Abstract
The Bühlmann CAST 2000 enzyme-linked immunosorbent assay is a potentially useful assay for measuring sulfidoleukotrienes released in vitro by allergen-challenged basophils. However, we observed that the positive-control reagent yielded positive signals in cell-free systems. These false-positive results depended on using a mouse anti-FcɛRI monoclonal antibody and were prevented by degranulation-inducing reagents other than mouse monoclonal antibodies.
Assays measuring sulfidoleukotrienes (sLT) released by blood basophils following in vitro challenge with specific substances are important diagnostic tools for immunoglobulin E (IgE)-dependent and IgE-independent allergies. As for the latter ones, to date basophil degranulation tests are the only available in vitro diagnostic tools for studying a large proportion of adverse reactions to drugs (anesthetics, nonsteroidal anti-inflammatory drugs, and antibiotics) and food additives (2, 7).
The Bühlmann CAST 2000 enzyme-linked immunosorbent assay (ELISA) (Bühlmann, Schoenenbuch, Switzerland) quantifies sLT released by blood basophils by a procedure suitable to clinical laboratories and has good predictive power for non-IgE-dependent reactions (1, 6).
We observed that the positive control reagent of CAST 2000, indicated by the manufacturer as an activating monoclonal antibody (MAb) to FcɛRI, also provoked sLT signals in cell-free samples or in cellular assays devoid of sLT. This applied to reagents belonging to different batches, which were purchased over a 3-year time period.
The principle part of CAST 2000 is covered by a patented technology (U.S. patent no. 5,487,977) and can be only partly extrapolated from the information made available by the manufacturer. Briefly, dextran gradient-enriched leukocytes are tested for specific allergen stimulation, basal sLT release, and maximum release following stimulation with an activating anti-FcɛRI MAb (the positive control reagent). The manufacturer informs that the CAST 2000 MAb also binds domain 1 of the FcɛRI α-chain in the presence of IgE. According to the manufacturer, 200 pg/ml sLT release is the minimum value to be considered positive. The ELISA is performed using microtiter wells precoated with an antimouse IgG antibody. In a single incubation step, the sample under analysis, a developing reagent (sLT covalently bound to alkaline phosphatase [sLT-AP]) and a MAb specific for sLT are added to the reaction well. The sLT in each sample compete with sLT-AP, and equilibrium is reached. The amount of sLT-AP captured by the specific MAb is inversely proportional to the amount of competing sLT, released by basophils in the sample. After washing, captured sLT-AP are measured using para-nitrophenyl-phosphate as a substrate. A calibration curve has to be determined for each analysis, using samples containing known amounts of sLT. We observed that for 164 subjects for whom routine allergological evaluation was requested over a 6-month period, values of sLT in positive control samples ranged from 1,088 to 6,473 pg/ml (median, 2,244; interquartile range, 1,828 to 2,800). Thus, surprisingly, all positive controls were well above the threshold value (200 pg/ml). Noticeably, when blood from 17 healthy individuals was tested for sLT release triggered by the positive control reagent, no significant difference was observed when comparing samples containing cell supernatants (median, 2,782 pg/ml; range, 1,903 to 4,835 pg/ml) with samples containing only the reaction buffer instead of the cell supernatants (median, 2,560 pg/ml; range, 1,670 to 3,678 pg/ml).
In order the study this problem, we used the following MAb as alternative controls (positive and negative): (i) 9E1, a mouse anti-FcɛRI MAb with characteristics similar to those of the CAST 2000 MAb (11, 12); (ii) OK-T4, an anti-human CD4 MAb (9) (protein A purified from hybridoma supernatant); (iii) AC38, an anti-idiotype MAb specific for antibody B1-8 (10) (a kind gift of Roberto Sitia, University Vita-Salute San Raffaele, Milan); (iv) anti-NIP (4-hydroxy-3-nitro-phenacetyl) human recombinant IgE MAb (8) (Serotec, Oxford, United Kingdom). After leukocyte incubation with anti-NIP IgE, bovine serum albumin (BSA) covalently linked to NIP (NIP-BSA) (stoichiometric ratio of NIP/BSA, >10) (Biosearch Technologies, Vacaville, CA) was added at 1 μg/ml to induce IgE cross-linking and basophil degranulation.
All MAb were added at 5 μg/ml, and the positive control was added under the conditions indicated by the manufacturer.
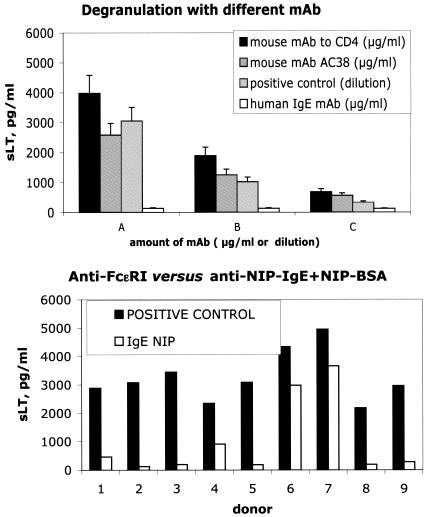
We found that with all mouse MAb but not with the human IgE MAb, a dose-dependent effect was observed in the sLT signal developed by the kit, suggesting a role for mouse MAb in these false-positive results (Fig. 1, top panel).
FIG. 1.
Top panel, CAST 2000 ELISA apparent sLT release (in pg/ml, on the y axis) in samples containing only buffer and MAb. Two different mouse MAb and a human IgE MAb (with specificities irrelevant to basophil activation) were used at 5, 1, or 0,2 μg/ml (A, B, and C, respectively, on the x axis). The positive control reagent provided with the kit was used at three different concentrations (1 to 5, 1 to 25, or 1 to 125, A, B, and C on the x axis, respectively). Bottom panel, sLT release (pg/ml) following maximum stimulation of blood basophils from nine healthy individuals (donor number on the x axis). Stimulation was achieved either with the positive control of the kit or with a human anti-NIP IgE MAb cross-linked with NIP-BSA.
Furthermore, degranulation of basophils from nine healthy individuals was induced by using either the anti-FcɛRI MAb provided with the kit (positive control) or human anti-NIP IgE plus NIP-BSA. The range of sLT concentration in the supernatant was 2,183 to 4,950 or 123 to 3,659 pg/ml, respectively (Fig. 1, bottom panel). Of note, when stimulated with anti-NIP IgE followed by NIP-BSA, two samples (numbers 2 and 8 in Fig. 1) yielded values below the threshold suggested as the lower acceptable limit for maximum degranulation (200 pg/ml).
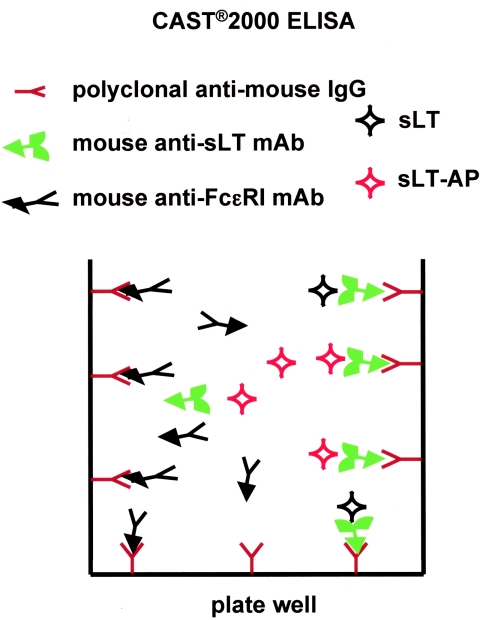
The main result of the present note is that the positive control of a common diagnostic kit (Bühlmann CAST 2000 ELISA), used to measure basophil degranulation via sLT release, does not provide reliable results, since it also yields a positive signal in the absence of sLT. This problem likely derives from the design of CAST 2000. The presence of the mouse anti-FcɛRI MAb in the positive control supernatant causes competition with the mouse anti-sLT MAb towards the solid-phase antimouse IgG antibodies. A diagram of the detection system is shown in Fig. 2. Our hypothesis is supported by the fact that several mouse IgG MAb, with either identical (9E1) or unrelated (OK-T4 and AC38) specificity, reproduced the false-positive result. In contrast, when a human IgE MAb was used, no false-positive results were detected.
FIG. 2.
Diagram of the CAST 2000 ELISA and the mechanism for the false-positive result generation. sLT covalently bound to alkaline phosphatase (sLT-AP) competes with sLT present in the sample (sLT) for binding to a mouse anti-sLT MAb. The immune complex (MAb-sLT) is captured by the antimouse antibody coated on the ELISA plate. The signal is inversely proportional to the amount of sLT. In the positive control sample, a mouse MAb (anti-FcɛRI) could compete with the anti-sLT MAb for the binding to the antimouse antibody coated on the ELISA plate, inducing a false-positive signal.
Concerning the alternatives for obtaining maximum stimulation, cross-linking human IgE via a multihapten carrier did not interfere with the CAST 2000 detection system. However, the presence of endogenous IgE bound to FcɛRI has to be taken into account as a factor potentially limiting the appropriate evaluation of maximum release.
The above-reported method for inducing maximum degranulation faithfully reproduces an IgE-induced activation. However, this might not be easy to transfer to the routine laboratory. A possible alternative would be the usage of the formyl-methionyl-leucyl-phenylalanine peptide in the same reaction condition of the anti-FcɛRI antibody. This peptide is a well-known nonspecific leukocyte activator (4), and it has been used in a commercial test (the BASOTEST), based on previous findings (5). However, several limitations have been reported for its usage, e.g., the fact that it does not mimic an IgE-induced activation. More importantly, the extent of basophil activation with N-formyl-methionyl-leucyl-phenylalanine does not parallel that observed with anti-IgE reagents, and it is often several times lower (3). Dedicated studies on cohorts of proper numerosity are needed to clarify the potential of this and other nonspecific, degranulation-inducing reagents.
The CAST 2000 could potentially be a useful assay for measuring the functional activity of the primary effector cells of allergy. However, the lack of a proper positive control precludes the detection of samples where degranulation already took place, either in vivo (for biological reasons) or ex vivo for the improper handling of the blood sample. Moreover, the correct measurement of maximum versus baseline degranulation could be exploited in unexplored situations of chronic mediator release by basophils, such as chronic idiopathic and physical urticarias.
REFERENCES
- 1.Czech, W., E. Schopf, and A. Kapp. 1995. Release of sulfidoleukotrienes in vitro: its relevance in the diagnosis of pseudoallergy to acetylsalicylic acid. Inflamm. Res. 44:291-295. [DOI] [PubMed] [Google Scholar]
- 2.de Weck, A. L., and M. L. Sanz. 2004. Cellular allergen stimulation test (CAST) 2003, a review. J. Investig. Allergol. Clin. Immunol. 14:253-273. [PubMed] [Google Scholar]
- 3.de Weck, A. L., and M. L. Sanz. 2002. Flow cytometric cellular allergen stimulation test (FAST/FLOW-CAST). ACI Int. 14:204-215. [Google Scholar]
- 4.Knol, E. F., L. Koenderman, F. P. Mul, A. J. Verhoeven, and D. Roos. 1991. Differential activation of human basophils by anti-IgE and formyl-methionyl-leucyl-phenylalanine. Indications for protein kinase C-dependent and -independent activation pathways. Eur. J. Immunol. 21:881-885. [DOI] [PubMed] [Google Scholar]
- 5.Knol, E. F., F. P. Mul, H. Jansen, J. Calafat, and D. Roos. 1991. Monitoring human basophil activation via CD63 monoclonal antibody 435. J. Allergy Clin. Immunol. 88:328-338. [DOI] [PubMed] [Google Scholar]
- 6.Kubota, Y., S. Imayama, A. Toshitani, H. Miyahara, T. Tanahashi, Y. Uemura, T. Koga, N. Sugawara, F. Kurimoto, and K. Hata. 1997. Sulfidoleukotriene release test (CAST) in hypersensitivity to nonsteroidal anti-inflammatory drugs. Int. Arch. Allergy Immunol. 114:361-366. [DOI] [PubMed] [Google Scholar]
- 7.Moneret-Vautrin, D. A., G. Kanny, and S. Fremont. 2003. Laboratory tests for diagnosis of food allergy: advantages, disadvantages and future perspectives. Allerg. Immunol. (Paris) 35:113-119. [PubMed] [Google Scholar]
- 8.Neuberger, M. S., G. T. Williams, E. B. Mitchell, S. S. Jouhal, J. G. Flanagan, and T. H. Rabbitts. 1985. A hapten-specific chimaeric IgE antibody with human physiological effector function. Nature 314:268-270. [DOI] [PubMed] [Google Scholar]
- 9.Reinherz, E. L., P. C. Kung, G. Goldstein, and S. F. Schlossman. 1979. Separation of functional subsets of human T cells by a monoclonal antibody. Proc. Natl. Acad. Sci. USA 76:4061-4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tesch, H., T. Takemori, and K. Rajewsky. 1983. The immune response against anti-idiotope antibodies II. The induction of antibodies bearing the target idiotope (Ab3 beta) depends on the frequency of the corresponding B cells. Eur. J. Immunol. 13:726-732. [DOI] [PubMed] [Google Scholar]
- 11.Vangelista, L., M. Cesco-Gaspere, D. Lamba, and O. Burrone. 2002. Efficient folding of the FɛRI alpha-chain membrane-proximal domain D2 depends on the presence of the N-terminal domain D1. J. Mol. Biol. 322:815-825. [DOI] [PubMed] [Google Scholar]
- 12.Vangelista, L., E. Soprana, M. Cesco-Gaspere, P. Mandiola, G. Di Lullo, R. N. Fucci, F. Codazzi, A. Palini, G. Paganelli, O. R. Burrone, and A. G. Siccardi. 2005. Membrane IgE binds and activates Fc epsilon RI in an antigen-independent manner. J. Immunol. 174:5602-5611. [DOI] [PubMed] [Google Scholar]