Abstract
Synthesis of inorganic polyphosphate (poly P) from the terminal phosphate of ATP is catalyzed reversibly by poly P kinase (PPK, now designated PPK1) initially isolated from Escherichia coli. PPK1 is highly conserved in many bacteria, including some of the major pathogens such as Pseudomonas aeruginosa. In a null mutant of P. aeruginosa lacking ppk1, we have discovered a previously uncharacterized PPK activity (designated PPK2) distinguished from PPK1 by the following: synthesis of poly P from GTP or ATP, a preference for Mn2+ over Mg2+, and a stimulation by poly P. The reverse reaction, a poly P-driven nucleoside diphosphate kinase synthesis of GTP from GDP, is 75-fold greater than the forward reaction, poly P synthesis from GTP. The gene encoding PPK2 (ppk2) was identified from the amino acid sequence of the protein purified near 1,000-fold, to homogeneity. The 5′-end is 177 bp upstream of the annotated genome sequence of a “conserved hypothetical protein”; ppk2 (1,074 bp) encodes a protein of 357 aa with a molecular mass of 40.8 kDa. Sequences homologous to PPK2 are present in two other proteins in P. aeruginosa, in two Archaea, and in 32 other bacteria (almost all with PPK1 as well); these include rhizobia, cyanobacteria, Streptomyces, and several pathogenic species. Distinctive features of the poly P-driven nucleoside diphosphate kinase activity and structural aspects of PPK2 are among the subjects of an accompanying report.
Inorganic polyphosphate (poly P) is a polymer of tens or hundreds of phosphate residues found in all cells (bacterial, fungal, plant, and animal) that have been examined (1–3). Among its many functions, poly P is needed for bacterial survival in the face of stress and stringencies and is required for the virulence of some pathogens (4–12).
The gene ppk1 encodes poly P kinase (PPK1), the enzyme that converts ATP to poly P among other activities (13). Null mutants of Pseudomonas aeruginosa PAO1 lacking ppk1 are deficient in motility, quorum sensing, biofilm formation, and virulence in mouse models (9, 10, 12). Despite the lack of detectable PPK1 activity (<1% of wild type), these mutants still possess as much as 20% of the wild-type levels of poly P (9).
The purpose of this study was to determine the source of poly P in the ppk1 mutants of P. aeruginosa. No clue could be obtained from extensive searches for homologies to PPK1 in the available databases. Thus, the putative PPK2 would have to be revealed by novel assays of poly P synthesis in cell-free extracts of the mutants and the subsequent isolation of the responsible enzyme.
In this report, we describe the identification and novel features of PPK2, its sequence in the P. aeruginosa genome, and the conservation of this sequence among 35 microorganisms, notably rhizobia, cyanobacteria, Streptomyces, several pathogenic species, and two Archaea.
Simultaneously with these studies, we pursued a very potent activity in P. aeruginosa that uses poly P as a donor to convert GDP to GTP (14). This activity, previously designated PNDK (poly P-driven nucleoside diphosphate kinase; ref. 15), has now been purified to homogeneity. Although seemingly different from the activity that synthesizes poly P from GTP, both activities reside in the very same protein, PPK2, encoded by ppk2. An account of this discovery is presented in the accompanying report (14).
Materials and Methods
Reagents.
The sources of the reagents and related supplies used in this study are as follows: creatine kinase, DNase I, and RNase A were from Boehringer Mannheim; ATP, GTP, creatine phosphate, poly P (types P15 and P75), and BSA were from Sigma; [γ-32P]ATP and [γ-32P]GTP were from Amersham Pharmacia; poly(vinylidene difluoride) membranes were from Bio-Rad. Purified P. aeruginosa PPK1 was a gift from S. Lee (ICOS Corporation, Bothell, WA).
Strains.
The strains used in this study are P. aeruginosa PAOM5 [PAO1 Δppk1:tet (Tcr)] (10) and Escherichia coli CF5802 [MG1655 Δppk1-Δppx:Kan (Kmr)] (16).
Assays for PPK Activities.
The PPK1 assay was performed as described (13). For the PPK2 assay, the reaction mixture (25 μl) contained 50 mM Hepes (pH 7.2), 100 mM (NH4)2SO4, 0.1 mM poly P75 (in phosphate residues), 10 mM MnCl2, and 2 mM [γ-32P]GTP (5–50 cpm/pmol GTP). After incubation for 5 min at 37°C, the reaction was stopped by adding 100 μl 2 mM BSA and 100 μl 7% percloride acid, then mixed well and put on ice. [32P]poly P was measured in a liquid scintillation counter as in the PPK1 assay by collecting on Whatman GF/C glass fiber filters and washing four times with 20 ml of washing solution (1 M HCL and 0.1 M pyrophosphate) followed by ethanol. One unit of enzyme is defined as the amount incorporating 1 pmol of phosphate into acid-insoluble poly P per min at 37°C.
Plasmid Construction.
To clone ppk2 from P. aeruginosa, PCR primers PPK2-F (TTccatggGAGAGGTGTAAGGCTTTCCT with an NcoI site near the 5′-end) and PPK2-R (TTggatccTGCCGTACAAGCAGATCGTGA with a BamHI site near the 5′-end) were used to amplify ppk2 from PAOM5 genome DNA, then cloned into the NcoI-BamHI site of pTrc99A (Ampr) (17) to form pTrc-PPK2.
Other Methods.
Protein mass spectrometry and amino terminal sequence analysis were done by the Stanford Protein and Nucleic Acid (PAN) facility. Protein on SDS/PAGE was digested by LysC for matrix-assisted laser desorption/ionization–time of flight (MALDI-TOF) mass spectrometry analysis. For amino-terminal sequence analysis, proteins were blotted onto poly(vinylidene difluoride) membrane according to the guidelines of the Stanford PAN facility. Sequencing was performed on an ABI sequenator (Applied Biosystems; model Procise 494) based on Edman degradation.
Results
Assay of PPK2.
The standard assay of PPK1 in lysates of the ppk1 null mutant in P. aeruginosa PAOM5 showed no activity (<1%) using the measure of conversion of [γ-32P]ATP to acid-insoluble poly P. By improving the separation of poly P, the sensitivity was increased so that as few as 200 cpm of 2 × 106 cpm [γ-32P]GTP added could be detected as poly P (expressed as 10 units/mg protein). By modifying cultural conditions and components of the assay mixture, such as replacing Mg2+ with Mn2+ and addition of poly P, a novel PPK2 activity in the lysate was increased 1,000-fold to 10,000 units/mg protein, a level comparable to that of PPK1 in wild-type lysates.
Purification.
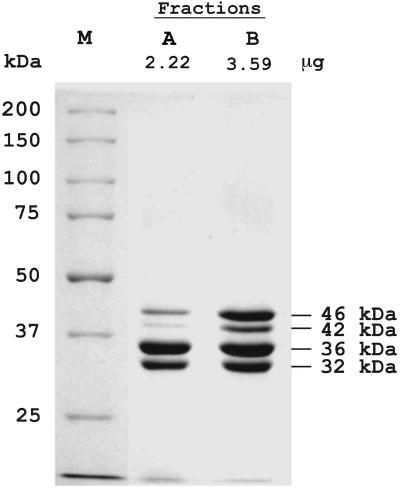
PPK2 was purified near 1,000-fold to apparent homogeneity from the P. aeruginosa PAOM5 soluble lysate by fractionation on heparin–Sepharose, GTP agarose, Mono Q, and Mono S columns. Yet, the purified PPK2 fractions with a specific activity of 5.5 × 106 units/mg protein showed on some occasions several bands on SDS/PAGE with molecular masses of ≈46, 42, 36, and 32 kDa (Fig. 1). By mass spectroscopy, all four bands referred to the same protein (see below), all with the same N-terminal sequence. The three bands with lower molecular mass were likely artifacts of acid-catalyzed cleavage of aspartyl-prolyl (D-P) bonds (19, 20).
Fig. 1.
SDS/PAGE analysis of purified PPK2. Samples were precipitated with 0.015% deoxycholate-5% trichloroacetic acid (18). Proteins were visualized by Coomassie blue staining. Samples of two different purification operations (A and B), both with nearly the same specific activity of 5.5 × 106 units/mg, were applied as indicated. M, protein molecular mass markers (Precision Protein Standards, Bio-Rad).
As described in detail in the accompanying report (14), a parallel purification of PPK2 to homogeneity was achieved by assay of the reverse reaction: poly P as a donor to [3H]GDP to form GTP with a 1,270-fold purification and a remarkable yield of 72% (14).
Requirements of PPK2.
Requirements of pure PPK2 for the synthesis of poly P (forward reaction) differed markedly from those for the utilization of poly P to make GTP from GDP (reverse reaction) (Table 1). Mn2+ (10 mM) was preferred over Mg2+ in poly P synthesis, but the opposite was true of poly P as a donor in GTP synthesis (14). GTP (Km, 0.68 mM; Vmax, 6.7 × 106 units/mg protein) and ATP (Km, 0.5 mM; Vmax, 7.6 × 106 units/mg protein) were equally active in poly P synthesis, as were GDP and ADP in GTP and ATP synthesis (14). Poly P stimulated poly P synthesis in the lysate 10-fold, but the effect was muted with the pure enzyme to which poly P15 had been added (1.5 μM in Pi residues when diluted 1,000-fold in the assay mixture). Poly P chains of 15–700 were all effective, but poly P3 was not.
Table 1.
P. aeruginosa PPK1 and PPK2 in the synthesis and utilization of poly P
| PPK1 | PPK2 | |
|---|---|---|
| Synthesis (NTP→poly P) | ||
| ATP or GTP | ATP only | ATP or GTP |
| Metal ion | Mg2+ | Mn2+ |
| Poly P stimulation | No | Yes |
| Vmax, units/mg | 2.1 × 106 | 6.7 × 106 |
| Poly P product, residues | 500–800 | 200–800 |
| Processivity | Yes | Yes |
| Utilization (Poly P→NTP) | ||
| ADP/GDP | 34 | 0.9 |
| Poly P size preference | Long | Short |
| Metal ion | Mg2+ | Mg2+ |
| Vmax, units/mg | 5.1 × 105 | 5.0 × 108 |
| Processivity | Yes | Yes |
| Synthesis/utilization | ||
| Vmax ratio | 4.1 | 0.013 |
His-tagged P. aeruginosa PPK1 was a gift from S. Lee (ICOS Corporation).
Processivity and Product Size.
The poly P-synthesizing reaction seems to be processive, because a maximal chain length of ≈700–800 residues appeared from the very beginning of the reaction (Fig. 2). Compared with E. coli PPK1, which produces a homogeneous product of 700–800 residues, the P. aeruginosa PPK1 and PPK2 products have a broader range: ≈500–800 for PPK1 and 200–800 for PPK2 (Fig. 3).
Fig. 2.
Processivity of PPK2 poly P synthesis. A 25-μl assay mixture contained 56 ng of PPK2. After incubation at 37°C for the indicated times, the products were separated by PAGE (20% gel) containing 7 M urea and visualized by PhosphorImager (Molecular Dynamics); the [γ-32P]GTP and poly P750 migrated on the gel as indicated.
Fig. 3.

Chain lengths of poly P synthesized by PPKs. PPK2 of P. aeruginosa (Pa; 140 ng); PPK1 of P. aeruginosa (Pa; 120 ng); PPK1 of E. coli (Ec; 29 ng). After 45 min at 37°C, the products were separated by PAGE (20% gel) containing 7 M urea and visualized by PhosphorImager (Molecular Dynamics); the positions to which [γ-32P]ATP and [γ-32P]GTP migrated on the gel are indicated.
Identification of the ppk2 Sequence.
Mass spectroscopic analysis of the purified PPK2 bands revealed a perfect match with a “conserved hypothetical protein” (GenBank accession no. NP_248831) of P. aeruginosa (21), but the amino-terminal sequence “SEEPTVSPP” detected in our isolated protein was absent in the annotated protein. Also absent from the annotated gene was a Shine–Delgarno (SD) sequence and the common ATG start codon. However, at 177 bp upstream in the same reading frame, we identified an ATG start codon with a strong SD sequence (AGGAGT) 11 bp upstream of it; translation of this alternative ORF gives exactly the same NH2-terminal sequence as that found in purified PPK2. Thus, PPK2 contains 357 aa with a molecular mass of 40.8 kDa encoded by a ppk2 of 1,074 bp (Fig. 4).
Fig. 4.
Nucleotide (ppk2) and the predicted amino acid (PPK2) sequences of P. aeruginosa. The nucleotide sequence (5′ to 3′) of the noncoding strand starts at nucleotide 1 and terminates at 1074. A possible Shine–Delgarno sequence (AGGAGT) for translation initiation starting at −11 bp is marked by a wavy line. The amino-terminal amino acid sequence determined from purified PPK2 (underlined) agrees with the deduced amino acid sequence from the start codon. The incorrect original annotation of the start codon at nucleotide 178 (GTG) is shaded. The P-loop sequence is marked by a dotted line, and the conserved amino acids are shaded. Three aspartyl-prolyl (D-P) bonds are identified in boxes.
Cloning of ppk2 and the Activity of the Gene Product.
The ppk2 was cloned into pTrc99A and transformed into E. coli CF5802. Both PPK2 poly P synthesis and utilization activities were detected in the lysate of isopropyl β-d-thiogalactoside (IPTG)-induced E. coli CF5802 (pTrc-PPK2) with a specific activity of 1.7 × 104 units/mg protein for synthesis and 1.3 × 106 units/mg protein for utilization, each about two to four times of the PPK2 activity in lysates of P. aeruginosa PAOM5; E. coli CF5802 (pTrc99A) containing only the vector, had no PPK2 activity [<0.01% of that of E. coli CF5802 (pTrc-PPK2)].
Conservation of the PPK2 Sequence.
The P. aeruginosa PPK2 sequence was used in blast searches of the NCBI, TIGR, EMBL, and DDBJ peptide sequence databases for homologs with an E value <1 × 10−10 (Table 2). In the P. aeruginosa genome (Fig. 5), two homologs are present: one of similar size (305 aa) and 51.5% identity and the other much larger (497 aa) with identity only in the carboxyl half of the sequence. Among other microorganisms, 51 homologs have been identified in 34 species; two of them are Archaea (M. acetivorans and M. mazei) each with two PPK2 homologs. Homologs are found in both Gram-positive and Gram-negative bacteria and, notably, include rhizobia, cyanobacteria, Streptomyces, and many pathogenic species. Most of the original annotations of these proteins are “conserved hypothetical protein” or “hypothetical protein,” many with a phosphate binding motif (P-loop) sequence, GXXXXGK (Fig. 4) commonly found in ATP- and GTP-binding proteins (22, 23).
Table 2.
PPK2 or PPK1 homologs among microorganisms
| PPK2 | PPK1 only | Neither PPK1 nor PPK2 |
|---|---|---|
| PPK2 and PPK1 | Genome complete | Aeropyrum pernix |
| Agrobacterium tumefaciens | Bacillus anthracis | Aquifex aeolicus |
| Brucella melitensis | Bacillus halodurans | Archaeoglobus fulgidus |
| Burkholderia fungorum(2) | Clostridium acetobutylicum | Bacillus subtilis |
| Campylobacter jejuni | E. coli | Borrelia burgdorferi |
| Caulobacter crescentus | Helicobacter pylori | Buchnera aphidicola str. Sg |
| Chlorobium tepidum | Mycobacterium leprae | Buchnera sp. APS |
| Chloroflexus aurantiacus | Neisseria meningitidis | Chlamydia muridarum |
| Deinococcus radiodurans | Salmonella typhi | Chlamydia trachomatis |
| Magnetospirillum magnetotacticum (2) | Salmonella typhimurium | Chlamydophila pneumoniae |
| Mesorhizobium loti(2) | Xylella fastidiosa | Clostridium perfringens |
| Methanosarcina acetivorans (2) | Yersinia pestis | Fusobacterium nucleatum |
| Methanosarcina mazei(2) | Haemophilus influenzae Rd | |
| Mycobacterium tuberculosis | Genome incomplete | Halobacterium sp. NRC-1 |
| Myxococcus xanthus | Acidithiobacillus ferrooxidans | Lactococcus lactis |
| Nostoc punctiforme (2) | Acinetobacter baumannii | Listeria innocua |
| Nostoc sp. PCC7120 (2) | Acinetobacter calcoaceticus | Listeria monocytogenes |
| Prochlorococcus marinus | Acinetobacter sp. ADP1 | Methanococcus jannaschi |
| P. aeruginosa(3) | Aphanizomenon sp. TR183 | Methanopyrus kandleri |
| Pseudomonas fluorescens(2) | Aphanizomenon baltica | Methanothermobacter |
| Ralstonia metallidurans (5) | Arthrobacter sp. KM | thermautotrophicus |
| Ralstonia solanacearum | Bordetella pertussis | Mycoplasma genitalium |
| Rhodobacter sphaeroides (2) | Burkholderia cepacia | Mycoplasma pneumoniae |
| Rhodopseudomonas palustris | Campylobacter coli | Mycoplasma pulmonis |
| Rhodospirillum rubrum | Cytophaga hutchinsonii | Pasteurella multocida |
| Sinorhizobium meliloti(3) | Dictyostelium discoideum | Pyobaculum aerophilum |
| Streptomyces coelicolor | Geobacter sulfurreducens | Pyrococcus abyssi |
| Synechococcus sp. WH 8102 | Haloferax volcanii | Pyrococcus furiosus |
| Synechocystis sp. PCC6803 | Klebsiella aerogenes | Pyrococcus horikoshii |
| Thermosynechococcus elongatus | Leuconostoc mesenteroides | Rickettsia conorii |
| Vibrio cholerae | Microbulbifer degradans | Rickettsia prowazekii |
| Xanthomonas axonopodis | Mycobacterium marinum | Saccharomyces cerevisiae |
| Xanthomonas campestris | Mycobacterium ulcerans | Schizosaccharomyces pombe |
| Neisseria gonorrhoea | Staphylococcus aureus | |
| Nitrosomonas europaea | Streptococcus pneumoniae | |
| PPK2 only | Nodularia spumigena | Streptococcus pyogenes |
| Corynebacterium glutamicum (2) | Oenococcus oeni | Sulfolobus solfataricus |
| MagnetococcusMC-1 (2) | Porphyromonas gingivalis | Sulfolobus tokodaii |
| Plectonema boryanum | Propionibacterium shermanii | Thermoanaerobacter tengcongensis |
| Rhodocyclus tenuis | Thermoplasma acidophilum | |
| Salmonella dublin | Thermoplasma volcanium | |
| Serratia marcescens | Thermotoga maritima | |
| Shigella flexneri | Treponema pallidum | |
| Streptomyces griseus | Ureaplasma urealyticum | |
| Streptomyces lividans | ||
| Thermobifida fusca |
Archaea.
The number in parentheses indicates the number of homologs of PPK2 in the organism.
Eukaryote.
Fig. 5.
Alignment of PPK2 and two homologs in P. aeruginosa. The identical amino acids are shaded in solid black. Hom1 is homolog 1 (hypothetical protein PA2428, GenBank accession no. NP_251118); Hom2 is homolog 2 (hypothetical protein PA3455, GenBank accession no. NP_252145).
The E. coli PPK1 sequence was also used for the same blast searches. Homologs were identified in 32 species that contained both PPK1 and PPK2; only three species with PPK2 lack PPK1. Among 43 species that contain only PPK1 are the slime mold D. discoideum and one Archae, H. volcanii. Lacking both PPK1 and PPK2 sequences in completely sequenced genomes are 14 of 17 Archael species, 27 of 55 bacterial species, and S. cerevisiae (Table 2).
Discussion
Certain mutants of P. aeruginosa lacked the highly conserved PPK1 that catalyzes the synthesis of poly P from ATP (10). The persistence of significant levels of poly P in these mutants sparked our search for the responsible enzyme activity. For lack of any clues from the PPK1 sequence in the available genomic databases, we resorted to classical biochemical approaches. We developed more sensitive activity assays to detect poly P, uncovered novel requirements for this activity, purified it to homogeneity and identified its gene. This activity in mutants and wild-type strains that synthesizes poly P from GTP or ATP is now designated PPK2 and the encoding gene is ppk2.
Concurrent with these studies, another very potent activity in P. aeruginosa was pursued in our laboratory, an activity that utilizes poly P as a donor to convert GDP to GTP (14). Some kinetic features made it seem that the poly P synthetic enzyme was separate from the poly P-driven NDK activity. Yet, on purification of each of these two activities to homogeneity, both proved to reside in the same protein (PPK2) and to derive from the same gene, ppk2. We recount here the properties of PPK2 as a poly P synthetase and the sequence of ppk2 and discuss the significance of its distribution in a wide variety of microorganisms. In the accompanying report (14), we focus on the remarkably strong capacity of PPK2 to use poly P in the synthesis of GTP and discuss its likely physiological importance.
Several characteristics distinguish the poly P synthetic activity of PPK2 from those of PPK1 in P. aeruginosa (Table 1); these distinctions also apply to the PPK1s of E. coli, V. cholerae, and H. pylori (11). PPK2 can use either GTP or ATP in the synthesis of poly P, but the PPK1s use only ATP. In cell lysates, PPK2 activity is stimulated 10-fold by the addition of poly P, but no such effect was observed with PPK1. Inasmuch as the poly P added to PPK2 was not extended (data not shown), its stimulatory effect may be to promote oligomerization and stability (14).
Mn2+ is preferred over Mg2+ by PPK2 for poly P synthesis activity, whereas the reverse is true of PPK1s. The optimal level of Mn2+ for PPK2 is reached at 10 mM, but levels of even 25 mM are tolerated. Whether Mn2+ serves by binding the nucleoside triphosphate or the active site of the enzyme or both are unknown. A requirement for Mn2+ is shared by many enzymes, including the NDK activity of pyruvate kinase, which is sustained by Mn2+ at the very low level of 0.05 mM (24). Mn2+ can also perform as an inorganic superoxide dismutase as in Lactobacillus plantarum where, as a complex of 30 mM Mn2+ with 60 mM poly P, it serves the cell in disposing of superoxide (25).
The processivity of E. coli PPK1 is extraordinary. The uniform product is 700–800 residues in average length; no shorter lengths are detectable in the course of the reaction. The P. aeruginosa PPK1 is also processive although the distribution of chain lengths (500–800) is somewhat broader. PPK2 is processive as well (Fig. 3) both in the synthesis of poly P and its utilization (14).
A remarkable feature of PPK2 is that the catalytic potency (Vmax and kcat) in the poly P-driven synthesis of GTP from GDP is far greater than the synthesis of poly P. The ratio of poly P utilization to poly P synthesis is 75 for PPK2, but only 0.07 for PPK1 of E. coli. Thus, the ratio with PPK2 differs from that with PPK1 >1,000-fold. Another distinctive characteristic is the selectivity for GDP vs. ADP as the substrate for the poly P-driven NDK activity. The ratio of GDP over ADP for PPK2 at Vmax is 1.1; by contrast, the PPK1s in their NDK actions strongly favor ADP over GDP with GDP/ADP ratios ranging from 0.015 for E. coli PPK1 to 0.00016 for V. cholerae PPK1.
A search of the available databases disclosed the wide conservation of the PPK2 sequence (Table 2). Of immediate interest are the two homologs in the P. aeruginosa genome. One is similar in size to PPK2, whereas the other is twice the size and homologous only in the carboxyl half of the molecule. It remains to be determined whether these homologs have PPK activity. Among bacterial species, 33 have PPK2 homologs as do two archaeal species; most possess PPK1 as well. Among those listed are some bacteria with developmental cycles (e.g., cyanobacteria, caulobacter, rhizobia) and several pathogens. Also listed are microorganisms that contain only PPK1 (uncompleted genome sequences may have a PPK2 homolog); also listed are microorganisms that have neither PPK1 nor PPK2. There are instances of closely related species in which only one possesses a PPK. As examples are the following: B. subtilis lacks any PPK but B. anthracis (with two virulence plasmids) has PPK1; M. tuberculosis has PPK1 and PPK2 but M. leprae has only PPK1; C. acetobutylicum has PPK1 but C. perfringens has neither PPK1 nor PPK2.
Further studies are now contemplated to prepare null mutants of PPK2 and its several homologs in conjunction with available ppk1, ndk mutants. The phenotypic features of these new constructs will be of considerable interest along with comparisons with clinical isolates of P. aeruginosa (e.g., strain 8830) as well as studies of the other microorganisms that possess PPK2.
At this stage in our studies, it seems likely that PPK1 in P. aeruginosa, and in other organisms, functions in the synthesis of poly P from ATP and that PPK2 functions by using poly P to generate GTP from GDP. In keeping with this proposed function for PPK2, it is significant that the appearance of the enzyme at the late-log stage (14) is at the very time when GTP is needed for the massive synthesis of alginate, the exopolysaccharide that covers the organism in a mucoid envelope (26, 27). PPK2 may also have other functions because some organisms (e.g., Streptomyces, etc.) that have PPK2 homologs do not have exopolysaccharide coats like alginate.
The fact that P. aeruginosa and many other organisms (Table 2) have multiple PPKs suggests that they attach importance to maintaining poly P levels and its use in a variety of functions. That PPK can serve as a target for discovery of antibiotics has already been demonstrated for PPK1 in P. aeruginosa (S. Lee, personal communication); PPK2 now invites a similar effort. Among considerations that favor PPK as a target for antibiotics is its distribution in a wide spectrum of pathogens, the loss of virulence in ppk mutants (9, 10, 12, 28, 29), and the expected lack of host toxicity because the PPK sequence seems to be absent in the sequences of yeast and animal cells.
Acknowledgments
To Leroy Bertsch we are indebted for advice and assistance in preparation of this paper. We thank Dr. Anand Sethuraman, who found a PPK activity in a ppk1 mutant of Klebsiella pneumoniae that used GTP and that alerted us to GTP as a possible donor for poly P synthesis. We thank the National Institute of General Medical Science of the National Institutes of Health for the support of our research and the Yamasa Corporation for fellowship support of K.I.
Abbreviations
poly P, inorganic polyphosphate
PPK, polyphosphate kinase
NDK, nucleoside diphosphate kinase
Data deposition: The sequence reported in this paper (ppk2) has been deposited in the GenBank database (accession no. AY168003).
References
- 1.Kulaev I. S., (1979) The Biochemistry of Inorganic Polyphosphates (Wiley, New York). [DOI] [PubMed]
- 2.Kulaev I. S. & Vagabov, V. M. (1983) Adv. Microbiol. 15, 731-738. [Google Scholar]
- 3.Wood H. G. & Clark, J. E. (1988) Annu. Rev. Biochem. 57, 235-260. [DOI] [PubMed] [Google Scholar]
- 4.Rao N. N. & Kornberg, A. (1996) J. Bacteriol. 178, 1394-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiba T., Tsutsumi, K., Yano, H., Ihara, Y., Kameda, A., Tanaka, K., Takahashi, H., Munekata, M., Rao, N. N. & Kornberg, A. (1997) Proc. Natl. Acad. Sci. USA 94, 11210-11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao N. N., Liu, S. & Kornberg, A. (1998) J. Bacteriol. 180, 2186-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ault-Riché D., Fraley, C. D., Tzeng, C. M. & Kornberg, A. (1998) J. Bacteriol. 180, 1841-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kornberg A., Rao, N. N. & Ault-Riché, D. (1999) Annu. Rev. Biochem. 68, 89-125. [DOI] [PubMed] [Google Scholar]
- 9.Rashid M. H., Rao, N. N. & Kornberg, A. (2000) J. Bacteriol. 182, 225-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rashid M. H. & Kornberg, A. (2000) Proc. Natl. Acad. Sci. USA 97, 4885-4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogawa N., Tzeng, C.-M., Fraley, C. D. & Kornberg, A. (2000) J. Bacteriol. 182, 6687-6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rashid M. H., Rumbaugh, K., Passador, L., Davies, D. G., Hamood, A. N., Iglewski, B. H. & Kornberg, A. (2000) Proc. Natl. Acad. Sci. USA 97, 9636-9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn K. & Kornberg, A. (1990) J. Biol. Chem. 265, 11734-11739. [PubMed] [Google Scholar]
- 14.Ishige K., Zhang, H. & Kornberg, A. (2002) Proc. Natl. Acad. Sci. USA 99, 16684-16688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishige K. & Noguchi, T. (2001) Biochem. Biophys. Res. Commun. 281, 821-826. [DOI] [PubMed] [Google Scholar]
- 16.Kuroda A., Murphy, H., Cashel, M. & Kornberg, A. (1997) J. Biol. Chem. 272, 21240-21243. [DOI] [PubMed] [Google Scholar]
- 17.Yanisch-Perron C., Vieira, J. & Messing, J. (1985) Gene 33, 103-119. [DOI] [PubMed] [Google Scholar]
- 18.Peterson G. L. (1997) Anal. Biochem. 83, 346-356. [DOI] [PubMed] [Google Scholar]
- 19.Piszkiewicz D., Landon, M. & Smith, E. L. (1970) Biochem. Biophys. Res. Commun. 40, 1173-1178. [DOI] [PubMed] [Google Scholar]
- 20.Marcus F. (1985) Int. J. Pept. Protein Res. 25, 542-546. [DOI] [PubMed] [Google Scholar]
- 21.Stover C. K., Pham, X. Q., Erwin, A. L., Mizoguchi, S. D., Warrener, P., Hickey, M. J., Brinkman, F. S., Hufnagle, W. O., Kowalik, D. J., Lagrou, M., et al. (2000) Nature 406, 959-964. [DOI] [PubMed] [Google Scholar]
- 22.Vetter I. R. & Wittinghofer, A. (1999) Q. Rev. Biophys. 32, 1-56. [DOI] [PubMed] [Google Scholar]
- 23.Saraste M., Sibbald, P. R. & Wittinghofer, A. (1990) Trends Biochem. Sci. 15, 430-434. [DOI] [PubMed] [Google Scholar]
- 24.Sundin G. W., Shanker, S., Chughani, S. A., Chopede, B. A., Kavanaughblack, A. & Chakrabarty, A. M. (1996) Mol. Microbiol. 20, 965-979. [DOI] [PubMed] [Google Scholar]
- 25.Archibald F. S. & Fridovich, I. (1982) Arch. Biochem. Biophys. 215, 589-596. [DOI] [PubMed] [Google Scholar]
- 26.Kim H.-Y., Schlictman, D., Shanker, S., Xie, Z., Chakrabarty, A. M. & Kornberg, A. (1998) Mol. Microbiol. 27, 717-725. [DOI] [PubMed] [Google Scholar]
- 27.Kamath S., Kapatral, V. & Chakrabarty, A. M. (1998) Mol. Microbiol. 30, 933-941. [DOI] [PubMed] [Google Scholar]
- 28.Tzeng C.-M. & Kornberg, A. (1998) Mol. Microbiol. 29, 381-382. [DOI] [PubMed] [Google Scholar]
- 29.Kornberg A. & Inorganic Polyphosphates (1999) in Progress in Molecular and Subcellular Biology, eds. Schröder, H. C. & Müller, W. E. G. (Springer, Berlin), Vol. 23, Chap. 1.