Abstract
Despite the success of multidrug therapy in reducing the number of registered leprosy cases worldwide, evidence suggests that Mycobacterium leprae continues to be transmitted. A serological diagnostic test capable of identifying and allowing treatment of early-stage disease could reduce transmission and prevent the onset of the disability, a common complication of the disease in later stages. Serological diagnosis based on antibody recognition of phenolic glycolipid I (PGL-I) cannot reliably identify individuals with lower bacterial indices (BI). One strategy that might improve this situation is the provision of highly specific serological antigens that may be combined with PGL-I to improve the sensitivity of diagnosis. Using serological expression cloning with a serum pool of untreated lepromatous leprosy (LL) patients, we identified 14 strongly reactive M. leprae proteins, 5 of which were previously unstudied. We present results suggesting that two of these proteins, ML0405 and ML2331, demonstrate the ability to specifically identify LL/borderline lepromatous (BL) patients on the basis of immunoglobulin G (IgG) reactivity. In a household contact study, LL index cases were identified on the basis of this reactivity, while household contacts of these patients demonstrated undetectable reactivity. At a serum dilution of 1:800, suitable to reduce background PGL-I IgM reactivity, two BL patients with a BI of <4 showed anti-human polyvalent immunoglobulin G, A, and M reactivity measured with a combination of ML0405, ML2331, and natural disaccharide O-linked human serum albumin (NDOHSA) (synthetic PGL-I) that was markedly higher than IgM reactivity to NDOHSA alone. We suggest that ML0405 and ML2331 may have utility in serological leprosy diagnosis.
Leprosy is a devastating human disease caused by infection with Mycobacterium leprae bacilli. The disease predominantly affects the skin, although during infection, significant nerve destruction leads to deformities of the hand, foot, face, and, in some cases, eye (1). The disease is represented by a clinical spectrum. Lepromatous leprosy/borderline lepromatous (LL/BL) patients represent one pole of the spectrum, demonstrating a high bacterial index (BI) and, as such, are classified as multibacillary (MB). LL/BL patients demonstrate high titers of M. leprae-specific antibodies and an absence of M. leprae-specific cell-mediated immunity. At the opposite pole, tuberculoid tuberculoid/borderline tuberculoid (TT/BT) patients demonstrate very low or absent BI and are designated paucibacillary. These individuals demonstrate significant M. leprae-specific cell- mediated immunity and very low or absent titers of M. leprae- specific antibodies.
Despite the success of multidrug therapy in reducing the number of registered leprosy cases worldwide, the annual rate of new case detection remains unchanged, at approximately 700,000 cases per year (33), with children representing 15% of new cases (18). This suggests that active transmission of M. leprae is still occurring, but the route and mechanism of this transmission is still unclear. Household contacts of individuals with MB disease have a higher risk of developing clinical leprosy than those of paucibacillary patients (7, 32), and this has been attributed to increased shedding of viable bacteria by MB patients (10, 26). Diagnosis of leprosy at early stages and subsequent treatment would prevent disability and may also help reduce transmission.
The presence of serum antibody to phenolic glycolipid I (PGL-I), an immunodominant M. leprae antigen, correlates with BI in MB patients, and enzyme-linked immunosorbent assay (ELISA), particle agglutination, dipstick, and rapid lateral-flow test formats have been developed for the detection of PGL-I immunoglobulin M (IgM) antibody (14, 15, 28). However, patients with a low BI often lack detectable antibody (2, 4). Additional serological antigens could improve the sensitivity and specificity of the PGL-I serological test, potentially improving the detection of leprosy. In numerous studies, lambda- gt11 M. leprae libraries have been screened to identify antigens based on reactivity to either LL/BL patient sera or mouse monoclonal antibodies raised against major abundant proteins purified from the bacillus (3, 13, 16, 23, 35). Thus far, no antigen identified by a genomic library screen has been successfully developed as a diagnostic reagent.
Recent advances in molecular biology have greatly facilitated the technique of expression cloning for prokaryotic organisms, refining the screening of whole genomes for the identification of protein antigens (20). Moreover, the use of pooled patient sera as a probe for expression cloning has led to the identification of novel antigens from a number of bacterial organisms (11, 19, 21). Our initial objective was to expand the number of identified M. leprae protein antigens by serological expression cloning with pooled serum from a discrete number of untreated LL/BL patients. We then carried out an analysis of these antigens to investigate their potential for serologically diagnosing leprosy.
MATERIALS AND METHODS
Patients.
Leprosy patient and household contact sera were obtained after drawing blood at the Leonard Wood Memorial Center for Leprosy Research, Cebu City, Philippines. All LL, BL, TT, and BT sera used in this study derived from recently diagnosed and untreated individuals. Leprosy was classified in each case by bacterial, histological, and clinical observations carried out by qualified personnel, with the BI recorded at the time of diagnosis. Sera from tuberculosis patients were obtained after drawing blood from Mycobacterium tuberculosis sputum-positive Seattle-based individuals with clinically confirmed pulmonary tuberculosis (PT). Normal sera were obtained after drawing blood from Seattle-based volunteers with no history of leprosy or tuberculosis infection. In all cases, drawing of blood was carried out with informed consent with local institutional review board approval in Seattle and local ethics committee approval in the Philippines.
M. leprae library construction.
M. leprae genomic library construction was carried out using the ZAP Express EcoRI predigested vector kit and Gigapack cloning kit according to the manufacturer's instructions (Stratagene, La Jolla, CA) and as previously described (20). M. leprae strain Thai-53 genomic DNA for library construction was kindly supplied by P. Brennan, Colorado State University, under NIAD, NIH, contract N01-AI-25469. To examine library quality, recombinant phages were obtained from plaques on an XLIB MRF′ Escherichia coli lawn on LB agar plates propagated with the appropriate antibiotics. Phage DNA was excised as a plasmid and digested with BamHI and HindIII restriction enzymes (NEB, Ipswich, MA). Digested DNA was resolved by 1% agarose gel electrophoresis to determine the M. leprae genomic DNA insert size.
Serological expression screening.
Serological expression screening was carried out with pooled sera from five untreated LL/BL patients as previously described (20). Briefly, 20,000 PFU of library phage in LB top agar were plated per each 30-cm LB agar plate and incubated at 37°C until plaque formation was visible (after approximately 3 h). Nitrocellulose filters soaked previously in 10 mM IPTG (isopropyl-β-d-thiogalactopyranoside) were then placed onto plates and incubated overnight at 42°C. Nitrocellulose filters were removed from top agar and blocked in phosphate-buffered saline buffer containing 0.1% (vol/vol) Tween 20 (PBST) and 1% bovine serum albumin (BSA). Subsequently, nitrocellulose filters were incubated in PBST containing a 1:100 dilution of LL/BL pooled sera at room temperature with gyration for 1 h, washed in PBST three times (10 min each), and incubated for 1 h with a 1:5,000 dilution of alkaline phosphatase (AP)-conjugated anti-human polyvalent immunoglobulin G, A, and M (IgGAM) antisera (Rockland, Gilbertsville, PA). Filters were then washed and developed using BCIP (5-bromo-4-chloro-3-indolyl phosphate)/nitroblue tetrazolium phosphatase substrate (KPL, Gaithersburg, MD). Positive plaques were removed from the original master plate by coring agar, and ∼100 phages replated on 15-cm agar plates were screened again by the same process described above to confirm reactivity with the serum. Plasmids derived from selected plaques were excised according to the manufacturer's instructions (Stratagene), and inserted M. leprae genomic DNA was sequenced in forward and reverse orientations using T3 promoter and M13-20 primers (NEB). Insert DNA was screened for homology against a database of M. leprae predicted proteins using BLASTX software (available at http://www.sanger.ac.uk/Projects/M_leprae/). We designated M. leprae coding sequences as putatively expressed if the insert contained the native ribosomal binding site and start codon or if the coding sequence was in frame with the inducible LacZ gene in the vector.
Cloning and purification of target antigens.
DNA encoding selected M. leprae proteins was PCR amplified from M. leprae Thai-53 genomic DNA using Pfx DNA polymerase (Invitrogen, Carlsbad, CA). PCR primers were designed to incorporate specific restriction enzyme sites 5′ and 3′ of the gene of interest and excluded in the target gene for directional cloning into the expression vector pET28a (Novagen, Madison, WI). After PCR amplification, purified PCR products were digested with restriction enzymes, ligated into pET28a using T4 DNA ligase (NEB), and then transformed into XL10G cells (Stratagene). Recombinant pET28a plasmid DNA was recovered from individual colonies grown on LB agar plates containing appropriate antibiotics and sequenced to confirm the correctly cloned coding sequence. The recombinant clones contain an N-terminal six-histidine tag followed by a thrombin cleavage site and the M. leprae gene of interest.
Recombinant plasmids were transformed into the E. coli BL21 derivative Rosetta2(DE3)(pLysS) (Novagen). Recombinant strains were cultured overnight at 37°C in 2× yeast tryptoe containing appropriate antibiotics, diluted 1/10 to 1/25 into fresh culture medium, grown to mid-log phase (optical density at 600 nm [OD600], 0.5 to 0.7), and induced by the addition of 1 mM IPTG. Cultures were grown for an additional 3 to 4 h, cells were harvested by centrifugation, and the bacterial pellets were stored at −20°C. Bacterial pellets were thawed and disrupted by sonication in 20 mM Tris (pH 8.0)-150 mM NaCl, followed by centrifugation to fractionate the soluble and insoluble material. Recombinant His-tagged protein products were isolated under native (soluble recombinant proteins) or denaturing (8 M urea) conditions using Ni-nitrilotriacetic acid metal ion affinity chromatography according to the manufacturer's instructions (QIAGEN, Valencia, CA). Protein fractions were eluted with an increasing imidazole gradient and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Affinity-purified protein fractions were combined and dialyzed against 20 mM Tris, pH 8.0, concentrated using Amicon Ultra 10-kDa-molecular-mass cutoff centrifugal filters (Millipore), and quantified using the BCA protein assay (Pierce, Rockford, IL).
Individual patient reactivity by ELISA.
Polysorp 96-well plates (Nunc, Rochester, NY) were coated with 1 μg/ml recombinant protein or 200 ng/ml NDOHSA, the synthetically derived B-cell epitope of PGL-I conjugated to human serum albumin (5), in bicarbonate buffer overnight at 4°C and blocked for 2 h at room temperature with PBST with 1% (wt/vol) BSA on a plate shaker. Serum diluted appropriately in PBST with 0.1% BSA was added to each well, and plates were incubated at room temperature for 2 h with shaking. Plates were washed with buffer only, and horseradish peroxidase-conjugated protein A (Zymed, Carlsbad, CA), IgM, IgG, or polyvalent immunoglobulins (IgGAM) (Sigma, St. Louis, MO), diluted 1:10,000 in PBST and 0.1% BSA, was added to each well and incubated at room temperature for 45 min with shaking. After washing, plates were developed with peroxidase color substrate (KPL), and the reaction was quenched by the addition of 1 N H2SO4 after 5 min. The corrected optical density of each well at 450 nm was read using a VERSAmax microplate reader (Molecular Devices, Sunnyvale, CA).
Western blot analysis.
Five micrograms of protein was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto nitrocellulose membranes using a Western blotting apparatus (Invitrogen). Nitrocellulose membranes were blocked overnight at 4°C in PBST containing 5% (wt/vol) nonfat skimmed milk, washed three times in PBST, and incubated with a 1:1,000 dilution of pooled LL/BL or TT/BT patient sera or a 1:500 dilution of monoclonal anti-His antibody (Novagen) in PBST at room temperature for 1 h. After subsequent washing with PBST, membranes were incubated with a 1:5,000 dilution of AP-conjugated anti-human IgGAM or a 1:2,000 dilution of AP-conjugated anti-mouse IgG at room temperature for 1 h, washed, and developed with BCIP/nitroblue tetrazolium phosphatase substrate (KPL).
Statistical analysis.
All statistical analyses were carried out using a two-tailed Student's t test (two-sample equal variance [homoscedastic]).
RESULTS
Serological expression cloning of LL/BL patients reveals recognition of predominantly membrane-bound and secreted protein sequences.
An M. leprae expression library was constructed using the ZAP Express system. To assess library quality, 20 random clones were excised and digested with restriction enzymes. The clones contained an average insert size of 1.4 kb of M. leprae genomic DNA (data not shown). A total of 200,000 PFU were subsequently screened with a serum pool from five untreated LL/BL patients. A serum dilution of 1:100 was selected for screening, as this titer gave the most easily identifiable positive signal relative to nonspecific reactivity in our experiments. After selection of positive plaques, excision of phage DNA, and sequencing of M. leprae genomic inserts, a number of clones encoding partial or full-length sequences of a discreet number of proteins were identified. The identities of 14 proteins, deriving from 38 distinct clones, are shown in Table 1. Two proteins were identified from single clones, and the remaining 12 proteins were identified from multiple clones. Five proteins were previously uncharacterized as serologically reactive. ML1213 shows conservation of an M. tuberculosis membrane protein, Rv1565c. ML2331 shares Pfam motifs with amidase-3, an N-acetylmuramoyl-l-alanine amidase, and ML1812 shares a Pfam motif with p60, both belonging to a superfamily of surface-expressed proteins of pathogenic bacteria (27). ML0405 and ML0568 show homology to M. tuberculosis proteins Rv3616c and Rv1435c, respectively, and have unknown functions. Both sequences were analyzed with PSORTb v.2, a computer algorithm that predicts gram-positive bacterial protein subcellular location (9). This algorithm identified five transmembrane helices located within the ML0405 protein sequence, giving a high probability of membrane location. The algorithm was unable to predict a subcellular location for ML0568.
TABLE 1.
Antigens of M. leprae identified by expression cloning using pooled LL/BL patient serad
| Antigen | No. of clonesa | Sizes (kDa) | TB homologue (% identity) | Description | Mean ELISA OD450 ± SDb
|
|
|---|---|---|---|---|---|---|
| LL/BL | NEC | |||||
| ML0050/ML0049 | 2 | 10, 9 | Rv3874 (40.0) | Secreted; CFP-10, ESAT-6 | 0.52 ± 0.87c | 0.06 ± 0.01 |
| Rv3875 (36.3) | ||||||
| ML0091 | 4 | 23.7 | Rv3810 (52.7) | Potentially secreted | ND | ND |
| ML0317 | 2 | 56.9 | Rv0440 (94.8) | GroEL2, 60-kDa chaperonin 2 | ND | ND |
| ML0405 | 2 | 40.7 | Rv3616c (62.7) | Previously unknown | 2.31 ± 0.45 | 0.13 ± 0.02 |
| ML0568 | 5 | 18.3 | Rv1435c (38.9) | Previously unknown | 0.34 ± 0.22 | 0.13 ± 0.04 |
| ML1213 | 3 | 80.1 | Rv1565c (76.5) | Previously unknown | 0.51 ± 0.21 | 0.23 ± 0.04 |
| ML2028 | 3 | 34.8 | Rv1886c (88) | Secreted; Ag85b | 2.29 ± 1.26 | 0.52 ± 0.21 |
| ML2055 | 6 | 29.5 | Rv1860 (66.8) | Membrane protein | 1.87 ± 1.19 | 0.19 ± 0.05 |
| ML2655 | 2 | 36.3 | Rv0129 (82.0) | Secreted; Ag85c | 2.82 ± 0.81 | 1.88 ± 0.28 |
| ML0097 | 1 | 35.4 | Rv3804c (82.0) | Secreted; Ag85a | ND | ND |
| ML1812 | 1 | 50.5 | Rv1477 (78.8) | Previously unknown | ND | ND |
| ML2331 | 2 | 26.5 | Rv3717 (82.0) | Previously unknown | 2.09 ± 1.07 | 0.11 ± 0.06 |
| ML2496 | 3 | 24.2 | Rv0351 (68.6) | DnaK, 70-kDa heat shock protein | ND | ND |
Number of different clones identified in the screen encoding the full or partial sequence of the same antigen.
OD450 ± standard deviation in ELISA developed with protein A at a serum dilution of 1:200 (minimum of n = 6 for each measurement). ND, not done.
ELISA results with ML0050-ML0049 fusion protein.
n = 5.
The remaining nine proteins had previously been described as serologically reactive. ML2028 (Ag85b), ML2655 (Ag85c), and ML0097 (Ag85a) are members of the Ag85 complex and are relatively well conserved among mycobacteria and dominant antigens in both leprosy and tuberculosis (17, 24). Two clones contained sequences of both ML0049 and ML0050 encoding the M. leprae ESAT-6 and CFP-10 homologues, respectively. These represent the only two sequences derived from an M. leprae genomic region-homologous RD-1 in M. tuberculosis, which is deleted from BCG vaccine strains. CFP-10 has previously been demonstrated to be serologically reactive in leprosy patients (31). ML0091 was identified as serologically reactive in a previous screen using a lambda-gt11 M. leprae DNA expression library (3). ML2055 is a surface-expressed protein that binds laminin-2 on Schwann cells to facilitate nerve invasion (30). GroEL2, a 60-kDa chaperonin 2 (ML0317), and DnaK, a 70-kDa heat shock protein (ML2496), are well established as immunodominant antigens during infection (22, 34). Taken together, these data indicate that the majority of proteins identified in this screen were putatively membrane bound or secreted (11 of 14 proteins).
ML0405, ML2331, and ML2055 are serologically recognized by LL/BL but not TT/BT patients.
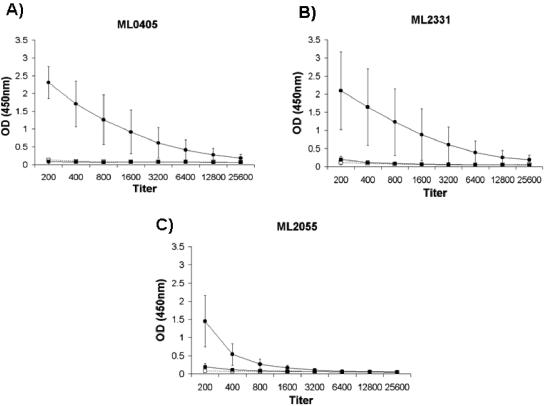
His-tagged recombinant derivatives of selected proteins were expressed, purified, and screened for reactivity with pooled LL/BL sera and nonendemic control (NEC) sera by ELISA at a serum dilution of 1:200. ML0405, ML2055, and ML2331 all showed the highest signal-to-background serological reactivity as recombinant proteins (Table 1). The recombinant proteins were further analyzed for reactivity by titrating ELISA of individual LL/BL sera pooled for expression cloning (n = 5), sera from a group of TT/BT patients (n = 6), and NEC sera (n = 8) (Fig. 1). ML0405 and ML2331 showed marked reactivity to LL/BL sera, with reactivity clearly detectable at a serum dilution of 1:1,600. The reactivity of ML2055 titrated out earlier than that observed for ML0405 and ML2331, with reactivity close to background levels at a serum dilution of 1:800. Low or absent reactivity to TT/BT or NEC sera was observed in all cases. Western blotting was performed to ensure that reactivity was specific to the purified His-tagged recombinant protein. Anti-His antibody and pooled LL/BL sera were identified by all three antigens, confirming that the serological reactivity observed is directed to the purified His-tagged recombinant protein in each case (Fig. 2).
FIG. 1.
(A to C) Reactivity of LL/BL, TT/BT, and NEC sera to selected full-length recombinant proteins by ELISA using protein A. (A) ML0405; (B) ML2331; (C) ML2055. Results are shown as mean ODs ± standard deviations. Closed circles, LL/BL sera (n = 5); closed squares, TT/BT sera (n = 6); open squares, normal sera (n = 6).
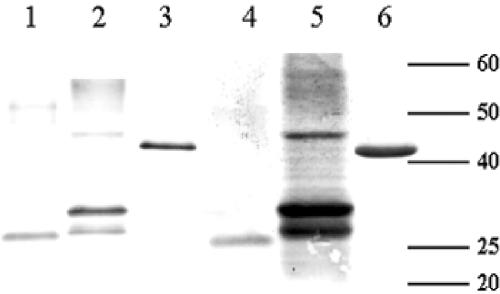
FIG. 2.
Reactivity of identified full-length recombinant proteins to pooled LL/BL sera by Western blot. Lanes 1 and 4, ML2331; lanes 2 and 5, ML0405; lanes 3 and 6, ML2055. Lanes 1 to 3 developed with anti-His monoclonal antibody (BD Biosciences). Lanes 4 to 6 developed with pooled LL patient sera used for expression cloning (n = 5). Positions of molecular mass markers are indicated in kilodaltons. The experiment was repeated three times, and a typical representation of the results is shown.
Individual LL/BL patients but not pulmonary tuberculosis patients show serological reactivity to ML2055, ML2331, and ML0405.
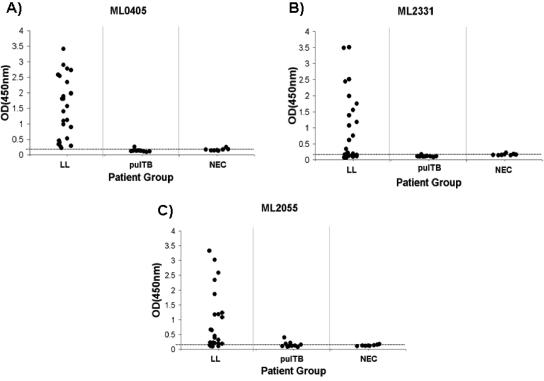
ML0405, ML2055, and ML2331 demonstrate significant homology to M. tuberculosis proteins (60 to 80%) (Table 1), and significant serological cross-reactivity with pulmonary TB patients could preclude their use as serological diagnostic reagents for leprosy. We screened individual pulmonary TB patient sera (n = 10) and NEC sera (n = 8) for reactivity to recombinant ML0405, ML2055, and ML2331 by ELISA. Of the 10 pulmonary TB patient sera screened, 6 were serologically reactive by ELISA to either TbF10, an M. tuberculosis fusion protein (12), or Rv3875 (Esat-6) (data not shown). In addition, we expanded our analysis of LL/BL patients with 24 individuals. All 24 LL/BL sera failed to recognize the M. tuberculosis antigen Rv3874 (CFP-10) when tested by ELISA (data not shown). Strikingly, ML2055, ML0405, and ML2331 all showed significantly higher reactivity to LL/BL patient sera than to pulmonary TB patient sera (P < 0.0005 in all cases) (Fig. 3). In each case, reactivity of pulmonary TB patient sera was not significantly different from that of NEC sera for ML2055, ML0405, and ML2331 (P > 0.1 in all cases).
FIG. 3.
(A to C) Reactivity of individual antigens to LL sera, pulmonary TB sera, and NEC serum to recombinant proteins measured by ELISA using protein A. LL (n = 24), pulmonary TB (n = 10), and NEC (normal) (n = 8) sera were tested. An arbitrary cutoff for reactivity of an OD of 0.2 is indicated by a horizontal line.
Household contacts of LL patients do not show serological reactivity to ML2331 and ML0405.
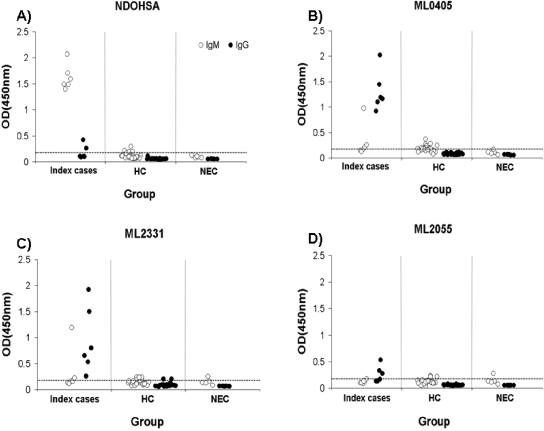
Reactivity of ML2055, ML2331, and ML0405 was tested in a household contact study within an area where leprosy is endemic. NDOHSA was employed as a control. Six families containing a single LL patient (BI of >5 at diagnosis) and at least four family members living in the same dwelling were selected. Serological reactivity of each LL patient serum sample and household contact serum sample to NDOHSA, ML2055, ML2331, and ML0405 was evaluated by IgG and IgM ELISA at a serum dilution of 1:200 (Fig. 4). All LL index cases demonstrated IgM antibodies to NDOHSA, and 7.1% of household contacts (2 of 28) showed IgM reactivity above the arbitrary cutoff value. All index cases showed the presence of IgG antibodies to ML0405 and ML2331, and 50% (3 of 6) showed the presence of IgG antibodies to ML2055. None of the household contact sera demonstrated IgG reactivity to ML0405, ML2331, or ML2055 measured above the cutoff. One of the six index case sera showed elevated IgM reactivity to ML0405 and ML2331, while the remainder showed IgM reactivity not significantly different from that of household contact serum or NEC serum. Thus, at least in the families tested, individuals with LL could be discriminated from household contacts by IgG reactivity to ML2331 or ML0405. These proteins could represent useful diagnostic tools in areas where leprosy is endemic.
FIG. 4.
IgM and IgG reactivity of six LL index cases, household contacts, and normal sera to (A) NDOHSA, (B) ML0405, (C) ML2331, and (D) ML2055, measured by ELISA. Serum dilution was 1:200. Number of index case serum samples was 6, the number of household contact (HC) serum samples was 28, and the number of NEC (normal) serum samples was 5. Each index case had a minimum of four household contacts included in this study. An arbitrary cutoff for reactivity of an OD of 0.2 is indicated by a horizontal line.
ML0405 and ML2331 show the potential to complement PGL-I in the serological diagnosis of BL leprosy.
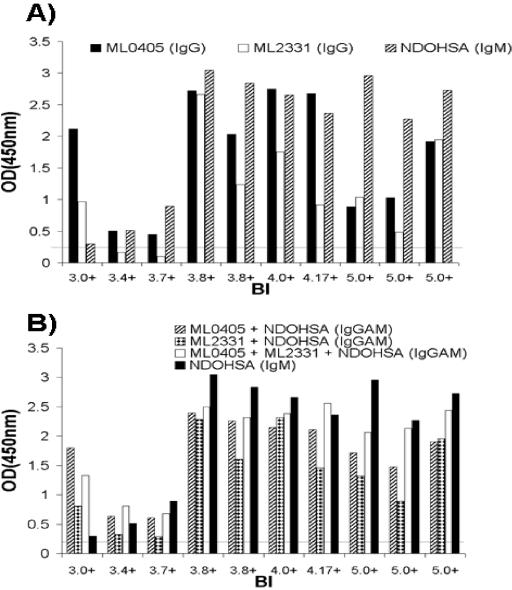
At serum dilutions of 1:800, all household contacts we tested showed IgM reactivity to NDOHSA, ML0405, and ML2331 that measured below the cutoff (OD of <0.2) (data not shown). All index cases in this study had a BI of >5, and since patients with a BI below 4 often demonstrate a lack of antibody to PGL-I, we examined whether ML0405 and ML2331 would aid serological diagnosis at this dilution with BL patients with a BI of <4. Ten sera were selected from patients with a BI between 3 and 5 and screened for IgM reactivity to NDOHSA and IgG reactivity to ML2331 or ML0405 by ELISA (Fig. 5A). At a serum dilution of 1:800, the patient with lowest BI (3.0) showed significant IgG reactivity to ML0405 but relatively low IgM reactivity to NDOHSA. In fact, the two patients with the lowest BI in this study showed IgGAM reactivity to a combination of all three antigens that was markedly higher than IgM reactivity to NDOHSA alone (Fig. 5B).
FIG. 5.
Potential utility of ML0405 and ML2331 in the serological diagnosis of BL patients. (A) ELISA of BL patients with a BI between 3 and 4.17 (n = 6) and LL patients with a BI of >5 at a serum dilution of 1:800. At this serum dilution, all household contact and NEC sera used in this study were tested at an OD of <0.2 (data not shown). At a lower BI, reactivity to NDOHSA (IgM) was reduced, and in one case (BI, 3.0), serum reactivity was markedly higher in response to ML0405 (IgG). (B) ELISA at the same serum dilution using combinations of antigens. In the patients with the lowest BI, a measured combination of all three antigens (IgGAM) improved signal compared to NDOHSA alone (IgM). An arbitrary cutoff for reactivity of an OD of 0.2 is indicated by a horizontal line.
DISCUSSION
Multidrug therapy for treatment of early or subclinical leprosy could have a significant impact on both the prevention of nerve damage within infected individuals and the transmission of the M. leprae bacillus to uninfected individuals. The presence of anti-PGL-I antibodies is not a reliable marker for the diagnosis of subclinical leprosy, since approximately 10% of an endemic population demonstrate PGL-I antibody, and the majority of these individuals (∼90%) do not progress to M. leprae infection (25). We carried out serological expression cloning and identified a total of 14 antigens that demonstrated serological reactivity to untreated MB leprosy patients. Five of these proteins are newly identified antigens. Of the antigens tested, ML0405, ML2331, and ML2055 demonstrated the highest serological reactivity for LL/BL sera compared to NEC sera. Importantly, pulmonary TB patients demonstrated low serological reactivity to these antigens in the form of purified recombinant proteins. However, TT/BT patients failed to show significant serological activity in response to these antigens, and diagnostic utility would therefore appear to be relevant only to patients with MB disease. Since MB patients represent a significant source of transmission, subclinical diagnosis and treatment of MB disease are likely to significantly benefit public health programs.
In a household contact study within an area where leprosy is endemic, using a serum dilution of 1:200, index cases showed significant levels of serum IgG that bound ML0405 and ML2331, whereas household contacts showed low or absent serological reactivity. The index cases all possessed IgM antibody that bound NDOHSA, but two household contacts showed IgM levels that measured above the arbitrary cutoff set in this experiment. At a serum dilution of 1:800, all household contacts demonstrated IgM levels against NDOHSA that were below the reactivity cutoff. At this serum dilution, in two cases of leprosy with a bacterial index below 4 (BI of 3 and 3.4), patients demonstrated markedly higher levels of antibody reactivity against a combination of ML0405, ML2331, and NDOHSA compared to IgM that was reactive to NDOHSA alone. Individuals with BI of this magnitude do not typically require serological diagnosis and are not representative of the early stage of MB disease. Despite this, these findings suggest that the sensitivity of serological diagnosis of leprosy can be improved by combining NDOHSA with additional antigens, and the impact of the addition of ML0405 and ML2331 to NDOHSA for the diagnosis of leprosy would be a worthwhile undertaking in a much larger study. Subsequent studies employing ML2331 and ML0405 might encompass the retrospective analysis of sera from household contacts that progressed to MB disease, similar to the analyses reported previously by Douglas et al. using PGL-I (6). In contrast to the IgM response that is elicited by NDOHSA, it is noteworthy that MB patient antibody directed to these antigens is primarily of the IgG subclass. Since the generation of specific IgG responses typically requires T-cell help, it is likely that at certain stages of infection, MB patients demonstrate specific T-cell reactivity to these antigens. Thus, the screening of strongly IgG-reactive antigens for T-cell reactivity may also represent a diagnostic rationale to detect early-stage MB leprosy.
The functions of ML0405 and ML2331 during human infection are unknown. However, recent data demonstrated that the M. tuberculosis homologue of ML0405, Rv3616c, is a virulence factor and is required for the secretion of Esat-6 and CFP-10 by M. tuberculosis (8, 29). Since ML0405 is recognized immunologically during leprosy, we speculate that ML0405 may play a similar role during M. leprae infection.
Taken together, our data indicate that further research on the roles of ML0405 and ML2331 is warranted and may lead to improved diagnosis of leprosy.
Acknowledgments
This work was funded by the American Leprosy Missions.
We wish to thank Raymond L. Houghton for advice on serological diagnosis of pulmonary tuberculosis and Malcolm S. Duthie for critical evaluation of the manuscript.
REFERENCES
- 1.Britton, W. J., and D. N. Lockwood. 2004. Leprosy. Lancet 363:1209-1219. [DOI] [PubMed] [Google Scholar]
- 2.Chanteau, S., P. Glaziou, C. Plichart, P. Luquiaud, R. Plichart, J. F. Faucher, and J. L. Cartel. 1993. Low predictive value of PGL-I serology for the early diagnosis of leprosy in family contacts: results of a 10-year prospective field study in French Polynesia. Int. J. Lepr. Other Mycobact. Dis. 61:533-541. [PubMed] [Google Scholar]
- 3.Cherayil, B. J., and R. A. Young. 1988. A 28-kDa protein from Mycobacterium leprae is a target of the human antibody response in lepromatous leprosy. J. Immunol. 141:4370-4375. [PubMed] [Google Scholar]
- 4.Cho, S. N., R. V. Cellona, L. G. Villahermosa, T. T. Fajardo, Jr., M. V. Balagon, R. M. Abalos, E. V. Tan, G. P. Walsh, J. D. Kim, and P. J. Brennan. 2001. Detection of phenolic glycolipid I of Mycobacterium leprae in sera from leprosy patients before and after start of multidrug therapy. Clin. Diagn. Lab. Immunol. 8:138-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho, S. N., T. Fujiwara, S. W. Hunter, T. H. Rea, R. H. Gelber, and P. J. Brennan. 1984. Use of an artificial antigen containing the 3,6-di-O-methyl-beta-D-glucopyranosyl epitope for the serodiagnosis of leprosy. J. Infect. Dis. 150:311-322. [DOI] [PubMed] [Google Scholar]
- 6.Douglas, J. T., R. V. Cellona, T. T. Fajardo, Jr., R. M. Abalos, M. V. Balagon, and P. R. Klatser. 2004. Prospective study of serological conversion as a risk factor for development of leprosy among household contacts. Clin. Diagn. Lab. Immunol. 11:897-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fine, P. E., J. A. Sterne, J. M. Ponnighaus, L. Bliss, J. Saui, A. Chihana, M. Munthali, and D. K. Warndorff. 1997. Household and dwelling contact as risk factors for leprosy in northern Malawi. Am. J. Epidemiol. 146:91-102. [DOI] [PubMed] [Google Scholar]
- 8.Fortune, S. M., A. Jaeger, D. A. Sarracino, M. R. Chase, C. M. Sassetti, D. R. Sherman, B. R. Bloom, and E. J. Rubin. 2005. Mutually dependent secretion of proteins required for mycobacterial virulence. Proc. Natl. Acad. Sci. USA 102:10676-10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardy, J. L., M. R. Laird, F. Chen, S. Rey, C. J. Walsh, M. Ester, and F. S. Brinkman. 2005. PSORTb v. 2.0: expanded prediction of bacterial protein subcellular localization and insights gained from comparative proteome analysis. Bioinformatics 21:617-623. [DOI] [PubMed] [Google Scholar]
- 10.Hatta, M., S. M. van Beers, B. Madjid, A. Djumadi, M. Y. de Wit, and P. R. Klatser. 1995. Distribution and persistence of Mycobacterium leprae nasal carriage among a population in which leprosy is endemic in Indonesia. Trans. R. Soc. Trop. Med. Hyg. 89:381-385. [DOI] [PubMed] [Google Scholar]
- 11.Homer, M. J., M. J. Lodes, L. D. Reynolds, Y. Zhang, J. F. Douglass, P. D. McNeill, R. L. Houghton, and D. H. Persing. 2003. Identification and characterization of putative secreted antigens from Babesia microti. J. Clin. Microbiol. 41:723-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houghton, R. L., M. J. Lodes, D. C. Dillon, L. D. Reynolds, C. H. Day, P. D. McNeill, R. C. Hendrickson, Y. A. Skeiky, D. P. Sampaio, R. Badaro, K. P. Lyashchenko, and S. G. Reed. 2002. Use of multiepitope polyproteins in serodiagnosis of active tuberculosis. Clin. Diagn. Lab. Immunol. 9:883-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Husson, R. N., and R. A. Young. 1987. Genes for the major protein antigens of Mycobacterium tuberculosis: the etiologic agents of tuberculosis and leprosy share an immunodominant antigen. Proc. Natl. Acad. Sci. USA 84:1679-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klatser, P. R. 2000. Use of a Mycobacterium leprae dipstick to classify patients with leprosy. Lepr. Rev. 71:S67-S72. [PubMed] [Google Scholar]
- 15.Kumar, S., K. D. Moudgil, A. H. Band, P. R. Naraynan, S. K. Gupta, A. K. Sharma, and G. P. Talwar. 1986. A dot enzyme immunoassay for detection of IgM antibodies against phenolic glycolipid-I in sera from leprosy patients. Indian J. Lepr. 58:185-190. [PubMed] [Google Scholar]
- 16.Laal, S., Y. D. Sharma, H. K. Prasad, A. Murtaza, S. Singh, S. Tangri, R. S. Misra, and I. Nath. 1991. Recombinant fusion protein identified by lepromatous sera mimics native Mycobacterium leprae in T-cell responses across the leprosy spectrum. Proc. Natl. Acad. Sci. USA 88:1054-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Launois, P., M. N. Niang, J. L. Sarthou, F. Rivier, A. Drowart, J. P. Van Vooren, J. Millan, and K. Huygen. 1993. T-cell stimulation with purified mycobacterial antigens in patients and healthy subjects infected with Mycobacterium leprae: secreted antigen 85 is another immunodominant antigen. Scand. J. Immunol. 38:167-176. [DOI] [PubMed] [Google Scholar]
- 18.Lockwood, D. N. 2002. Leprosy elimination—a virtual phenomenon or a reality? BMJ 324:1516-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lodes, M. J., Y. Cong, C. O. Elson, R. Mohamath, C. J. Landers, S. R. Targan, M. Fort, and R. M. Hershberg. 2004. Bacterial flagellin is a dominant antigen in Crohn disease. J. Clin. Investig. 113:1296-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lodes, M. J., D. C. Dillon, R. L. Houghton, and Y. A. Skeiky. 2004. Expression cloning. Methods Mol. Med. 94:91-106. [DOI] [PubMed] [Google Scholar]
- 21.Lodes, M. J., D. C. Dillon, R. Mohamath, C. H. Day, D. R. Benson, L. D. Reynolds, P. McNeill, D. P. Sampaio, Y. A. Skeiky, R. Badaro, D. H. Persing, S. G. Reed, and R. L. Houghton. 2001. Serological expression cloning and immunological evaluation of MTB48, a novel Mycobacterium tuberculosis antigen. J. Clin. Microbiol. 39:2485-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKenzie, K. R., E. Adams, W. J. Britton, R. J. Garsia, and A. Basten. 1991. Sequence and immunogenicity of the 70-kDa heat shock protein of Mycobacterium leprae. J. Immunol. 147:312-319. [PubMed] [Google Scholar]
- 23.Mustafa, A. S., A. Deggerdal, K. E. Lundin, R. M. Meloen, T. M. Shinnick, and F. Oftung. 1994. An HLA-DRw53-restricted T-cell epitope from a novel Mycobacterium leprae protein antigen important to the human memory T-cell repertoire against M. leprae. Infect. Immun. 62:5595-5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naito, M., S. Izumi, and T. Yamada. 1998. Two-dimensional electrophoretic analysis of humoral responses to culture filtrate of Mycobacterium bovis BCG in patients with leprosy and tuberculosis. Int. J. Lepr. Other Mycobact. Dis. 66:208-213. [PubMed] [Google Scholar]
- 25.Oskam, L., E. Slim, and S. Buhrer-Sekula. 2003. Serology: recent developments, strengths, limitations and prospects: a state of the art overview. Lepr. Rev. 74:196-205. [PubMed] [Google Scholar]
- 26.Ramaprasad, P., A. Fernando, S. Madhale, J. R. Rao, V. K. Edward, P. D. Samson, P. R. Klatser, M. Y. de Wit, W. C. Smith, and I. A. Cree. 1997. Transmission and protection in leprosy: indications of the role of mucosal immunity. Lepr. Rev. 68:301-315. [DOI] [PubMed] [Google Scholar]
- 27.Rigden, D. J., M. J. Jedrzejas, and M. Y. Galperin. 2003. Amidase domains from bacterial and phage autolysins define a family of gamma-D,L-glutamate-specific amidohydrolases. Trends Biochem. Sci. 28:230-234. [DOI] [PubMed] [Google Scholar]
- 28.Roche, P. W., S. S. Failbus, W. J. Britton, and R. Cole. 1999. Rapid method for diagnosis of leprosy by measurements of antibodies to the M. leprae 35-kDa protein: comparison with PGL-I antibodies detected by ELISA and “dipstick” methods. Int. J. Lepr. Other Mycobact. Dis. 67:279-286. [PubMed] [Google Scholar]
- 29.Sassetti, C. M., and E. J. Rubin. 2003. Genetic requirements for mycobacterial survival during infection. Proc. Natl. Acad. Sci. USA 100:12989-12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimoji, Y., V. Ng, K. Matsumura, V. A. Fischetti, and A. Rambukkana. 1999. A 21-kDa surface protein of Mycobacterium leprae binds peripheral nerve laminin-2 and mediates Schwann cell invasion. Proc. Natl. Acad. Sci. USA 96:9857-9862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spencer, J. S., H. J. Kim, A. M. Marques, M. Gonzalez-Juarerro, M. C. Lima, V. D. Vissa, R. W. Truman, M. L. Gennaro, S. N. Cho, S. T. Cole, and P. J. Brennan. 2004. Comparative analysis of B- and T-cell epitopes of Mycobacterium leprae and Mycobacterium tuberculosis culture filtrate protein 10. Infect. Immun. 72:3161-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Beers, S. M., M. Hatta, and P. R. Klatser. 1999. Patient contact is the major determinant in incident leprosy: implications for future control. Int. J. Lepr. Other Mycobact. Dis. 67:119-128. [PubMed] [Google Scholar]
- 33.World Health Organization. 2002. Leprosy. Global situation. Wkly. Epidemiol. Rec. 77:1-8. [PubMed] [Google Scholar]
- 34.Young, D., R. Lathigra, R. Hendrix, D. Sweetser, and R. A. Young. 1988. Stress proteins are immune targets in leprosy and tuberculosis. Proc. Natl. Acad. Sci. USA 85:4267-4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young, R. A., V. Mehra, D. Sweetser, T. Buchanan, J. Clark-Curtiss, R. W. Davis, and B. R. Bloom. 1985. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature 316:450-452. [DOI] [PubMed] [Google Scholar]