virus in immortalized mouse hepatocyte cultures. J. Virol. 76:5646-5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakai, Y., A. Masamune, A. Satoh, J. Nishihira, T. Yamagiwa, and T. Shimosegawa. 2003. Macrophage migration inhibitory factor is a critical mediator of severe acute pancreatitis. Gastroenterology 124:725-736. [DOI] [PubMed] [Google Scholar]
- 24.Takai, S., K. Kimura, M. Nagaki, S. Satake, K. Kakimi, and H. Moriwaki. 2005. Blockade of neutrophil elastase attenuates severe liver injury in hepatitis B transgenic mice. J. Virol. 79:15142-15150. [DOI] [PMC free article] [PubMed] [Google Scholar]