Abstract
A comparative study was conducted between two laboratories in order to evaluate the differences between two enzyme-linked immunosorbent assay (ELISA) techniques for the detection of pneumococcal anti-capsular polysaccharide antibodies. One laboratory used an assay including heterologous 22F polysaccharide inhibition, and the other laboratory employed a non-22F reference assay. After conjugate immunization, 30 pediatric post-primary immunization sera with antipolysaccharide concentrations ranging from <0.05 to 15 μg/ml were analyzed. Aggregate reverse cumulative distribution curves combining concentrations of antibodies against serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F revealed similar results for both methods at antibody levels of >1 μg/ml. However, at antibody levels of <1 μg/ml, the distribution curve measured with the 22F inhibition ELISA shifted toward lower levels. This observation suggests that the 22F inhibition assay is more specific at low antibody concentrations, which was confirmed by heterologous polysaccharide inhibition experiments. Translation of low antibody levels suggested that the proposed threshold concentration of 0.35 μg/ml determined with the non-22F ELISA corresponded to a concentration of 0.20 μg/ml with the 22F inhibition ELISA. Pneumococcal antipolysaccharide ELISA including 22F inhibition can be recommended as a reference method.
Pneumococcal polysaccharide (PS) antibody levels are the most commonly used correlate of clinical protection against invasive pneumococcal disease in infants after immunization with conjugate vaccines. Currently, the primary method for assessing pneumococcal capsular PS immunoglobulin G (IgG) antibodies is the enzyme-linked immunosorbent assay (ELISA). An aggregate threshold antibody concentration across the seven serotypes of 0.20 μg/ml (5) was initially proposed on the basis of the data generated in the pivotal seven-valent pneumococcal conjugate vaccine efficacy trial conducted in northern California (1). The value was revised after analysis of the pooled serology data of three clinical efficacy trials, conducted in northern California, among Navajo Indians and in Soweto, South Africa (1, 5, 7, 8). A new aggregate antibody concentration of 0.35 μg/ml was recommended by the WHO (12) as an estimate of the threshold concentration for protection against invasive disease. This refined threshold value is also recommended as the reference antibody concentration for demonstration of the noninferiority of new pediatric candidate vaccines compared with the licensed seven-valent conjugate vaccine.
Comparison of antibody concentrations determined by an alternative method and the reference method may result in defining a new threshold concentration (12). Before adopting an alternative assay, it is important to assess whether the analytical accuracy of the novel procedure is at least equivalent to the standard assay. Two WHO reference laboratories, located at the Institute of Child Health (ICH; London, United Kingdom) and the Department of Pathology at the University of Birmingham (Birmingham, Alabama), have been established to guide assay development and assist other laboratories in standardizing their own ELISA method and to ensure that the serological data obtained with alternative assays are comparable. The pneumococcal ELISA protocol used by these reference laboratories shows excellent correlation with the assay employed during the three clinical efficacy trials. This protocol encompasses the inhibition with cell wall PS (CPS) to reduce the detection of non-capsular-PS-specific antibodies (9).
It has recently been shown that competitive inhibition of antibody binding by a heterologous PS (22F) increases the ratio of functional to nonfunctional antibodies with reference to opsonophagocytic activity (2). The mechanism for this increased ratio relates to the adsorption of nonfunctional antibodies such as those directed against common protein and nonprotective PS epitopes. Following the recommendation of the WHO in 2000 (WHO Workshop, Geneva, Switzerland), a 22F inhibition ELISA was applied in the present study, aiming to increase the serotype specificity of pneumococcal anti-PS measurement. The specificity of the 22F inhibition ELISA method was evaluated with various serum samples by using inhibition with various heterologous PSs. The new assay was compared with the reference non-22F ELISA (9) employed at the WHO reference laboratory at the ICH and indeed demonstrated increased specificity at anti-PS levels of <1 μg/ml, resulting in a new aggregate threshold antibody concentration.
(The results of this comparative study were presented in part at the 4th International Symposium on Pneumococci and Pneumococcal Diseases in Helsinki, Finland, May 2004.)
MATERIALS AND METHODS
Serum samples A.
For the interlaboratory 22F-non-22F ELISA comparison, 30 pediatric sera were obtained from two different studies after pneumococcal conjugate immunization, covering an anti-PS concentration range of <0.05 to 15 μg/ml (as measured by non-22F ELISA). Twenty samples were from a study conducted with German infants who received an experimental pneumococcal conjugate vaccine containing PSs from 11 different Streptococcus pneumoniae serotypes (1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F), each conjugated to Haemophilus influenzae-derived protein D. The vaccine was given as a primary vaccination course to infants in their third, fourth, and fifth months of life, coadministered with a DTPa-HBV-IPV/Hib vaccine (Infanrix Hexa; GlaxoSmithKline Biologicals [GSK], Rixensart, Belgium). Ten samples were from a study conducted with Lithuanian infants who were immunized at 3, 4.5, and 6 months of age with the same 11-valent pneumococcal conjugate vaccine coadministered with DTPa-IPV/Hib vaccine (Infanrix IPV/Hib; GSK). In all cases, serum samples were taken 1 month after administration of the third dose.
Serum samples B.
A second 22F-non-22F ELISA comparison was performed in order to evaluate differences between pre- and post-primary immunization serum samples for which serotype 6B is shown as an example. The tests were performed with 75 preimmunization and 79 postimmunization sera from Belgian infants immunized at 2, 3, and 4 months of age with the same 11-valent vaccine or the licensed seven-valent pneumococcal conjugate vaccine (serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F) coadministered with Infanrix Hexa.
Serum samples C.
The effect of the 22F inhibition ELISA on the apparent antibody level was evaluated by using pediatric serum samples from the German study described above, including seven control sera from infants immunized with Infanrix Hexa alone, and the 20 postconjugate sera as already described. The sera were tested at the WHO reference laboratory by using both the original non-22F ELISA (9) and the 22F inhibition ELISA.
Serum samples D.
The specificity of the non-22F and 22F inhibition ELISAs was assessed with pediatric and adult serum samples from a variety of different studies, obtained pre- or post-pneumococcal conjugate and PS immunizations. The PS4 ELISA was taken as an example, with anti-PS4 serum concentrations ranging from 0.5 to 4.25 μg/ml. The details of the studies are not relevant because each sample was analyzed with and without 22F as a paired comparison.
Non-22F ELISA.
The non-22F pneumococcal ELISA employed was equivalent to the standard protocol applied by Wyeth Lederle Vaccines (Pearl River, NY) during the three efficacy trials establishing the aggregate threshold of 0.35 μg/ml. The WHO reference laboratory for pneumococcal antibody assays at the ICH in-transferred the Wyeth assay in and demonstrated excellent agreement (data not shown). Briefly, serotype-specific pneumococcal PSs (American Type Culture Collection [ATCC], Manassas, Va.) were adsorbed onto medium-binding plates (Greiner, Gloucester, United Kingdom) at 37°C for 5 h. Serum samples were diluted with PBST (phosphate-buffered saline-Tween) containing 10 μg/ml of cell wall PS (Statens Serum Institut, Copenhagen, Denmark) and incubated for 30 min at room temperature. The standard serum 89-SF (courtesy of Carl Frasch, Food and Drug Administration) was incubated with 10 μg/ml of CPS-PBST. Sera were serially diluted and added to the coated and washed plates, which were incubated for 2 h at room temperature. After a further washing step, an alkaline phosphatase-labeled goat anti-human IgG (Biosource, Camarillo, CA) was added and the mixture was incubated for 2 h at room temperature and developed with p-nitrophenyl phosphate substrate in diethanolamine buffer. Plates were read at 405 nm with a 630-nm background filter after 2 h. Serotype-specific IgG concentrations of the samples were expressed in micrograms per milliliter on the basis of the standard 89-SF serum. The limit of quantification of the ELISA was 0.016 to 0.036 μg/ml of IgG, depending on the serotype.
22F inhibition ELISA.
The two laboratories involved in this study employed two different 22F inhibition ELISAs to measure anti-PS IgG antibodies in human sera. (i) The 22F inhibition ELISA method used by the laboratory at GSK was essentially based on an assay proposed in 2001 by Concepcion and Frasch (2). Briefly, purified pneumococcal PSs (GSK) were mixed with methylated human serum albumin and adsorbed onto Nunc Maxisorp (Nunc, Roskilde, Denmark) high-binding microtiter plates overnight at 4°C. The plates were blocked with 10% fetal bovine serum (FBS) in PBS for 1 h at room temperature with agitation. Serum samples were diluted in PBS containing 10% FBS, 10 μg/ml of CPS (Statens Serum Institut), and 2 μg/ml of pneumococcal PS of serotype 22F (ATCC) and further diluted on the microtiter plates with the same buffer. An internal reference calibrated against standard serum 89-SF using the serotype-specific IgG concentrations in 89-SF (9) was treated in the same way and included on every plate. After washing, the bound antibodies were detected by using peroxidase-conjugated anti-human IgG monoclonal antibody (Stratech Scientific Ltd., Soham, United Kingdom) diluted in 10% FBS (in PBS) and incubated for 1 h at room temperature with agitation. The color was developed by using a ready-to-use single component tetramethylbenzidine peroxidase enzyme immunoassay substrate kit (Bio-Rad, Hercules, CA) in the dark at room temperature. The reaction was stopped with H2SO4 at 0.18 M, and the optical density at 450 nm was read. Serotype-specific IgG concentrations (in micrograms per milliliter) in the samples were calculated by referencing optical density points within defined limits to the internal reference serum curve, which was modeled by a four-parameter logistic log equation calculated with SoftMax Pro (Molecular Devices, Sunnyvale, CA) software. The cutoff for the ELISA was 0.05 μg/ml of IgG for all serotypes, taking into account the limit of detection and the limit of quantification. When purified pneumococcal PSs from different companies (GSK and ATCC) were compared, they were both equally effective as coating antigens in ELISAs to measure specific IgG levels in human sera. (ii) An alternative 22F ELISA method was applied by the WHO reference laboratory at the ICH. The assay has been described in the Training Manual for Enzyme Linked Immunosorbent Assay for the Quantitation of S. pneumoniae Serotype Specific IgG (Pn PS ELISA) (http://www.vaccine.uab.edu/). It was developed based on the reference non-22F ELISA described above, with the modification that unknown samples were incubated with 5 μg/ml of 22F PS and 10 μg/ml of CPS.
Specificity of non-22F and 22F inhibition ELISAs.
The specificity of the non-22F and 22F inhibition ELISAs was evaluated by paired comparison experiments performed with PSs from different serotypes. Pediatric and adult serum samples were incubated for 1 h at 37°C in the absence and in the presence of homologous PS (PS4) or heterologous PS (6B, 9V, 14, 18C, 19F, or 23F) at concentrations of PS ranging between 0.5 and 4.25 μg/ml, depending on the serotype. Inhibition was expressed as the mean ratio of antibodies measured in neutralized sera versus sera not neutralized with homologous or heterologous PS. The ratio equals 1 for no inhibition and close to 0 for full inhibition. After incubation, antibody concentrations in both neutralized and nonneutralized samples were determined by non-22F and 22F ELISAs.
Statistical methods. (i) Calculation of a new threshold antibody concentration for the 22F inhibition ELISA.
IgGs to PS 4, 6B, 9V, 14, 18C, 19F, and 23F were measured in 30 samples (samples A) on two occasions with the non-22F ELISA (ICH laboratory) and on two or three occasions with the 22F ELISA (GSK laboratory). Geometric mean concentrations (GMCs) of these repeats were calculated for each PS per laboratory. To illustrate the antibody response after pneumococcal immunization, an aggregate reverse cumulative distribution curve (RCDC) was plotted for each ELISA method with the antibody concentrations of all seven PSs combined. The new antibody threshold for the 22F inhibition ELISA was determined for the same response level (percent) as the threshold of 0.35 μg/ml established with the reference non-22F ELISA.
(ii) Comparison of antibody concentrations generated by two different ELISA methods.
Antibody concentrations were assayed in pediatric samples (samples A) with the non-22F ELISA (ICH laboratory) and with the 22F ELISA (GSK laboratory). The difference between concentration measurements with and without 22F was then evaluated on the basis of log10-transformed double-positive results (above the assay cutoff for both laboratories) for each PS. From the mean difference and the standard deviation, the geometric mean ratio and 95% confidence interval (CI) between results were calculated by transforming back into the original units.
(iii) Evaluation of the specificity of the ELISA method.
The effect of the ELISA method on the ratio of antibodies measured in PS-neutralized sera versus nonneutralized sera (samples D) was tested by analysis of variance using the MIXED procedure of the SAS System (SAS Institute, Inc., Cary, NC). The ratio was shown not to be affected by the treatment factor “sample type” (pediatric or adult) or “PS” (no significant interaction). Therefore, mean ratios were calculated for both the 22F and non-22F ELISAs with all samples combined.
Samples for which the interrepeat coefficient of variation was greater than 100% were eliminated from all of the statistical analyses described in the present paper (one sample for PS4 and one sample for PS19F).
RESULTS
RCDCs of pediatric postimmunization pneumococcal PS antibody concentrations (samples A).
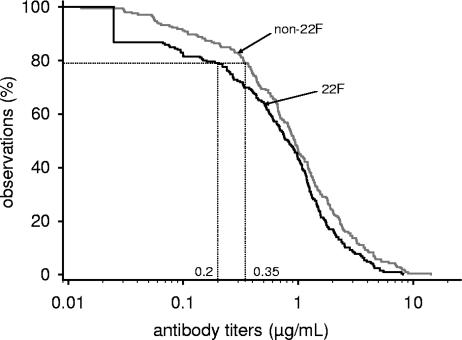
Distributions of antibody concentrations measured in the absence and in the presence of 22F were analyzed by RCDCs (10). The aggregate RCDCs across the seven anti-PS responses for the non-22F ELISA (ICH laboratory) versus the 22F inhibition ELISA (GSK laboratory) are presented in Fig. 1. Aggregate RCDCs combining concentrations of antibodies against serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F revealed similar results for both methods at antibody levels of >1 μg/ml. However, at antibody concentrations of <1 μg/ml, the distribution curve measured with the 22F inhibition ELISA shifted toward lower levels. Antibody concentrations of ≥0.35 μg/ml were exhibited by 78.9% of the results obtained with the non-22F assay. In contrast, the corresponding concentration in the 22F inhibition ELISA was 0.20 μg/ml.
FIG. 1.
Aggregate RCDCs of pediatric post-conjugate pneumococcal anti-PS ELISA comparing two assays. Curves were calculated from antibodies to serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F based on 22F inhibition ELISA (GSK laboratory) or non-22F ELISA (ICH laboratory). Thirty serum samples were obtained after primary immunization with pneumococcal conjugate vaccine. Concentrations of ≥0.35 μg/ml were exhibited by 78.9% of the observations obtained with the non-22F reference ELISA. This percentage corresponds to a threshold of 0.20 μg/ml compared to the 22F inhibition ELISA curve.
RCDCs of pre- and postimmunization antibody concentrations (samples B).
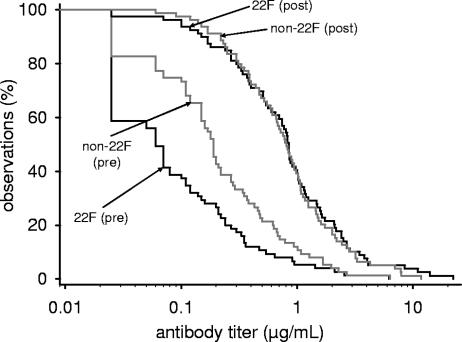
The effect of 22F adsorption on apparent antibody concentrations was measured in sera from infants pre- and post-primary vaccination and evaluated by analyzing RCDCs of IgG concentrations derived from anti-PS 6B ELISA as an example (Fig. 2). At the assay cutoff value (0.05 μg/ml), 84 and 56% of the prevaccination subjects had detectable antibodies according to the non-22F and 22F inhibition ELISAs, respectively. For post-primary vaccination subjects, detectable antibodies were found in 100 and 97% of the samples. From Fig. 2, it can be concluded that the antibody distribution curve shifted toward lower concentrations when measured by the 22F inhibition assay. However, this effect was observed more clearly with prevaccination than with postvaccination samples.
FIG. 2.
RCDCs for anti-PS6B comparing pediatric sera pre- and post-conjugate pneumococcal vaccination. Anti-PS IgG ELISA (GSK laboratory) was performed with and without neutralization with PS 22F. The tests were performed with 75 preimmunization and 79 postimmunization sera from Belgian infants immunized at 2, 3, and 4 months of age with an 11-valent or a seven-valent pneumococcal conjugate vaccine coadministered with a DTPa-HBV-IPV/Hib vaccine.
Comparison of antibody concentrations generated by two different ELISA methods (samples A).
Antipneumococcal antibodies were measured in pediatric samples by two different ELISA methods, one without 22F (at ICH) and the other including 22F (at GSK) to remove nonspecific antibodies (Table 1). The geometric mean ratio of the IgG antibody concentrations measured under both conditions was calculated. A ratio of 1 would suggest no difference between the two measurements. However, the ELISA 22F/non-22F antibody ratio was below 1 for all PSs but one (PS18C), ranging between 0.50 (PS6B) and 0.89 (PS4). This decrease in antibody concentrations observed with the 22F inhibition ELISA was statistically significant for PS6B, PS14, PS19F, and PS23F.
TABLE 1.
Comparison of 22F and non-22F ELISAsa
| Parameter | PS4 | PS6B | PS9V | PS14 | PS18C | PS19F | PS23F |
|---|---|---|---|---|---|---|---|
| Mean 22F/non-22F ratio | 0.89 | 0.50 | 0.87 | 0.70 | 1.03 | 0.73 | 0.56 |
| 95% CI | 0.79, 1.00 | 0.40, 0.62 | 0.73, 1.04 | 0.65, 0.76 | 0.89, 1.20 | 0.65, 0.81 | 0.41, 0.75 |
| No. of double-positive samples | 27 | 24 | 27 | 30 | 26 | 24 | 21 |
The reference non-22F ELISA (ICH laboratory) and the 22F inhibition ELISA (GSK laboratory) were compared by using anti-PS 4, 6B, 9V, 14, 18C, 19F, and 23F IgG ELISA titers from 30 post-primary immunization pediatric samples. Only the samples for which the antibody concentration was equal to or greater than the assay cutoff at both laboratories were considered for the statistical analyses (number of double-positive samples). Values are expressed as the mean 22F/non-22F ELISA antibody concentration ratio. A mean ratio and 95% CI below 1 indicates that the 22F ELISA method gives significantly lower results.
Effect of removal of nonspecific antibodies by 22F (samples C).
The effect of 22F PS on antibodies measurd in pediatric samples was further evaluated (ICH laboratory). Serum samples obtained 1 month after primary vaccination with either pneumococcal conjugate vaccine or control vaccine were measured on two occasions by both the non-22F and 22F inhibition ELISAs. The average of the log10-transformed data was calculated for each sample and represents the final concentration. Table 2 presents the average percentage of 22F inhibition for groups of serum samples taken after immunization (6 months of age) with either control vaccine or pneumococcal conjugate vaccine. In general, samples of all categories displayed a titer reduction for all PSs when measured by the 22F inhibition ELISA. Up to 42% of the positive findings according to the non-22F ELISA were not detected by the 22F ELISA. Inhibition of anti-PS levels after 22F adsorption was generally more pronounced in the control group than in the pneumococcal conjugate group.
TABLE 2.
Effect of adsorption with 22F of serum samples from infants vaccinated with conjugate pneumococcal vaccine or control vaccinea
| PS and post-primary immunization group | ELISA | GMC (μg/ml) | LL | UL | % Inhibition by 22F |
|---|---|---|---|---|---|
| 4 | |||||
| Control | Non-22F | 0.21 | 0.08 | 0.55 | 32 |
| 22F | 0.14 | 0.06 | 0.37 | ||
| Pneumo | Non-22F | 0.72 | 0.40 | 1.27 | 16 |
| 22F | 0.60 | 0.34 | 1.06 | ||
| 6B | |||||
| Control | Non-22F | 0.47 | 0.17 | 1.28 | 41 |
| 22F | 0.28 | 0.10 | 0.76 | ||
| Pneumo | Non-22F | 0.40 | 0.22 | 0.72 | 39 |
| 22F | 0.24 | 0.13 | 0.44 | ||
| 9V | |||||
| Control | Non-22F | 0.27 | 0.10 | 0.73 | 33 |
| 22F | 0.18 | 0.07 | 0.49 | ||
| Pneumo | Non-22F | 0.57 | 0.32 | 1.02 | 16 |
| 22F | 0.48 | 0.27 | 0.86 | ||
| 14 | |||||
| Control | Non-22F | 0.79 | 0.31 | 2.06 | 17 |
| 22F | 0.66 | 0.25 | 1.72 | ||
| Pneumo | Non-22F | 2.73 | 1.55 | 4.80 | 14 |
| 22F | 2.36 | 1.34 | 4.15 | ||
| 18C | |||||
| Control | Non-22F | 0.15 | 0.06 | 0.39 | 30 |
| 22F | 0.10 | 0.04 | 0.27 | ||
| Pneumo | Non-22F | 0.36 | 0.20 | 0.64 | 22 |
| 22F | 0.28 | 0.16 | 0.50 | ||
| 19F | |||||
| Control | Non-22F | 1.29 | 0.52 | 3.20 | 31 |
| 22F | 0.89 | 0.36 | 2.21 | ||
| Pneumo | Non-22F | 1.63 | 0.94 | 2.83 | 18 |
| 22F | 1.33 | 0.77 | 2.31 | ||
| 23F | |||||
| Control | Non-22F | 0.41 | 0.14 | 1.21 | 42 |
| 22F | 0.24 | 0.08 | 0.70 | ||
| Pneumo | Non-22F | 0.26 | 0.14 | 0.49 | 26 |
| 22F | 0.19 | 0.10 | 0.36 |
Samples were from a study conducted with German infants who received an experimental 11-valent pneumococcal conjugate vaccine coadministered with a DTPa-HBV-IPV/Hib vaccine (Pneumo, n = 20) or the DTPa-HBV-IPV/Hib vaccine alone (Control, n = 7). Samples were taken 1 month after administration of the third of three vaccinations. LL and UL, lower and upper limits of the 95% CI, respectively. For comparisons between the control and pneumococcal vaccine groups, samples in the same concentration range were used when possible. % Inhibition = 100 − [GMC in presence of 22F/GMC in absence of 22F× 100].
Specificity of the assays (samples D).
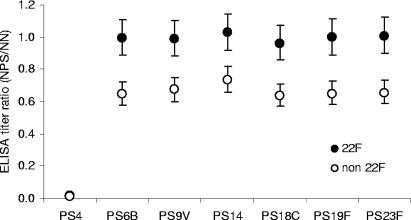
The specificity of the 22F inhibition ELISA was evaluated by inhibition experiments with homologous (PS4) and heterologous PSs, with the PS4 IgG ELISA given as an example (Fig. 3). In terms of PS inhibition, the statistical analysis revealed no difference between the effects of different heterologous PSs or between pediatric and adult serum samples. Therefore, results obtained with serum samples from various sources were pooled and expressed as the mean ratio of antibody titers measured in the absence versus in the presence of heterologous PS. Inhibition of antibody detection in the serum samples after preincubation with homologous PS4 was almost 100% with both the non-22F and 22F ELISAs. The mean antibody titer ratio, calculated with all samples and inhibition with heterologous PS combined, was close to 1 for the 22F ELISA (0.99; 95% CI, 0.89 to 1.12) and 33% lower for the non-22F ELISA (0.66; 95% CI, 0.59 to 0.75). This difference was highly significant (P = 0.0003), indicating that the non-22F assay is susceptible to heterologous inhibition.
FIG. 3.
Inhibition experiments with homologous or heterologous PS. Serum samples from different sources were analyzed pairwise for anti-PS4 antibodies after preincubation without or with homologous PS4 and different heterologous PSs (6B, 9V, 14, 18C, 19F, and 23F). Results obtained with the 22F and non-22F ELISAs are compared and expressed for each ELISA method as the ratio of antibody concentrations measured in the absence and in the presence of inhibitory PS. NN, nonneutralized sera; NPS, sera neutralized with homologous or heterologous PS.
DISCUSSION
The PS capsule of S. pneumoniae confers serotype-specific protection and is the primary virulence factor for invasive pneumococcal disease (6). At present, IgG pneumococcal anti-capsular antibody concentrations measured by ELISA offer the most quantitative in vitro measurement of short-term protection. It is currently understood that protective immunity to invasive pneumococcal disease is only established when antibody concentrations reach a particular threshold level (5). Based on the consensus that a 0.35-μg/ml IgG antibody level (as determined by non-22F ELISA) represents a reasonable estimate of a surrogate marker for protection against invasive disease, new pneumococcal vaccines will have to demonstrate immunological noninferiority to the licensed seven-valent pneumococcal conjugate vaccine with this threshold as the primary endpoint. However, the assay used to measure anti-PS IgG antibodies in human serum has recently been improved by the addition of the heterologous 22F PS to the sample diluent, which significantly enhanced the serotype specificity of the assay through adsorption of non-PS-specific antibodies. When employing the 22F inhibition ELISA, it is necessary to adapt the established threshold that is based upon the non-22F ELISA. Aggregate RCDCs of antipneumococcal PS antibodies measured in pediatric samples after conjugate immunization were used to compare the two ELISA methods. The comparison revealed that the 22F adsorption of pediatric post-conjugate immunization sera was a more specific measurement at anti-PS antibody levels below 1 μg/ml. The proposed threshold of 0.35 μg/ml, as measured by way of non-22F anti-PS ELISA, was found to be comparable to 0.20 μg/ml for the 22F inhibition ELISA.
The type specificity of the anti-PS IgG ELISA was assessed by studying inhibition with homologous and heterologous PSs. Human sera from various origins (adults and infants, pre- and post-pneumococcal conjugate and PS immunization) were used, and the inhibition was calculated for both the non-22F and 22F inhibition ELISAs. Inhibition caused by heterologous PS was evidenced in the non-22F rather than in the 22F assay, indicating that parts of the antibody levels in the non-22F ELISA were nonspecific for PS. The type specificity of the 22F assay was therefore improved compared to that of the non-22F ELISA, thus confirming previous findings (2).
Use of the 22F inhibition ELISA resulted in a reduction in the antibody level compared to that obtained with the non-22F ELISA. This effect was most pronounced in nonimmunized infants and adults and can be explained by the neutralization or removal of non-PS-specific antibodies by 22F PS. If not removed, these antibodies, and thus the count of antibodies in the serum, are overestimated. In infant sera, the non-PS-specific antibodies were mainly present in the preimmune samples, while post-conjugate immunization sera showed good specificity in either assay, which is in agreement with the findings from other studies (2, 3, 11). Consistently, the percentage of 22F inhibition was higher in the control group than in the group of sera obtained after pneumococcal vaccination.
Natural antibodies contribute to the non-PS-specific inhibitions observed. In infants, non-PS-specific antibodies originate from placental transfer of maternal antibodies (4). After conjugate immunization, the natural antibodies become masked by the increasing amount of PS-specific antibodies induced. An increased ratio of non-PS-specific to PS-specific antibodies accounts for the shift of the RCDC of the antibody concentration toward lower values at antibody concentrations of <1 μg/ml observed after 22F inhibition ELISA.
In summary, we showed that the serotype specificity of the 22F assay was improved compared with that of the non-22F ELISA. By removing non-PS-specific antibodies, the 22F inhibition ELISA will lead to lower (but real) anti-PS levels in the critical range of <1 μg/ml. The differences observed between the two assays, particularly in low-range pediatric antibody levels, prompted us to propose an alternative threshold of 0.20 μg/ml for the 22F-ELISA. In conjunction with the more specific ELISA, this lower threshold antibody concentration can be adopted for noninferiority analyses comparing new pneumococcal conjugate vaccines with the licensed seven-valent vaccine (12).
Acknowledgments
We thank Nathalie Durant, Mohamed Benaata, and Dany DeGrave for scientific support; David Le Tallec and Dominique Derreumaux for statistical analyses; and Ulrike Krause for editorial assistance.
REFERENCES
- 1.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, K. Edwards, and Northern California Kaiser Permanente Vaccine Study Center Group. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 2.Concepcion, N. F., and C. E. Frasch. 2001. Pneumococcal type 22F polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 8:266-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feikin, D. R., C. M. Elie, M. B. Goetz, J. L. Lennox, G. M. Carlone, S. Romero-Steiner, P. F. Holder, W. A. O'Brien, C. G. Whitney, J. C. Butler, and R. F. Breiman. 2004. Specificity of the antibody response to the pneumococcal polysaccharide and conjugate vaccines in human immunodeficiency virus-infected adults. Clin. Diagn. Lab. Immunol. 11:137-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inostroza, J., S. Villanueva, K. Mason, L. E. Leiva, and R. U. Sorensen. 2005. Effects of absorption with pneumococcal type 22F polysaccharide on maternal, cord blood, and infant immunoglobulin G antipneumococcal polysaccharide antibodies. Clin. Diagn. Lab. Immunol. 12:722-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jódar, L., J. Butler, G. Carlone, R. Dagan, D. Goldblatt, H. Käyhty, K. Klugman, B. Plikaytis, G. Siber, R. Kohberger, I. Chang, and T. Cherian. 2003. Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine 21:3265-3272. [DOI] [PubMed] [Google Scholar]
- 6.Kelly, T., J. P. Dillard, and J. Yother. 1994. Effect of genetic switching of capsular type on virulence of Streptococcus pneumoniae. Infect. Immun. 62:1813-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klugman, K. P., S. A. Madhi, R. E. Huebner, R. Kohberger, N. Mbelle, and N. Pierce. 2003. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N. Engl. J. Med. 349:1341-1348. [DOI] [PubMed] [Google Scholar]
- 8.O'Brien, K. L., L. H. Moulton, R. Reid, R. Weatherholtz, J. Oski, L. Brown, G. Kumar, A. Parkinson, D. Hu, J. Hackell, I. Chang, R. Kohberger, G. Siber, and M. Santosham. 2003. Efficacy and safety of seven-valent conjugate pneumococcal vaccine in American Indian children: group randomised trial. Lancet 362:355-361. [DOI] [PubMed] [Google Scholar]
- 9.Quataert, S. A., C. S. Kirch, L. J. Quackenbush Wiedl, D. C. Phipps, S. Strohmeyer, C. O. Cimino, J. Skuse, and D. V. Madore. 1995. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin. Diagn. Lab. Immunol. 2:590-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reed, G. F., B. D. Meade, and M. C. Steinhoff. 1995. The reverse cumulative distribution plot: a graphic method for exploratory analysis of antibody data. Pediatrics 96:600-603. [PubMed] [Google Scholar]
- 11.Soininen, A., G. van den Dobbelsteen, L. Oomen, and H. Käyhty. 2000. Are the enzyme immunoassays for antibodies to pneumococcal capsular polysaccharides serotype specific? Clin. Diagn. Lab. Immunol. 7:468-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. 2005. Pneumococcal conjugate vaccines. Recommendations for the production and control of pneumococcal conjugate vaccines. WHO Tech. Rep. Ser. 927(Annex 2):64-98. [Google Scholar]