Abstract
Although tuberculin skin testing has been a hallmark of bovine tuberculosis eradication campaigns, it lacks sensitivity, can be confounded by exposure to nontuberculous mycobacteria, and cannot be repeated for 60 days due to desensitization. To overcome these difficulties, an effective whole-blood cellular immunoassay for bovine gamma interferon (IFN-γ) has been developed. The IFN-γ test is commonly used in conjunction with tuberculin skin testing as a confirmatory test following a positive response to the caudal fold test (CFT). The present study was conducted to determine the effect of different tuberculin skin-testing regimens on IFN-γ and antibody production by using calves that were experimentally infected with Mycobacterium bovis. Holstein calves were CFT tested 60 days after inoculation and the comparative cervical test (CCT) was conducted 7 (7-day CCT) or 55 (55-day CCT) days after the CFT. In both the 7-day CCT and 55-day CCT groups, IFN-γ responses increased 3 days after the CFT; this was immediately followed by a decrease to pre-skin test levels 7 days after the CFT. In both groups, the application of the CCT at 7 or 55 days after the CFT resulted in no significant increase in IFN-γ production. The administration of the CFT and the CCT to M. bovis-inoculated cattle boosted antibody responses to M. bovis PPD, rMPB83, ESAT-6, and the fusion protein Acr1-MPB83. The boosting effect was more pronounced in the 55-day CCT group. Increases in either IFN-γ or antibody production were not seen in noninoculated cattle. Measurement of both IFN-γ and antibody responses after skin testing may be useful in identifying M. bovis-infected cattle; however, the timing of collection of such samples may influence interpretation.
Mycobacterium bovis and M. tuberculosis are the primary causes of tuberculosis in cattle and humans, respectively. In 1890, Robert Koch demonstrated that intradermal instillation of live or killed tubercle bacilli or their extracts could elicit a delayed-type hypersensitivity response in guinea pigs that were experimentally infected with tubercle bacilli. Koch recognized the diagnostic value of such a response and termed the reaction the “tuberculin skin test” (22). The tuberculin skin test has remained the primary diagnostic test for tuberculosis in both cattle and humans since that time. Although Koch initially used a poorly characterized concentrate of filtrate from heat-killed liquid cultures of M. tuberculosis, termed “old tuberculin” today, a purified protein derivative (PPD) from M. bovis or M. avium is used in the tuberculin skin testing of cattle (22). PPDs are crude antigen preparations derived from heat-killed cultures of mycobacteria. Purified protein derivative contains a mixture of proteins, polypeptides, nucleic acids, and substantial amounts of polysaccharides (1). The currently acceptable tuberculin skin test involves the intradermal injection of PPD from M. bovis or M. avium and the subsequent detection of swelling and induration at the injection site 72 h later.
In the United States, two types of tuberculin skin tests are used in cattle. The first is the caudal fold test (CFT), where PPD is injected into a fold of skin at the base of the tail. Skin measurements are not recorded; however, any palpable swelling or induration 72 h after injection is considered a positive reaction and the animal is considered a “reactor.” Since animals that are infected or exposed to various nontuberculous mycobacteria (e.g., M. avium subsp. avium, M. avium subsp. paratuberculosis, M. kansasii, etc.) may show positive reactions on the CFT, a follow-up test, known as the comparative cervical test (CCT), is used after the CFT to differentiate true M. bovis infection from exposure to nontuberculous mycobacteria. To perform the CCT, two sites are shaved on the lateral side of the neck. Mycobacterium bovis PPD is injected into one site, and M. avium PPD is injected into the second site. The change in skin thickness due to swelling or induration is measured at both sites from preinjection to 72 h postinjection. The relative change in skin thickness at the two sites is used to differentiate true M. bovis infection from infection with nontuberculous mycobacteria. Although the identification and removal of tuberculous cattle through such testing have been effective in reducing the prevalence of bovine tuberculosis in most developed countries, problems do exist with such tests.
Studies have shown that tuberculin skin testing cannot be repeated within 60 days of initial tuberculin skin tests without suppression of responses that may erroneously categorize infected animals as noninfected (23). Such suppression is present as early as 7 days after the initial test and may not return to normal for 50 to 60 days (5, 23). Therefore, retesting of animals that yield marginal or ambiguous results is not generally done for approximately 60 days after the last tuberculin skin test.
In the United States, the estimated sensitivity values for the CFT and the CCT are 80.4 to 88.4% and 75%, respectively, and the specificity values are 96% and 98%, respectively (29). Early studies in Australia determined the sensitivity and specificity of the CFT to be 72% and 98.8%, respectively (6). The specificity of tuberculin skin testing can be reduced by exposure to nontuberculous mycobacteria, including M. avium subsp. avium and M. avium subsp. paratuberculosis.
To overcome some of the problems associated with tuberculin skin testing, a whole-blood cellular assay using a sandwich enzyme immunoassay for bovine gamma interferon (IFN-γ) was previously developed and found effective in the diagnosis of M. bovis infection in cattle (25, 32). The IFN-γ immunoassay for cattle uses two monoclonal antibodies, specific for bovine gamma interferon, that do not detect bovine alpha or beta interferons. The antibodies also recognize gamma interferon from sheep, goat, and buffalo but not pig, deer, or human (25).
Previous studies have demonstrated the sensitivity and specificity of the IFN-γ assay to be 81.8% and 99.1%, respectively, with slight variations depending on the study (26, 31, 33). In the same group of cattle from one of the above studies, the sensitivity and specificity of tuberculin skin testing were 68.1% and 96.7%, respectively (33). The IFN-γ assay has been adopted in many countries and approved for use in the diagnosis of M. bovis infection in cattle. However, in most countries, it is approved for use in conjunction with tuberculin skin testing and is most commonly used in place of the CCT as a confirmatory test following the CFT. In the United States, blood for the IFN-γ assay may be collected 3 to 30 days after injection of the CFT.
Various studies have been conducted in multiple countries to investigate the effect of prior tuberculin skin testing on IFN-γ production and the interpretation of IFN-γ assays (Table 1). Likely due to the highly variable conditions under which these studies were conducted, results have differed and range from no effect to an elevation or suppression in IFN-γ production (5, 26, 27, 30, 31, 34).
TABLE 1.
Summary of previous studies on the effect of tuberculin skin testing on IFN-γ responses in tuberculous mycobacterium-infected cattle
| Effect of tuberculin skin testing on IFN-γ responses | Reference |
|---|---|
| No significant change | Thom et al. (27) |
| No significant change | Gormley et al. (11a) |
| Increased 3 to 28 days after testing | Whipple et al. (30) |
| No effect 8 to 28 days after testing (no pretest sampled collected) | Ryan et al. (26) |
| No effect at 7 days after testing | Doherty et al. (5) |
| Increased 8 days after testing | Buddle et al. (2) |
| Decreased 3 to 7 days after testing followed by a boost, not returning to pretest levels at 59 days after testing | Wood and Rothel (31) |
| Marked decline for 7 days followed by an enhancement in response but not reaching pretest levels for 59 days | Rothel et al. (25) |
Serological tests to detect tuberculosis have long been desired due to their relative simplicity, robustness, and noninvasive nature relative to tests such as the tuberculin skin test. Early serological studies of bovine tuberculosis involved assays using unfractionated, highly cross-reactive antigen preparations, such as PPD, culture filtrates, or sonicates of M. bovis (4, 13, 18). Most of these tests displayed cross-reactivity with nontuberculous mycobacteria, decreasing their specificity. More recent investigations have focused on responses to specific proteins that are isolated from M. bovis culture filtrate, such as MPB70, MPB64, MPB83, and others (4, 7, 8, 14).
In general, serological assays for M. bovis infection are considered to be of lower sensitivity due to the theory that, early in M. bovis infection, cell-mediated responses predominate and that high antibody titers are seen in only the latter stages of disease. Some studies have shown that antibodies to various M. bovis components appear at different stages of disease and that elevated antibody titers may be correlated with advanced disease severity (7, 8, 12, 17). It has also been shown that tuberculin skin testing boosts antibody responses in M. bovis-infected cattle (13, 14, 19, 27). The objective of the present study was to investigate the effects of tuberculin skin testing (i.e., injection of PPD) on IFN-γ and antibody responses using two different testing regimens that are used in the United States.
MATERIALS AND METHODS
Animals.
Twenty-nine female and castrated male, 4-month-old, Holstein calves from tuberculosis-free sources were divided into four groups: inoculated 7-day CCT (n = 10), noninoculated control 7-day CCT (n = 5), inoculated 55-day CCT (n = 9), and noninoculated control 55-day CCT (n = 5). Cattle to be inoculated were inoculated intratonsillarly with 5 × 103 CFU of M. bovis strain 1315 into each tonsillar crypt for a total dose of 1 × 104 CFU, as described previously (27). Strain 1315 was originally isolated in 1995 from a white-tailed deer in Michigan. For intratonsillar inoculation, cattle were sedated by intravenous (i.v.) injection of xylazine (0.025 mg/kg of body weight) (Mobay Corporation, Shawnee, KS). After inoculation, the effects of xylazine were reversed by i.v. injection of tolazoline (4 mg/kg) (Lloyd Laboratories, Shenandoah, IA).
Animals were housed in biosecurity level 3 housing and fed commercial pelleted feed with free access to water. An outline of all procedures was approved by the National Animal Disease Center Institutional Animal Care and Use Committee prior to the beginning of the experiment.
Inoculum preparation.
Inoculum consisted of mid-log-phase M. bovis grown in Middlebrook's 7H9 liquid medium supplemented with 10% oleic acid-albumin-dextrose complex (OADC; Difco, Detroit, MI) plus 0.05% Tween 80 (Sigma Chemical Co., St. Louis, MO) grown for 10 days at 37°C. To harvest bacilli from the culture medium, cells were pelleted by centrifugation at 750 × g, washed twice with 1 ml of phosphate-buffered saline solution (PBS, 0.01 M [pH 7.2]), and diluted to the appropriate density. Enumeration of bacilli was performed by serial dilution plate counting on Middlebrook's 7H11 selective medium (Becton Dickinson, Cockeysville, MD). Inoculum was stored in 1-ml aliquots at −80°C until used. Twenty-four hours after freezing, a single 1-ml aliquot was removed and bacilli were enumerated by plate counting of serial dilutions on Middlebrook's 7H11 medium. At the time of inoculation, aliquots of inoculum were thawed and diluted to the appropriate concentration based on plate counts as described above. Plate counts were repeated on the day of inoculation to retrospectively confirm inoculum dosage.
Tuberculin skin testing.
The CFT was administered to all calves 55 days after inoculation according to USDA guidelines. Briefly, 100 μl (1.0 mg/ml) of M. bovis PPD (National Veterinary Services Laboratories, Ames, IA) was injected intradermally in the right caudal fold. Seventy-two hours later, the injection site was evaluated visually and by palpation for swelling and induration. Seven days after injection of the CFT, cattle in the inoculated 7-day CCT and noninoculated control 7-day CCT groups were administered the CCT test according to USDA guidelines. Briefly, after the clipping of hair from two sites on the lateral neck, 100 μl (1.0 mg/ml) of M. bovis PPD and 100 μl (0.4 mg/ml) of M. avium PPD (National Veterinary Services Laboratories) were injected intradermally into separate sites on the lateral side of the neck. Seventy-two hours later, injection sites were evaluated visually, by palpation, and by skin thickness measurements to the nearest millimeter, which were obtained using calipers. Change in skin thickness due to swelling or induration was calculated by subtracting the preinjection skin thickness from the postinjection skin thickness that was obtained 72 h after injection. Cattle in the inoculated 55-day CCT and noninoculated control 55-day CCT groups were evaluated by the CCT (as described above) 55 days after administration of the CFT.
Gamma interferon production.
Prior to inoculation and monthly thereafter, blood was collected for the evaluation of cell-mediated and humoral immune responses. Blood was also collected on the day of PPD injection and 3, 7, 10, and 15 days after PPD injection from all groups. Cell-mediated immune responses were evaluated by antigen-specific IFN-γ production using a commercially available kit (Bovigam; Pfizer, Inc.). For the IFN-γ assay, heparinized blood samples were dispensed in 1.5-ml aliquots into individual wells of a 24-well plate (Falcon 353047; Becton Dickinson and Company, Franklin Lakes, N.J.). Whole blood was incubated for 24 h with appropriate antigens. Plasma was harvested and stored at −80°C. IFN-γ concentrations in stimulated plasma were determined by using a commercial enzyme-linked immunosorbent assay (Bovigam; Pfizer, Inc.). Absorbancies of test samples were read at 450 nm by using an enzyme-linked immunosorbent assay plate reader (Molecular Devices, Menlo Park, CA.). Duplicate samples for individual treatments were analyzed.
Serology.
Antibody responses were evaluated for a panel of M. tuberculosis complex antigens by using the multiantigen print immunoassay as previously described (21). The following recombinant antigens of M. bovis were purified to near homogeneity as polyhistidine-tagged proteins (Rv numbers according to the classification of Cole et al. [3]): ESAT-6 (Rv3875), CFP-10 (Rv3874), and polyepitope fusions CFP-10-ESAT-6 and Acr1-MPB83, kindly provided by Peter Andersen, Statens Serum Institute, Copenhagen, Denmark; MPB59 (Rv1886c), MPB64 (Rv1980c), MPB70 (Rv2875), and MPB83 (Rv2873), kindly provided by John Pollock, Veterinary Sciences Division, Stormont, Belfast, United Kingdom (18); alpha-crystallin (Acr1, Rv3391) and the 38-kDa protein (PstS1, Rv0934), purchased from Standard Diagnostics, Seoul, South Korea. M. bovis culture filtrate was obtained from a field strain of M. bovis (T/91/1378; Veterinary Sciences Division, Belfast, United Kingdom) and kindly provided by John Pollock. Antigens were immobilized on nitrocellulose membranes (Protran BA83; Schleicher and Schuell) as narrow bands by using a semiautomatic air brush printing device (Linomat IV; Camag) at 50 μg/ml in PBS. After antigen printing, the membrane was cut perpendicular to the antigen bands into strips that were 4 mm wide. Nitrocellulose strips were blocked for 1 h with 1% nonfat skim milk in 0.15 M phosphate-buffered saline plus 0.05% Tween 20 (PBS-T). Strips were incubated for 1 h with the serum samples diluted 1:50 in blocking buffer, followed by extensive washing with PBS-T. The strips were incubated for 1 h with alkaline-phosphatase-labeled protein G (Sigma, St. Louis, MO) diluted 1:1,000 in blocking buffer and were washed again in PBS-T. Antibodies bound to printed antigens were visualized with 3,3′,5,5′-tetramethyl benzidine (Kirkegaard and Perry Laboratories, Gaithersburg, MD). The entire procedure was performed at room temperature.
Necropsy and tissue sampling.
One hundred thirty days after inoculation, all calves were euthanized by i.v. sodium pentobarbital. At necropsy, the following tissues or fluids were collected and processed for the isolation of M. bovis and microscopic analysis as described previously (27): palatine tonsil, lung, liver, mandibular, parotid, medial retropharyngeal, tracheobronchial, mediastinal, hepatic, mesenteric, and prefemoral lymph nodes. Lymph nodes were cross-sectioned at 0.5-cm intervals and examined. Each lung lobe was examined separately and cross-sectioned at 0.5- to 1.0-cm intervals. Lungs and lymph nodes were subjected to semiquantitative scoring of gross lesions in a method adapted from Vordermeier et al. (28). Lung lobes (left cranial, left caudal, right cranial, right caudal, middle, and accessory) were subjected to the following scoring system: 0, no visible lesions; 1, no external gross lesions but lesions seen upon slicing; 2, <5 gross lesions of <10 mm in diameter; 3, >5 gross lesions of <10 mm in diameter; 4, >1 distinct gross lesion of >10 mm in diameter; 5, coalescing gross lesions. The scoring of lymph node gross lesions was based on the following scoring system: 0, no visible lesions; 1, small focal lesion of 1 to 2 mm in diameter; 2, several small foci; 3, extensive lesions. Tissues collected for microscopic analysis were fixed by immersion in 10% neutral buffered formalin and included all tissues that were collected for bacteriologic examination. For microscopic examination, formalin-fixed tissues were processed by routine paraffin-embedment techniques, cut in 5-μm sections and stained with hematoxylin and eosin. Adjacent sections were cut from samples containing lesions suggestive of tuberculosis (caseonecrotic granulomata) and stained by the Ziehl-Neelsen technique for the identification of acid-fast bacteria.
Statistical analysis.
Means of IFN-γ responses between groups were compared using one-way repeated measures analysis of variance (GraphPad Prism; GraphPad Software, San Diego, CA). Differences between means were then compared by using the Bonferroni method. Means of lesion scores between groups were compared using Student's t test. A P value of <0.05 was considered significant.
RESULTS
Intradermal skin testing.
Fifty-eight days after inoculation, all experimentally infected cattle were categorized as reactors by using the CFT. At the same time point, 9/10 noninoculated control animals were categorized as nonreactors, while 1/10 noninoculated control animals was categorized as a reactor. In practice, under current USDA guidelines, all animals that are categorized as reactors by using the CFT are subsequently tested by the CCT using one of the two methods described in Materials and Methods.
The 10 animals in the inoculated 7-day CCT group were categorized as reactors to M. bovis PPD according to USDA guidelines for the interpretation of the CCT. Conversely, the five calves in the noninoculated control 7-day CCT group were categorized as nonreactors to M. bovis PPD, including the animal that had been categorized as a reactor by the CFT. This particular animal had a change in skin thickness at the M. avium PPD injection site two- to eightfold greater than those of the other noninoculated control calves tested at this time point, suggesting prior exposure to nontuberculous mycobacteria.
All eight (one calf died unexpectedly from nontuberculosis-related causes 11 weeks after inoculation) calves in the inoculated 55-day CCT group were categorized as reactors to M. bovis PPD according to the guidelines for the interpretation of the CCT. Conversely, all five calves in the noninoculated control 55-day CCT group were categorized as nonreactors to M. bovis PPD.
Gamma interferon production.
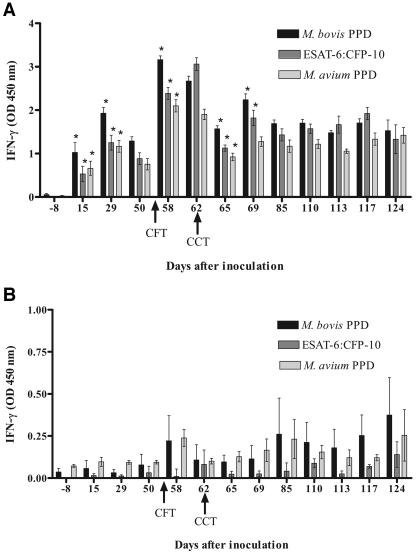
In calves in the inoculated 7-day CCT group, IFN-γ responses to M. bovis PPD, rESAT-6-CFP-10, and M. avium PPD were qualitatively similar but differed quantitatively (Fig. 1A). Following the administration of the CFT at 55 days after inoculation, there was a significant increase in IFN-γ production at 58 and 62 days after inoculation; this was immediately followed by a significant decrease in production 65 days after inoculation. Values from 65 days after inoculation to the termination of the study were not significantly different from pre-skin test values. Similar changes in IFN-γ response were seen when rESAT-6-CFP-10 and M. avium PPD were used as antigens instead of M. bovis PPD (Fig. 1A).
FIG. 1.
Longitudinal IFN-γ response to M. bovis PPD, M. avium PPD, or rESAT-6-CFP-10 fusion protein. Values represent the mean responses to each antigen (24-h stimulation) minus the response to medium alone ± standard error of the mean (error bars) for 10 M. bovis-inoculated calves (A) or 5 noninoculated control calves (B). Day 0 represents the day of inoculation. Arrows represent time points when purified protein derivative was injected for tuberculin skin testing. *, significantly different than the previous time point (P < 0.05). OD, optical density.
In noninoculated-control, 7-day CCT calves, responses to both M. bovis PPD and M. avium PPD followed the same pattern, with an increased response after the CFT; however, values did not differ significantly over the course of the study (Fig. 2A). Responses to rESAT-6-CFP-10 were significantly lower at all time points than those seen in response to M. bovis and M. avium PPDs.
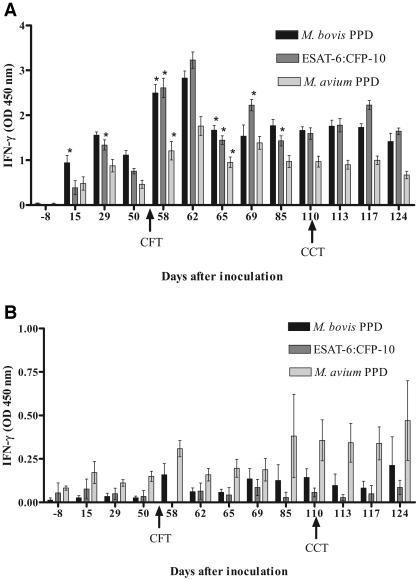
FIG. 2.
Longitudinal IFN-γ response to M. bovis PPD, M. avium PPD, or rESAT-6-CFP-10 fusion protein. Values represent the mean responses to each antigen (24-h stimulation) minus the response to medium alone ± standard error of the mean (error bars) for eight to nine M. bovis-inoculated calves (A) or five noninoculated control calves (B). Day 0 represents the day of inoculation. Arrows represent time points when purified protein derivative was injected for tuberculin skin testing. *, significantly different than previous time point (P < 0.05). OD, optical density.
In the inoculated 55-day CCT group, IFN-γ responses to M. bovis PPD, M. avium PPD and rESAT-6-CFP-10 were qualitatively similar but differed quantitatively. Similar to the inoculated 7-day CCT group, following the administration of the CFT at 55 days after inoculation, there was a significant increase in IFN-γ production in response to all three antigens, peaking at 58 to 62 days after inoculation, followed by a significant decrease in production 65 days after inoculation (Fig. 2A). Values from 65 days after inoculation to the termination of the study were not significantly different from pre-CFT values. The administration of the CCT at 110 days after inoculation resulted in no significant change in IFN-γ production in response to M. bovis PPD, M. avium PPD, or rESAT-6-CFP-10 stimulation.
In the noninoculated control 55-day CCT group responses to M. bovis PPD, M. avium PPD and rESAT-6-CFP-10 did not increase significantly after the CFT or CCT compared to pre-skin test levels. Values did not differ significantly over the course of the study (Fig. 2B). Responses to both M. bovis PPD and rESAT-6-CFP-10 were lower than those seen for M. avium PPD.
Serology.
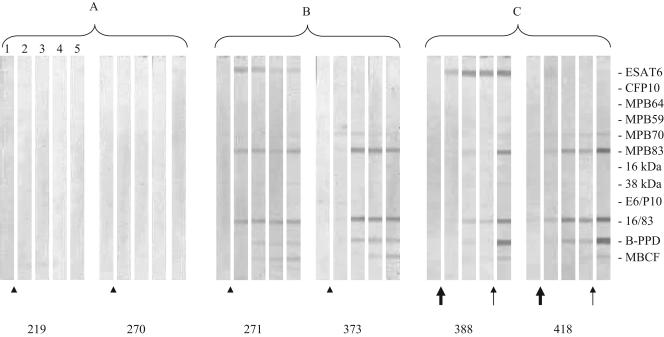
Skin testing boosted antibody responses in M. bovis-infected cattle. Intriguingly, this effect was most pronounced in the inoculated 55-day CCT group after the CCT at 110 days after inoculation (Fig. 3). In contrast, in the inoculated 7-day CCT group, the application of the CCT 7 days after the CFT boosted responses but, thereafter, antibody levels remained unchanged throughout the study. In all M. bovis-inoculated cattle, antibodies to MPB83, the fusion protein MPB83-Acr1, and M. bovis PPD were noted after tuberculin skin testing. Antibody responses to M. bovis culture filtrate and ESAT-6 were seen after tuberculin skin testing in 10 of 18 and 2 of 18 cattle, respectively. Tuberculin skin testing induced no antibody responses in noninoculated cattle.
FIG. 3.
Antibody responses to recombinant antigens detected by multiantigen print immunoassay in cattle that were experimentally inoculated with M. bovis. Arrows in the lower margin indicate time points when purified protein derivatives were injected for tuberculin skin testing (arrowhead indicates CFT and CCT conducted 7 days apart; large arrow indicates CFT only; thin arrow indicates CCT only). Printed antigens are shown in the right margin. Strips represent different time points during infection when serum samples were collected. Representative responses by two different calves in the noninoculated control 7-day CCT group (no. 219 and no. 270) (A), inoculated 7-day CCT group (no. 271 and no. 373) (B), and inoculated 55-day CCT group (no. 388 and no. 418) (C). Lane 1, postinoculation day (PID) −8; lane 2, PID 69; lane 3, PID 85; lane 4, PID 110; lane 5, PID 124.
Necropsy.
All M. bovis-inoculated cattle developed gross and microscopic lesions that were consistent with tuberculosis (i.e., caseonecrotic granulomas with acid-fast bacilli) in at least one lymph node. The medial retropharyngeal lymph node was affected in all experimentally inoculated cattle. Other sites with gross or microscopic lesions included tonsil (11/18), mandibular lymph node (7/18), mediastinal lymph node (5/18), lung (5/18), tracheobronchial lymph node (4/18), liver (4/18), and mesenteric lymph node (1/18). The distribution of lesions and mean lymph node lesion scores did not differ between groups that were inoculated with M. bovis (Table 2). Mean lung lesion scores were significantly greater in the 55-day-CCT-inoculated group. Lesions were not seen and M. bovis was not isolated from control cattle.
TABLE 2.
Mean lesion scores from Mycobacterium bovis-inoculated and tuberculin skin-tested cattlea
| Group | Mean LN lesion score ± SEb | Lung lesion score |
|---|---|---|
| 7-day CCT | 0.70 ± 0.14 | 0.08 ± 0.05 |
| 55-day CCT | 0.74 ± 0.14 | 0.38 ± 0.08c |
Data represent means of 8 to 10 calves and were obtained by using the CFT followed 7 or 55 days later by the CCT.
LN, lymph node.
P value was 0.01 compared to that for the 7-day CCT group.
DISCUSSION
In M. bovis-inoculated cattle, but not noninoculated cattle, the injection of PPD for the CFT induced a significant boost in IFN-γ production in peripheral blood mononuclear cells in response to incubation with M. bovis PPD, M. avium PPD, and rESAT-6-CFP-10. These findings are similar to those seen by other investigators (2, 30). Specifically, our findings are most similar to those reported by Buddle et al. (2), where IFN-γ responses were measured after tuberculin skin testing in M. bovis BCG-vaccinated, M. bovis-inoculated cattle. However, unlike the findings reported by Whipple et al. (30), where the IFN-γ boosting effects of PPD administration were seen 3 to 28 days after injection, in the present study, the boosting effects were seen for only 3 to 7 days. Differences between results seen by Whipple et al. and those in the present study may be due to cattle age differences (adult cattle versus 4-month-old calves) or the use of cattle that were sensitized with killed M. bovis strain AN5 in the former study rather than M. bovis-inoculated cattle as were used in the present study.
This is the first study to demonstrate a boost in IFN-γ production by peripheral blood mononuclear cells in response to stimulation with rESAT-6-CFP-10. In M. bovis-inoculated cattle, responses to rESAT-6-CFP-10 were similar to those seen in response to M. bovis PPD stimulation. However, significantly decreased responses to rESAT-6-CFP-10 were seen compared to those with M. bovis PPD stimulation in noninoculated cattle, suggesting that specificity could be enhanced by using specific antigens, such as rESAT-6-CFP-10, in the IFN-γ assay.
Differences between the results that were obtained in the present study and those reported previously (5, 26, 27, 30, 31, 34) may be due to differences in route of inoculation, natural infection versus experimental inoculation, stage of disease progression, methods and dosages of PPD used for skin testing, or differences in methods used to conduct or interpret the IFN-γ assay. All calves in the present study were experimentally inoculated and evaluated at the same time point after inoculation. It is possible that results may differ if cattle are evaluated at various stages of disease progression. In the present study, two specific methods for use of the IFN-γ assay in relation to tuberculin skin testing were evaluated. These methods were performed according to USDA guidelines. Dosages of PPD and methods used for tuberculin skin testing were also those approved by USDA.
Boosting of IFN-γ responses after injection of PPD in M. bovis-inoculated but not noninoculated control animals potentially increases test sensitivity, enhancing the ability to identify M. bovis-infected cattle. In the United States, current recommendations are to collect blood for the IFN-γ assay 3 to 30 days after injection of PPD for the CFT. The present study did not support a rationale for the 3- to- 30-day time frame but, rather, suggests that, in order to benefit from the boosting effect of tuberculin skin testing, blood should be collected 3 to 7 days after the injection of PPD for the CFT.
Significant IFN-γ responses to all three antigens used in the study were seen by 15 days after experimental inoculation with M. bovis. Elevations in IFN-γ within 2 to 4 weeks of experimental inoculation are consistent with other studies using cattle that were experimentally inoculated with M. bovis (2, 27). Similarly, hypersensitivity to tuberculin, resulting in positive tuberculin skin test results, usually occurs in cattle within 3 to 6 weeks after inoculation (6).
Although responses to M. bovis PPD and M. avium PPD increased after the CFT in noninoculated cattle, the increases were not significant relative to pretuberculin skin test responses. Of note, however, was that responses to rESAT-6-CFP-10 were less affected by tuberculin skin testing than were responses to either of the PPD preparations. Minimizing the effect of tuberculin skin testing on noninoculated cattle would decrease the potential for false-positive results that may be created by application of the caudal fold test. Such issues become important as the prevalence of tuberculosis decreases and the positive predictive value of a positive tuberculin skin test also decreases. The application of highly specific ancillary tests, such as the IFN-γ assay using specific antigens such as rESAT-6-CFP-10, will be warranted to clarify the true status of tuberculin skin test reactors.
An advantage of the IFN-γ assay relative to tuberculin skin testing is that both sensitivity and specificity of the assay can be adjusted by altering the criterion for defining a positive reactor (29, 33). This could be especially helpful in some situations, such as during an eradication campaign where maximum sensitivity is desired. The interpretation of results can be adjusted to maximize sensitivity at the cost of increasing the number of false positives.
Antibodies to MPB83 were boosted by tuberculin skin testing. MPB83 is a constitutively expressed, highly immunogenic, cell wall-associated glycolipoprotein, with high homology to MPB70 (15, 16). Both MPB83 and MPB70 are major constituents of M. bovis PPD. Elevated antibodies to MPB83 have previously been seen after tuberculin skin testing in cattle that were experimentally infected with M. bovis (20). Moreover, a positive relationship was seen between disease severity, bacterial burdens in lymph nodes, and MPB83 antibody responses (20). Similar to the findings in the present study, Lyashchenko et al. noted a positive correlation between post-tuberculin skin testing antibody responses to MPB83 and IFN-γ responses to ESAT-6 (20). Although antibodies to the MPB83-Acr1 fusion protein were seen after tuberculin skin testing in M. bovis-inoculated cattle in the present study, these same cattle did not develop antibodies to the Acr1 protein alone, suggesting that MPB83 is the major immunogenic component of the MPB83-Acr1 fusion protein. A similar failure to induce antibodies to Acr1 has been demonstrated previously (20).
Tuberculin skin testing also resulted in a boosting of antibody production to ESAT-6. Similar findings have been described previously (20). The protein ESAT-6 is cosecreted by members of the M. tuberculosis complex in a tight 1:1 complex with CFP-10 (24). Genes for both ESAT-6 and CFP-10 are absent in many nontuberculous mycobacteria as well as in the vaccine M. bovis BCG. However, esat-6 and cfp-10 are present in a subset of nontuberculous mycobacteria, such as M. kansasii, M. marinum, M. leprae, and M. smegmatis (9, 10, 11; N. C. Gey van Pittius, R. M. Warren, and P. D. van Helden, Letter, Infect. Immun. 70:6509-6510, 2002). Therefore, infection with or exposure to nontuberculous mycobacteria that produced ESAT-6 or CFP-10 could confound diagnostic tests based on these proteins.
The present study revealed different patterns of the tuberculin skin test-induced boost of antibody responses in inoculated 7-day CCT and 55-day CCT groups. Cattle in the latter group showed more striking anamnestic responses following CCT. Intriguingly, significantly higher lung pathology scores were found in this group compared to those of 7-day CCT animals. While reasons for this difference remain unclear, this finding is consistent with previous observations that greater skin test-boosted antibody responses are associated with severity of disease (20).
No antibody was found in noninoculated control cattle, neither before nor after tuberculin skin testing. Thus, serological assays may have enhanced sensitivity if used in conjunction with tuberculin skin testing, whereas specificity should not be compromised.
In general, the current study demonstrates that the boosting effect of tuberculin skin testing on both IFN-γ and antibody production takes place in only infected animals, is highly specific, and could be further explored for development of more sensitive diagnostic algorithms (i.e., caudal fold testing followed by IFN-γ analysis and serology).
Acknowledgments
We thank Peter Andersen and John Pollock for supplying recombinant antigens; Larry Wright, Dennis Weuve, Don Robinson, Doug Ewing, and Todd Holtz for animal care; and Becky Lyon, Shelly Zimmerman, Jessica Pollock, and Peter Lasley for technical assistance.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
REFERENCES
- 1.Angus, R. D. 1978. Production of reference PPD tuberculins for veterinary use in the United States. J. Biol. Stand. 6:221-227. [DOI] [PubMed] [Google Scholar]
- 2.Buddle, B. M., G. W. deLisle, A. Pfeffer, and F. W. Aldwell. 1995. Immunological responses and protection against Mycobacterium bovis in calves vaccinated with a low dose of BCG. Vaccine 13:1123-1130. [DOI] [PubMed] [Google Scholar]
- 3.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 4.Daniel, T. M., and S. M. Debanne. 1987. The serodiagnosis of tuberculosis and other mycobacterial diseases by enzyme-linked immunosorbent assay. Am. Rev. Respir. Dis. 135:1137-1151. [DOI] [PubMed] [Google Scholar]
- 5.Doherty, M. L., L. Monaghan, H. F. Bassett, and P. J. Quinn. 1995. Effect of recent injection of purified protein derivative on diagnostic tests for tuberculosis in cattle infected with Mycobacterium bovis. Res. Vet. Sci. 58:217-221. [DOI] [PubMed] [Google Scholar]
- 6.Francis, J., R. J. Seiler, I. W. Wilkei, D. O'Boyle, M. J. Lumsden, and A. J. Frost. 1978. The sensitivity and specificity of various tuberculin tests using bovine PPD and other tuberculins. Vet. Rec. 103:420-435. [DOI] [PubMed] [Google Scholar]
- 7.Fifis, T., J. S. Rothel, and P. R. Wood. 1994. Soluble Mycobacterium bovis protein antigens: studies on their purification and immunological evaluation. Vet. Microbiol. 40:65-81. [DOI] [PubMed] [Google Scholar]
- 8.Fifis, T., L. A. Corner, J. S. Rothel, and P. R. Wood. 1994. Cellular and humoral immune responses of cattle to purified Mycobacterium bovis antigens. Scand. J. Immunol. 39:267-274. [DOI] [PubMed] [Google Scholar]
- 9.Geluk, A., K. E. van Meijgaarden, K. L. Franken, B. Wieles, S. M. Arend, W. R. Faber, B. Naafs, and T. J. Ottenhoff. 2004. Immunological cross-reactivity of the Mycobacterium leprae CFP-10 with its homologue in Mycobacterium tuberculosis. Scand. J. Immunol. 59:66-70. [DOI] [PubMed] [Google Scholar]
- 10.Geluk, A., K. E. van Meijgaarden, K. L. Franken, Y. W. Subronto, B. Wieles, S. M. Arend, E. P. Sampaio, T. de Boer, W. R. Faber, B. Naafs, and T. H. Ottenfoff. 2002. Identification and characterization of the ESAT-6 homologue of Mycobacterium leprae and T-cell cross-reactivity with Mycobacterium tuberculosis. Infect. Immun. 70:2544-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gey van Pittius, N. C., J. Gamieldien, W. Hide, G. D. Brown, R. J. Siezen, and A. D. Beyers. 2001. The ESAT-6 gene cluster of Mycobacterium tuberculosis and other high G+C gram-positive bacteria. Genome Biol. 2:RESEARCH0044. [Online.] http://gemonebiology.com/2001/2/10/research/0044. [DOI] [PMC free article] [PubMed]
- 11a.Gormley, E., M. B. Doyle, K. McGill, E. Costello, M. Good, and J. D. Collins. 2004. The effect of the tuberculin test and the consequences of a delay in blood culture on the sensitivity of a gamma-interferon assay for the detection of Mycobacterium bovis in cattle. Vet. Immunol. Immunopathol. 102:413-420. [DOI] [PubMed] [Google Scholar]
- 12.Hanna, J., S. D. Neill, and J. J. O'Brien. 1989. Use of PPD and phosphatide antigens to detect the serological response in experimental bovine tuberculosis. Res. Vet. Sci. 47:43-47. [PubMed] [Google Scholar]
- 13.Hanna, J., S. D. Neill, and J. J. O'Brien. 1992. ELISA tests for antibodies in experimental bovine tuberculosis. Vet. Microbiol. 31:243-249. [DOI] [PubMed] [Google Scholar]
- 14.Harboe, M., H. Wiker, R. Duncan, M. M. Garcia, T. W. Dukes, B. W. Brooks, C. Turcotte, and S. Nagai. 1990. Protein G-based enzyme linked immunosorbent assay for anti-MPB70 antibodies in bovine tuberculosis. J. Clin. Microbiol. 28:913-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harboe, M., S. Nagai, H. G. Wiker, K. Sletten, and S. Haga. 1995. Homology between the MPB70 and MPB83 proteins of Mycobacterium bovis BCG. Scand. J. Immunol. 42:46-51. [DOI] [PubMed] [Google Scholar]
- 16.Hewinson, R. G., S. L. Michell, W. P. Russell, R. A. McAdam, and W. R. Jacobs. 1996. Molecular characterization of MPT83: a seroreactive antigen of Mycobacterium tuberculosis with homology to MPT70. Scand. J. Immunol. 43:490-493. [DOI] [PubMed] [Google Scholar]
- 17.Lenzini, L., P. Rotolli, and L. Rotolli. 1977. The spectrum of human tuberculosis. Clin. Exp. Immunol. 27:230-237. [PMC free article] [PubMed] [Google Scholar]
- 18.Lepper, A. W. D., L. A. Corner, and C. W. Pearson. 1977. Serological responses in experimental bovine tuberculosis. Aust. Vet. J. 53:301-305. [DOI] [PubMed] [Google Scholar]
- 19.Lightbody, K. A., R. A. Skuce, S. D. Neill, and J. M. Pollock. 1998. Mycobacterial antigen-specific antibody responses in bovine tuberculosis: an ELISA with potential to confirm disease status. Vet. Rec. 142:295-300. [DOI] [PubMed] [Google Scholar]
- 20.Lyashchenko, K., A. O. Whelan, R. Greenwald, J. M. Pollock, P. Andersen, R. G. Hewinson, and H. M. Vordermeier. 2004. Association of tuberculin-boosted antibody responses with pathology and cell-mediated immunity in cattle vaccinated with Mycobacterium bovis BCG and infected with M. bovis. Infect. Immun. 72:2462-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyashchenko, K. P., M. Singh, R. Colangeli, and M. L. Gennaro. 2000. A multi-antigen print immunoassay for the development of serological diagnosis of infectious diseases. J. Immunol. Methods 242:91-100. [DOI] [PubMed] [Google Scholar]
- 22.Pritchard, D. G. 1988. A century of bovine tuberculosis 1888-1988: conquest and controversy. J. Comp. Pathol. 99:357-399. [DOI] [PubMed] [Google Scholar]
- 23.Radunz, B. L., and A. W. D. Lepper. 1985. Suppression of skin reactivity to bovine tuberculin in repeat tests. Aust. Vet. J. 62:191-194. [DOI] [PubMed] [Google Scholar]
- 24.Renshaw, P. S., P. Panagiotidou, A. Whelan, S. V. Gordon, R. G. Hewinson, R. A. Williamson, and M. D. Carr. 2002. Conclusive evidence that the major T-cell antigens of the Mycobacterium tuberculosis complex ESAT-6 and CFP-10 for a tight 1:1 complex and characterization of the structural properties of ESAT-6, CFP-10, and the ESAT-6:CFP-10 complex. Implications for pathogenesis and virulence. J. Biol. Chem. 277:21598-21603. [DOI] [PubMed] [Google Scholar]
- 25.Rothel, J. S., S. L. Jones, L. A. Corner, J. C. Cox, and P. R. Wood. 1990. A sandwich enzyme immunoassay for bovine interferon-γ and its use for the detection of tuberculosis in cattle. Aust. Vet. J. 67:134-137. [DOI] [PubMed] [Google Scholar]
- 26.Ryan, T. J., B. M. Buddle, and G. W. deLisle. 2000. An evaluation of the gamma interferon test for detecting bovine tuberculosis in cattle 8 to 28 days after tuberculin skin testing. Res. Vet. Sci. 69:57-61. [DOI] [PubMed] [Google Scholar]
- 27.Thom, J., J. H. Morgan, J. C. Hope, B. Villarreal-Ramos, M. Martin, and C. J. Howard. 2004. The effect of repeated tuberculin skin testing of cattle on immune responses and disease following experimental infection with Mycobacterium bovis. Vet. Immunol. Immunopathol. 102:399-412. [DOI] [PubMed] [Google Scholar]
- 28.Vordermeier, H. M., M. A. Chambers, P. J. Cockle, A. O. Whelan, J. Simmons, and R. G. Hewinson. 2002. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 70:3026-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whipple, D. L., C. A. Bolin, A. J. Davis, J. L. Jarnagin, D. C. Johnson, R. S. Nabors, J. B. Payeur, D. A. Saari, A. J. Wilson, and M. M. Wolf. 1995. Comparison of the sensitivity of the caudal fold test and a commercial γ-interferon assay for diagnosis of bovine tuberculosis. Am. J. Vet. Res. 56:415-419. [PubMed] [Google Scholar]
- 30.Whipple, D. L., M. V. Palmer, R. E. Slaughter, and S. L. Jones. 2001. Comparison of purified protein derivatives and effect of skin testing on results of a commercial gamma interferon assay for diagnosis of tuberculosis in cattle. Am. J. Vet. Res. 13:117-122. [DOI] [PubMed] [Google Scholar]
- 31.Wood, P. R., and J. S. Rothel. 1994. In vitro immunodiagnostic assays for bovine tuberculosis. Vet. Microbiol. 40:125-135. [DOI] [PubMed] [Google Scholar]
- 32.Wood, P. R., and S. L. Jones. 2001. Bovigam: an in vitro cellular diagnostic test for bovine tuberculosis. Tuberculosis 81:147-155. [DOI] [PubMed] [Google Scholar]
- 33.Wood, P. R., L. A. Corner, J. S. Rothel, C. Baldock, S. L. Jones, D. B. Cousins, B. S. McCormick, B. R. Francis, J. Creeper, and N. E. Tweedle. 1991. Field comparison of the interferon gamma assay and the intradermal tuberculin test for the diagnosis of bovine tuberculosis. Aust. Vet. J. 68:286-290. [DOI] [PubMed] [Google Scholar]
- 34.Wood, P. R., L. A. Corner, J. S. Rothel, J. L. Ripper, T. Fifis, B. S. McCormick, B. Francis, L. Melville, K. Small, K. De Witte, J. Tolson, T. J., Ryan, G. W. deLisle, J. C. Cox, and S. L. Jones. 1992. A field evaluation of serological and cellular diagnostic tests for bovine tuberculosis. Vet. Microbiol. 31:71-79. [DOI] [PubMed] [Google Scholar]