Abstract
We report the isolation and characterization of a new line of mutant Chinese hamster ovary cells, designated SRD-5, that are resistant to 25HC, a potent suppressor of cleavage of sterol regulatory element-binding proteins (SREBPs) in mammalian cells. In SRD-5 cells, SREBPs are cleaved constitutively, generating transcriptionally active nuclear SREBP even in the presence of sterols. Sequence analysis of SREBP cleavage-activating protein (SCAP) transcripts from SRD-5 cells revealed the presence of a mutation in one SCAP allele that results in substitution of a conserved Leu by Phe at amino acid 315 within the sterol-sensing domain. Sterols fail to inhibit the packaging of SREBP/SCAP(L315F) complexes into budding vesicles in vitro. Sterols also fail to induce binding of SCAP(L315F) to insig-1 or insig-2, two proteins that function in the sterol-mediated retention of SREBP/SCAP complexes in the endoplasmic reticulum. Similar findings were observed for SCAP(D443N) and SCAP(Y298C), both of which cause a sterol-resistant phenotype. Thus, three different point mutations, each within the sterol-sensing domain of SCAP, prevent sterol-induced binding of SCAP to insig proteins and abolish feedback regulation of SREBP processing by sterols.
In animal cells, lipid homeostasis is maintained by a feedback mechanism regulating the transcription of genes involved in lipid synthesis and uptake (1). Sterol regulatory element-binding proteins (SREBPs) comprise a family of membrane-bound transcription factors that regulate the coordinated expression of these genes. SREBPs are synthesized as 120-kDa integral membrane proteins of the endoplasmic reticulum (ER) that consist of three domains. Both the NH2-terminal transcription factor domain and the COOH-terminal regulatory domain face the cytoplasm. These are connected by a third domain consisting of two transmembrane helices that are separated by a loop of ≈30 amino acids that projects into the lumen of the ER. In the ER, SREBPs form a complex with an escort factor, SREBP cleavage- activating protein (SCAP), via their respective COOH-terminal domains (2). When cellular lipid levels are high, the SREBP/SCAP complex remains in the ER; when lipid levels are low, SREBP/SCAP complexes are packaged into budding vesicles and transit to the Golgi apparatus (3, 4).
Packaging of SREBP/SCAP complexes into vesicles is the crucial event in feedback regulation of cholesterol homeostasis (3). SREBPs exit the ER in response to cellular demand for cholesterol and transit to the Golgi apparatus. Once there, the luminal loop of SREBP is cleaved by site-1-protease (S1P), separating the two halves of SREBP. These remain bound to the membrane because each half retains a single transmembrane domain. The amino-terminal half then serves as a substrate for site-2-protease (S2P), a hydrophobic metalloprotease that cleaves it at a site 3 aa into the transmembrane domain (5), freeing the transcriptionally active nuclear form of SREBP.
Identification and functional analysis of the protein machinery responsible for maintaining cholesterol homeostasis have been greatly facilitated by the availability of mutant somatic cells harboring defects in cholesterol homeostasis (6). Mutant Chinese hamster ovary (CHO) cells that can survive in the presence of 25-hydroxycholesterol (25HC) have substantially enhanced our understanding of the pathway. 25HC is a potent suppressor of SREBP processing (7) and, therefore, efficiently suppresses both de novo synthesis of cholesterol and its exogenous uptake. Owing to its additional hydroxyl group, 25HC is functionally unable to substitute for cholesterol in cell membranes. Thus, wild-type cells grown in the presence of 25HC eventually die owing to depletion of cellular cholesterol.
Mutant cells resistant to killing by chronic treatment with 25HC fall into two classes based on the nature of the mutation involved: (i) rearrangements in the gene encoding SREBP-2 such that the transcription factor domain is synthesized without a transmembrane domain, thereby bypassing the regulatory machinery, and (ii) point mutations within the sterol-sensing domain of SCAP (6).
SCAP is a 1,276-aa polytopic transmembrane protein composed of an NH2-terminal region consisting of eight transmembrane helices and a soluble COOH-terminal domain consisting of multiple copies of a WD40 repeat motif, which mediates protein–protein interactions (8). Transmembrane helices 2–6 (amino acids 280–448) form a sterol-sensing domain that is essential for proper transduction of the regulatory signals originating from cellular lipid levels. The rate-limiting enzyme of cholesterol biosynthesis, 3-hydroxy-3-methylglutaryl CoA reductase (1), contains a similar sterol-sensing domain that is required for the sterol-regulated stability of the enzyme (9).
The cDNA encoding SCAP was cloned by using an expression assay. Owing to a guanine-to-adenine alteration, producing a D443N mutation in the sterol-sensing domain, mutant SCAP conferred sterol insensitivity in a dominant fashion on wild-type cells (10). Subsequently, another independently isolated, sterol-insensitive cell line was shown to harbor a Y298C point mutation within the sterol-sensing domain of SCAP (11). Both of these amino acid substitutions also interfere with the ability of the SCAP sterol-sensing domain to undergo a conformational change when cholesterol is added to ER membranes (12).
Yang et al. (13) recently identified insig-1 as a protein in mammalian cells to which the sterol-sensing domain of SCAP binds, thereby retaining the SREBP/SCAP complex in the ER. A highly similar protein, insig-2, plays a similar role (14). There are important differences between the two insig proteins: (i) insig-1 is encoded by a gene that is transcriptionally activated by nuclear SREBP (but the gene encoding insig-2 is not) and (ii) insig-1, when expressed at high levels, can interact with SCAP in the absence of sterols, whereas the interaction between insig-2 and SCAP absolutely requires the presence of sterols (14). The Y298C mutation in the sterol-sensing domain of SCAP that renders SREBP cleavage insensitive to sterols blocks the interaction of SCAP and insig (13).
Here, we present characterization of a new dominant, sterol-insensitive allele of SCAP and show that it too has defects in the regulation of packaging into ER-derived transport vesicles and in interaction with both insig-1 and insig-2. We also show that SCAP(D443N) likewise fails to interact with insig. This brings to three the number of distinct mutations within the SCAP sterol-sensing domain that disrupt interaction between SCAP and insig and result in a dominant, sterol-insensitive form of SCAP.
Materials and Methods
Materials.
Mouse mAbs IgG-1D2 against NH2 terminus of human SREBP-2 (14), IgG-7D4 against the NH2 terminus of hamster SREBP-2 (15), IgG-9D5 against hamster SCAP (2), and IgG-9E10 against c-Myc (13) have been described. We obtained mouse mAb anti-KDEL IgG from StressGen Biotechnologies (Victoria, Canada); rabbit polyclonal anti-Myc antibodies from Upstate Biotechnology (Lake Placid, NY); horseradish peroxidase-conjugated, affinity-purified donkey anti-mouse IgG from Jackson ImmunoResearch; BCA Kit and SuperSignal Substrate System from Pierce; QuikChange XL Site-Directed Mutagenesis Kit from Stratagene; and FuGENE 6 reagent from Roche (Gipf-Oberfrick, Switzerland). All other reagents were obtained from reported sources (3, 13, 14, 16, 17).
Tissue Culture Medium.
Medium A contains a 1:1 mixture of Ham's F-12 medium and DMEM containing 100 units/ml penicillin and 100 μg/ml streptomycin sulfate. Medium B contains medium A supplemented with 5% (vol/vol) FCS, 5 μg/ml cholesterol, 1 mM sodium mevalonate, and 20 mM sodium oleate. Medium C contains medium A supplemented with 5% (vol/vol) newborn calf lipoprotein-deficient serum, 50 μM sodium compactin, and 50 μM sodium mevalonate.
Cell Culture.
Cells were grown in monolayer at 37°C in an atmosphere of 8–9% CO2. CHO-7 cells are a line of CHOK1 cells selected for growth in lipoprotein-deficient serum (18). SRD-9 cells are a line of mutant 25HC-resistant hamster cells (11). SRD-13A cells are a line of cholesterol and unsaturated fatty acid auxotrophic hamster cells deficient in SCAP (17). CHO-7 cells were maintained in medium A containing 5% newborn calf lipoprotein-deficient serum. SRD-9 cells were maintained in medium A containing 5% (vol/vol) fetal calf lipoprotein-deficient serum with 0.3 μg/ml 25HC. SRD-13A cells were maintained in medium B.
Mutagenesis and Isolation of Sterol-Resistant SRD-5 Cells.
CHO-7 cells were plated on day 0 at 3 × 105 cells per 75-cm2 flask in medium D [medium A containing 2 mM glutamine, 10 mM Hepes, and 10% (vol/vol) newborn calf lipoprotein-deficient serum]. On day 1, the medium was replaced with fresh medium D supplemented with 0.4 mg/ml nitrosoethylurea (Sigma). After 24 h, the cells were washed three times with PBS and refed with medium D supplemented with 0.2 mM mevalonate. On day 4, cells were washed, refed with medium D, and grown for 7 days to allow expression of altered phenotypes. On day 10, pools of mutagenized cells were plated on six dishes at 3 × 105 cells per l00-mm dish in medium D supplemented with 1 μg/ml 25HC. On day 22, surviving colonies were isolated with cloning cylinders, transferred to 24-well plates in medium D containing 1 μg/ml 25HC, and allowed to proliferate. After determining that supplementation with Hepes and mevalonate was dispensable, the cells were maintained further in medium A containing 5% fetal calf lipoprotein-deficient serum and 1 μg/ml 25HC.
In Vitro Vesicle-Formation Assay.
The protocol used in this study was described (3). Briefly, the 1.6 × 104 g fraction of membranes was isolated from hamster cells treated in the absence or presence of sterols and incubated with nucleoside triphosphates and rat liver cytosol to generate ER-derived transport vesicles, which then were separated from donor membranes by differential centrifugation. Vesicles and donor membranes were analyzed by SDS/PAGE and immunoblotting to measure incorporation of proteins into ER-derived transport vesicles.
Cloning of Mutant SCAP from SRD-5 Cells.
cDNA encoding the sterol-sensing domain of SCAP was amplified by PCR by using first-strand cDNAs made from total RNA of CHO-7 and SRD-5 cells. The following pair of forward and reverse primers was used for PCR: 5′-TTAGCCTGCTGCTACCCTCTGCTGAAG-3′ and 5′-TACCAGGATGCCAATCCAAACAACGGTACC-3′. Resulting PCR products were subcloned into pCRII vector (Invitrogen), and 12 individual clones were sequenced to identify potential mutations in the sterol-sensing domain of SCAP.
Plasmids.
The following recombinant expression plasmids were described: pTK-HSV-BP2, encoding wild-type herpes simplex virus-tagged human SREBP-2 under control of the weak thymidine kinase (TK) promoter (10); pTK3-SCAP and pTK3-SCAP(Y298C), encoding wild-type and mutant hamster SCAP, respectively, under control of the TK promoter (11); pCMV-SCAP and pCMV-SCAP(Y298C), encoding wild-type and mutant hamster SCAP, respectively, under control of the strong cytomegalovirus (CMV) promoter (2); and pCMV-Insig-1-Myc and pCMV-Insig-2-Myc, encoding Myc-tagged human insig-1 and human insig-2, respectively, under control of the CMV promoter (14).
pTK3-SCAP(L315F) and pCMV-SCAP(L315F) encode mutant hamster SCAP under control of the TK and CMV promoters, respectively. Plasmids were generated by in vitro site-directed mutagenesis, by using QuikChange XL Site-Directed Mutagenesis Kit with pCMV-SCAP as a template and the following primers: 5′-GTCTAAGTGGGGATTCGCCCTGGCAGC-3′ and 5′-GCTGCCAGGGCGAATCCCCACTTAGAC-3′. Mutagenesis was confirmed by sequencing. The BstEII-FseI fragment was released from the mutated plasmid and used to replace the corresponding fragments in pTK3-SCAP and pCMV-SCAP. These plasmids were sequenced in their entirety.
Transient Transfection of SRD-13A Cells.
On day 0, SRD-13A cells were plated at 4 × 105 cells per 60-mm dish or 8 × 105 cells per 100-mm dish in medium B. On day 2, cells were transfected with the indicated plasmids by using FuGENE 6 as described (17). The total amount of DNA in each transfection was adjusted to 5 μg per dish by addition of pTK vector and/or pcDNA3 vector. After transfection, cells were incubated at 37°C for 12–14 h in medium A supplemented with 5% (vol/vol) FCS. On day 3, the cells were switched to medium C containing 1% (wt/vol) hydroxypropyl-β-cyclodextrin. After incubation at 37°C for 1 h, cells were washed twice with PBS and switched to medium C in the absence or presence of sterols added in ethanol [final concentration, 0.5% (vol/vol)]. After incubation for 5 h, cells were washed twice with PBS and harvested for preparation of cell extracts for immunoblot analysis and immunoprecipitation (see below). The pellets from two 60-mm dishes of transfected SRD-13A cells were suspended in 0.2 ml of buffer A [10 mM Tris⋅HCl, pH 7.4/100 mM NaCl/1% (wt/vol) SDS] containing protease inhibitors (10 μg/ml leupeptin/5 μg/ml pepstatin A/2 μg/ml aprotinin/25 μg/ml N-acetyl-leucinal-leucinal-norleucinal) as described (3). Protein concentration of the extracts was determined by using the BCA Kit, after which the extracts were mixed with 5× SDS loading buffer [150 mM Tris⋅HCl, pH 6.8/15% (vol/vol) SDS/25% (vol/vol) glycerol/0.02% (wt/vol) bromophenol blue/12.5% (vol/vol) 2-mercaptoethanol]. After boiling for 5 min, the extracts were subjected to SDS/PAGE and immunoblot analysis.
Immunoprecipitation.
The pellets from two 100-mm dishes of transfected SRD-13A cells were suspended in 1 ml of buffer B [50 mM Hepes-KOH, pH 7.4/100 mM NaCl/1.5 mM MgCl2/0.1% (vol/vol) Nonidet P-40] containing protease inhibitors (as above). Cell lysates were prepared and immunoprecipitation was performed by using 20 μg of control nonimmune IgG or polyclonal anti-Myc IgG together with 50 μl of protein A/G-agarose beads (Santa Cruz Biotechnology) as described (14). After centrifugation, the resulting supernatant was mixed with 5× SDS loading buffer. The pelleted beads were washed four times for 15 min at 4°C with 1 ml of buffer B and resuspended in 100 μl of buffer A containing protease inhibitors and mixed with 5× SDS loading buffer. Supernatants and pellets were boiled for 5 min and subjected to SDS/PAGE and immunoblot analysis.
Immunoblot Analysis.
Gels were calibrated with prestained molecular weight markers (Bio-Rad), and antibodies were used at the following concentrations: anti-human SREBP-2 IgG-1D2, 1–5 μg/ml; anti-hamster SREBP-2 IgG-7D4, 5 μg/ml; anti-SCAP IgG-9D5, 5 μg/ml; anti-Myc IgG-9E10, 1 μg/ml; anti-KDEL IgG, 1 μg/ml; and anti-mouse IgG, 0.2 μl/ml. Bound antibodies were visualized by chemiluminescence by using the SuperSignal Substrate System according to the manufacturer's instructions. Filters were exposed to Kodak X-Omat Blue XB-1 films at room temperature for 1–120 s.
Results
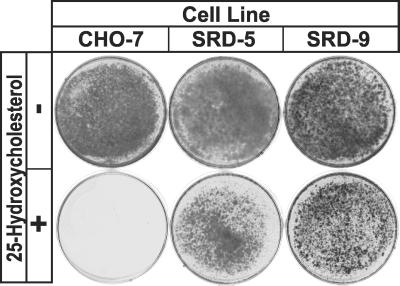
Chronic treatment with 25HC, a potent suppressor of SREBP processing, kills wild-type mammalian cells grown in lipoprotein-deficient serum. Under these conditions, cholesterol synthesis and uptake are impaired severely and the cells are starved for cholesterol because 25HC cannot substitute for cholesterol as a structural component of cell membranes (6). SRD-5 cells were isolated by selecting mutagenized CHO-7 cells for growth in the presence of 1 μg/ml 25HC. We compared the growth of SRD-5 cells with wild-type CHO-7 cells and with SRD-9 cells, a previously described line of 25HC-resistant mutant cells (11). SRD-9 cells have been shown to harbor a point mutation in one allele of SCAP, resulting in a Y298C substitution in the sterol-sensing domain (11). Parental CHO-7 cells are killed by chronic treatment with 25HC. In contrast, both SRD-5 and SRD-9 cells grow well even in the presence of 25HC (Fig. 1).
Fig 1.
Growth of wild-type CHO-7 and mutant SRD-5 and SRD-9 cells in the presence or absence of 25HC. On day 0, cells were set up at the following densities: CHO-7 and SRD-9, 4 × 104 cells per 60-mm dish; and SRD-5, 9 × 104 cells per 60-mm dish. On day 1, the cells were washed once with PBS and refed every 2–3 days with medium A supplemented with 5% fetal calf lipoprotein-deficient serum in the absence or presence of 0.3 μg/ml 25HC. On day 14, the cells were washed, fixed in 95% ethanol, and stained with crystal violet.
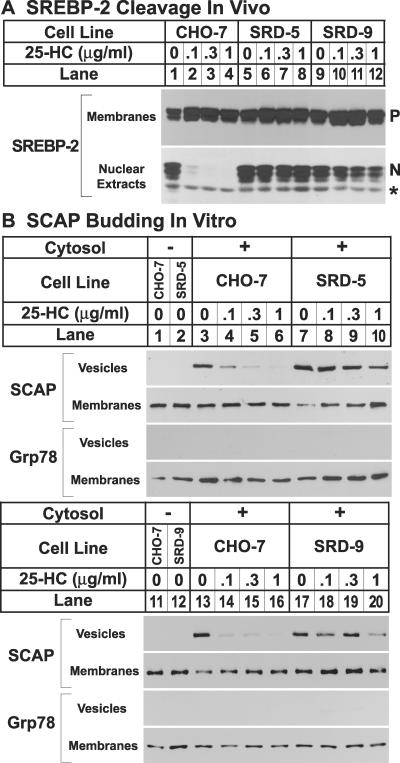
In SRD-9 cells, processing of SREBP-2 is resistant to suppression by 25HC (11). To determine whether processing of SREBP-2 is affected in SRD-5 cells, cells were incubated with 25HC and accumulation of SREBP-2 in membranes and nuclear extracts was analyzed by immunoblotting (Fig. 2A). When the parental CHO-7 cells were incubated with increasing concentrations of 25HC, the amount of SREBP-2 in nuclear extracts declined dramatically, even at the lowest concentration tested (0.1 μg/ml; Fig. 2A, lane 2). Processing of SREBP-2 in the SRD-5 and SRD-9 cells was markedly resistant to 25HC (lanes 6–8 and 10–12).
Fig 2.
(A) SREBP-2 cleavage in wild-type CHO-7 cells and mutant SRD-5 and SRD-9 cells. On day 0, cells were set up at the following densities: CHO-7, 5 × 105 cells per 100-mm dish; and SRD-5 and SRD-9, 8 × 105 cells per 100-mm dish. On day 2, cells were washed once with PBS and refed with medium C in the absence or presence of indicated concentrations of 25HC. Cells that received 25HC also received 10 μg/ml cholesterol. On day 3, the cells were harvested, and membranes and nuclear extracts were prepared as described (10). Aliquots of membranes (25 μg of protein) and nuclear extracts (50 μg of protein) were subjected to SDS/PAGE and immunoblot analysis with 5 μg/ml IgG-7D4 (SREBP-2). N and P denote the nuclear and precursor forms of SREBP-2, respectively. The asterisk (*) denotes a crossreacting protein of unknown identity. (B) Constitutive exit of SCAP from the ER in mutant SRD-5 and SRD-9 cells, as determined by in vitro vesicle-formation assay. On day 0, cells were set up at the following densities: CHO-7, 5 × 105 cells per 100-mm dish; SRD-5, 12 × 105 cells per 100-mm dish; and SRD-9, 8 × 105 cells per 100-mm dish. On day 3, the cells were switched to medium C containing 1% hydroxypropyl-β-cyclodextrin and incubated for 1 h at 37°C. Cells then were washed twice with PBS and switched to medium B in the absence or presence of the indicated concentrations of 25HC. Cells that received 25HC also received 10 μg/ml cholesterol. After incubation for 5 h at 37°C, cells were harvested for membrane preparation. Membranes were incubated in vitro under conditions that permit vesicle budding as described (3). Aliquots of the membranes (80 μg of protein) were incubated in the absence (lanes 1 and 2) or presence (lanes 3–10) of rat liver cytosol for 15 min at 28°C. Vesicle and membrane fractions were separated by centrifugation, and aliquots containing 100% of the vesicle protein and 20% of the membrane protein were subjected to SDS/PAGE and immunoblot analysis with IgG-9D5 (SCAP) and anti-KDEL antibody (grp78).
To characterize the regulatory defect in SRD-5 cells in vitro, we performed a vesicle-formation assay (Fig. 2B). Incubating cells with increasing concentrations of 25HC caused SCAP to be excluded from budding vesicles when ER membranes from wild-type CHO-7 cells were incubated in vitro (Fig. 2B, lanes 4–6). SRD-5-derived ER membranes showed substantial resistance to inhibition by 25HC (Fig. 2B, lanes 8–10). We blotted with antibody against Grp78, an ER-resident membrane protein, as a control for integrity of the donor membranes (3). Similar results were obtained when we compared CHO-7 and SRD-9 cells (Fig. 2B, lanes 14–16 and 18–20, respectively).
To determine whether the SRD-5 cells have a point mutation in SCAP, we amplified the sterol-sensing domain region by using a first-strand cDNA made from total RNA. We subcloned amplified PCR products and determined nucleotide sequences of 12 individual clones. Sequencing revealed a C-to-T substitution in codon 315 of SCAP, changing a Leu (CTC) to Phe (TTC) (Fig. 3A). This change was observed in 7 of 12 independent clones, indicating that SRD-5 cells were heterozygous for the L315F mutation (Fig. 3B) and suggesting a dominant mutation that confers sterol resistance.
Fig 3.
Identification of SCAP mutation in SRD-5 cells. (A) Nucleotide and deduced amino acid sequences of wild-type SCAP transcript (Wild Type) and mutant SCAP transcript (Mutant) from SRD-5 cells. The mutated nucleotide and resulting predicted amino acid are indicated by black boxes. (B) Location of these mutations in sterol-sensing domain (shaded) of hamster SCAP. The newly identified mutation, L315F, and the two reported mutations, D443N (10) and Y298C (8), are denoted by arrows. Residues conserved in SCAP proteins from human, hamster, C. elegans, and D. melanogaster are shown in red. Sequences of SCAP proteins from human, hamster, C. elegans, and D. melanogaster were aligned by using the CLUSTALW program (DNASTAR, Madison, WI). GenBank accession nos. are D83782, U67060, 247808, and U38238, respectively.
Fig. 3B shows that position 315 is located at the beginning of the third transmembrane domain of hamster SCAP, within the sterol-sensing domain. Residues conserved in SCAP proteins from human, hamster, Caenorhabditis elegans, and Drosophila melanogaster are shown in red. The Leu at position 315 is conserved in all species examined (Fig. 3B). This is also true of the Tyr-298 that is mutated in SRD-9 cells. The Asp-443 in hamster SCAP is conserved in mammalian species; the corresponding residue is Asn in SCAP from D. melanogaster, an organism in which SREBP processing is not responsive to sterols (19).
Recently, insig-1 and insig-2 were identified as proteins of the ER membrane that bind SCAP via its sterol-sensing domain and enable the sterol-mediated retention of the SREBP/SCAP complex in the ER (13, 14). Addition of sterols to cultured cells enhances the binding of the SREBP/SCAP complex to insig proteins as determined by coimmunoprecipitation. This binding does not occur when SCAP harbors the Y298C mutation, resulting in sterol-resistant cleavage of SREBP-2 (13, 14). To address whether the L315F mutation interferes with SCAP binding to insig proteins, we performed the experiments shown in Fig. 4.
Fig 4.
Reduced interaction of SCAP(Y298C) and SCAP(L315F) with insig proteins. (A) On day 0, SRD-13A cells were set up in medium C at 4 × 105 cells per 60-mm dish. On day 2, the cells were transfected with the following plasmids: 2 μg of pTK-HSV-BP2; 0.5 μg of pCMV-SCAP, pCMV-SCAP(Y298C), or pCMV-SCAP(L315F); and 0.1 μg of pCMV-Insig-1-Myc (Upper) or 1 μg of pCMV-Insig-2-Myc (Lower). On day 3, cells were switched to medium B containing 1% hydroxypropyl-β-cyclodextrin and incubated for 1 h at 37°C. Cells then were washed twice with PBS and switched to medium B in the absence or presence of 0.1 μg/ml 25HC plus 10 μg/ml cholesterol (sterols). After incubation for 5 h, cells were harvested, and total cell extracts were prepared as described in Materials and Methods. Aliquots of the extracts (50 μg) were subjected to SDS/PAGE and immunoblot analysis with IgG-1D2 (SREBP-2), IgG-9D5 (SCAP), and IgG-9E10 (insig-1 and insig-2). N and P denote the cleaved nuclear and uncleaved precursor forms of SREBP-2, respectively. (B) On day 0, SRD-13A cells were set up in medium C at 8 × 105 cells per 100-mm dish. On day 2, the cells were transfected as in A. On day 3, cells were treated as in A. After incubation for 5 h, cells were harvested for immunoprecipitation with polyclonal anti-Myc IgG (lanes 1–7) or control nonimmune IgG (lanes 8–10) as described in Materials and Methods. Immunoprecipitated pellets (representing 0.5 dish of cells; Pt) and supernatants (0.05 dish of cells; Sp) were subjected to SDS/PAGE and immunoblot analysis with IgG-1D2 (SREBP-2), IgG-9D5 (SCAP), and IgG-9E10 (insig-1 and insig-2). (C) All three sterol-resistant SCAP mutants show reduced interaction with insig proteins, as determined by immunoprecipitation and as performed in B. Cells were transfected with 0.5 μg of the indicated SCAP plasmids.
In the experiment of Fig. 4A, we transfected SCAP-deficient SRD-13A cells (17) with cDNAs encoding either wild-type or mutant SCAP under control of the strong CMV promoter, which overproduces SCAP (Fig. 4A Upper). The cells were cotransfected with cDNAs encoding epitope-tagged SREBP-2 and insig-1. Cells were incubated in the absence or presence of sterols. Total cell extracts were analyzed by SDS/PAGE and immunoblotting with various antibodies. When SCAP was overexpressed, we observed an increase in the precursor form of SREBP-2 as well as generation of the nuclear form of SREBP-2 because of the stabilizing and escorting functions of SCAP, respectively (Fig. 4A Upper, lanes 3 and 4). The nuclear form of SREBP-2 did not decrease when sterols were added (lanes 3 and 4), indicating that the retention process was saturated by the excess SCAP. Insig-1 restored sterol regulation of SREBP-2 processing when cells expressed wild-type SCAP (lanes 5 and 6), but not when they expressed SCAP(Y298C) (lanes 9 and 10) or SCAP(L315F) (lanes 13 and 14). Similar results were obtained when insig-2 was used (Fig. 4A, lanes 17–20, 23, 24, 27, and 28). These results suggest that, just as for the Y298C mutation of SCAP, L315F interferes with interaction between SCAP and insig proteins.
Coimmunoprecipitation experiments demonstrated reduced interaction between mutant SCAP and insig proteins. When Myc-tagged insig-1 was cotransfected, anti-Myc antibody brought down wild-type SCAP and SREBP-2 in the presence of sterols (Fig. 4B, lane 5). There was no immunoprecipitation when cells expressed SCAP(Y298C) or SCAP(L315F) (lanes 6 and 7). Similar results were obtained when insig-1 was replaced with insig-2 (Fig. 4B, lanes 15–17).
SCAP (D443N) was the first sterol-resistant mutation identified in the SCAP locus. Its interaction with insig has not been examined previously. Therefore, to complete our studies, we performed the immunoprecipitation experiment shown in Fig. 4C. SCAP(D443N) prevents interaction with insig (Fig. 4C, lanes 7 and 16) just as observed for the Y298C and L315F mutations (lanes 8, 9, 17, and 18).
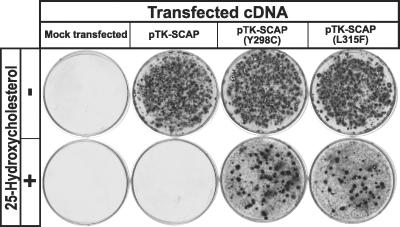
To confirm that the SCAP(L315F) mutation is capable of causing a dominant sterol-resistant phenotype, we performed a growth experiment in which wild-type CHO-7 cells were transfected with cDNAs encoding wild-type SCAP, SCAP(Y298C), or SCAP(L315F) (Fig. 5). The cells then were grown in the presence of 25HC and the absence of cholesterol. Transfection of wild-type SCAP did not permit growth under these conditions, but both SCAP(Y298C) and SCAP(L315F) restored growth.
Fig 5.
Expression of transfected SCAP(L315F) is sufficient to confer a 25HC-resistant phenotype to CHO-7 cells. On day 0, CHO-7 cells were set up at 4 × 105 cells per 60-mm dish. On day 2, the cells were transfected with 0.3 μg of pTK-SCAP, pTK-SCAP(Y298C), or pTK-SCAP(L315F). Mock-transfected cells received no plasmid. All plasmids contained the G418-resistance gene neo. On day 3, cells were switched to medium A containing 5% FCS and 0.7 mg/ml G418 and refed every 2–3 days. No cells survived in mock-transfected dishes. On day 14, cells were switched to medium A containing 5% newborn calf lipoprotein-deficient serum and 0.5 mg/ml G418 in the absence (−) or presence (+) of 1 μg/ml 25HC and refed every 2–3 days. On day 24, cells were washed, fixed in 95% ethanol, and stained with crystal violet.
Discussion
The present work reports a previously undescribed point mutation in the sterol-sensing domain of SCAP that accounts for the sterol resistance of SRD-5 cells. Introduction of this mutation into an otherwise wild-type SCAP cDNA enables it to confer sterol resistance on recipient cells (Figs. 4 and 5). The mutated residue, Leu-315, is absolutely conserved among SCAP proteins from invertebrates and vertebrates (Fig. 3). The two previously identified sterol-resistant point mutations in the sterol-sensing domain of SCAP also affect residues that are absolutely conserved in mammalian SCAPs.
In mammalian cells, sterols promote the interaction of insig proteins with SCAP, facilitating retention of SREBP/SCAP complexes in the ER (13, 14). Binding of insig proteins to SCAP does not occur when SCAP harbors any of three point mutations: Y298C, D443N, and L315F. The finding that all three mutations block binding to insigs and all three mutations lead to sterol-resistant processing of SREBPs provides strong evidence that sterol-induced binding of SCAP to insigs is a crucial event in sterol-mediated feedback regulation in mammalian cells.
Currently, we do not know whether interaction between SCAP and insig proteins is direct or whether it requires additional, unknown proteins. However, the mutations identified in the SCAP sterol-sensing domain will enable a better understanding of the structural basis of its function in protein–protein interaction and in monitoring membrane status.
Acknowledgments
We thank Drs. Michael Brown and Joseph Goldstein for advice and support; Jim Metherall for isolating the SRD-5 cells in the course of a screen for sterol-resistant mutants conducted in the Brown and Goldstein laboratory in 1988; Russell DeBose-Boyd and Peter Espenshade for helpful discussions; Anand Sitram and Tuyet Dang for excellent technical assistance; Lisa Beatty and Angela Carroll for invaluable help with tissue culture; Jeff Cormier and Melody Kerr for DNA sequencing; and Carla Dorsett for help with gels. This research was supported by National Institutes of Health Grant HL-20948, the Perot Family Foundation, and the Keck Foundation.
Abbreviations
CHO, Chinese hamster ovary
CMV, cytomegalovirus
ER, endoplasmic reticulum
25HC, 25-hydroxycholesterol
SREBP, sterol regulatory element-binding protein
SCAP, SREBP cleavage-activating protein
TK, thymidine kinase
References
- 1.Brown M. S. & Goldstein, J. L. (1999) Proc. Natl. Acad. Sci. USA 96, 11041-11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakai J., Nohturfft, A., Cheng, D., Ho, Y. K., Brown, M. S. & Goldstein, J. L. (1997) J. Biol. Chem. 272, 20213-20221. [DOI] [PubMed] [Google Scholar]
- 3.Nohturfft A., Yabe, D., Goldstein, J. L., Brown, M. S. & Espenshade, P. J. (2000) Cell 102, 315-323. [DOI] [PubMed] [Google Scholar]
- 4.Espenshade P. J., Li, W.-P. & Yabe, D. (2002) Proc. Natl. Acad. Sci. USA 99, 11694-11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan E. A., Dave, U. P., Sakai, J., Goldstein, J. L. & Brown, M. S. (1998) J. Biol. Chem. 273, 17801-17809. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein J. L., Rawson, R. B. & Brown, M. S. (2002) Arch. Biochem. Biophys. 397, 139-148. [DOI] [PubMed] [Google Scholar]
- 7.Wang X., Sato, R., Brown, M. S., Hua, X. & Goldstein, J. L. (1994) Cell 77, 53-62. [DOI] [PubMed] [Google Scholar]
- 8.Nohturfft A., Brown, M. S. & Goldstein, J. L. (1998) J. Biol. Chem. 273, 17243-17250. [DOI] [PubMed] [Google Scholar]
- 9.Gil G., Faust, J. R., Chin, D. J., Goldstein, J. L. & Brown, M. S. (1985) Cell 41, 249-258. [DOI] [PubMed] [Google Scholar]
- 10.Hua X., Nohturfft, A., Goldstein, J. L. & Brown, M. S. (1996) Cell 87, 415-426. [DOI] [PubMed] [Google Scholar]
- 11.Nohturfft A., Brown, M. S. & Goldstein, J. L. (1998) Proc. Natl. Acad. Sci. USA 95, 12848-12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown A., Sun, L., Feramisco, J., Brown, M. & Goldstein, J. (2002) Mol. Cell 10, 237-245. [DOI] [PubMed] [Google Scholar]
- 13.Yang T., Espenshade, P., Wright, M., Yabe, D., Gong, Y., Aebersold, R., Goldstein, J. & Brown, M. (2002) Cell 110, 489-500. [DOI] [PubMed] [Google Scholar]
- 14.Yabe D., Brown, M. S. & Goldstein, J. L. (2002) Proc. Natl. Acad. Sci. USA 99, 12753-12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J., Brown, M. S., Ho, Y. K. & Goldstein, J. L. (1995) J. Biol. Chem. 270, 12152-12161. [DOI] [PubMed] [Google Scholar]
- 16.Sakai J., Rawson, R. B., Espenshade, P. J., Cheng, D., Seegmiller, A. C., Goldstein, J. L. & Brown, M. S. (1998) Mol. Cell 2, 505-514. [DOI] [PubMed] [Google Scholar]
- 17.Rawson R. B., DeBose-Boyd, R., Goldstein, J. L. & Brown, M. S. (1999) J. Biol. Chem. 274, 28549-28556. [DOI] [PubMed] [Google Scholar]
- 18.Metherall J. E., Goldstein, J. L., Luskey, K. L. & Brown, M. S. (1989) J. Biol. Chem. 264, 15634-15641. [PubMed] [Google Scholar]
- 19.Seegmiller A. C., Dobrosotskaya, I., Goldstein, J. L., Ho, Y. K., Brown, M. S. & Rawson, R. B. (2002) Dev. Cell 2, 229-238. [DOI] [PubMed] [Google Scholar]