Abstract
Free radicals have been implicated in the pathogenesis of an increasing number of diseases. Lipids, which undergo peroxidation, are major targets of free radical attack. We report the discovery of a pathway of lipid peroxidation that forms a series of isomers in vivo that are characterized by a substituted tetrahydrofuran ring structure, termed isofurans (IsoFs). We have proposed two distinct pathways by which IsoFs can be formed based on 18O2 and H218O labeling studies. Measurement of F2-isoprostanes (IsoPs), prostaglandin F2-like compounds formed nonenzymatically as products of lipid peroxidation, is considered one of the most reliable approaches for assessing oxidative stress status in vivo. However, one limitation with this approach is that the formation of IsoPs becomes limited at high oxygen tension. In contrast, the formation of IsoFs becomes increasingly favored as oxygen tension increases. IsoFs are present at readily detectable levels in normal fluids and tissues, and levels increase dramatically in CCl4-treated rats, an animal model of oxidant injury. The ratio of IsoFs to IsoPs in major organs varies according to normal steady-state tissue oxygenation. In addition, IsoFs show a marked increase early in the course of hyperoxia-induced lung injury, whereas IsoPs do not significantly increase. We propose that combined measurement of IsoFs and IsoPs should provide a more reliable index of oxidant stress severity than quantification of either alone because of the opposing modulation of the two pathways by oxygen tension, which can vary widely in different organs and disease states.
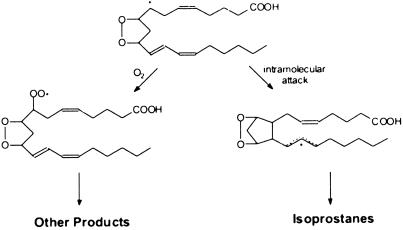
Free radicals have been implicated in an increasing number of diverse diseases. Lipids, which undergo peroxidation, are major targets of free radical attack. After the initial formation of lipid hydroperoxides, complex secondary reactions ensue, leading to the formation of a plethora of downstream products. One such example is F2-isoprostanes (F2-IsoPs). F2-IsoPs are prostaglandin F2-like compounds that are formed nonenzymatically in vivo by free radical-induced peroxidation of arachidonic acid (1). Measurement of F2-IsoPs has emerged as one of the most reliable approaches for assessing oxidative stress in vivo (2, 3). However, we have long recognized a potential limitation of measuring F2-IsoPs for assessing oxidative stress related to the mechanism of formation. In the pathway of IsoP formation, a carbon-centered radical must undergo intramolecular attack of a double bond to form a cyclopentane ring. Competing with this reaction is attack of the carbon-centered radical by molecular O2, which precludes the formation of IsoPs (Fig. 1). Thus, it is reasonable to predict that at high oxygen concentrations IsoP formation would become limited, whereas products formed subsequent to the addition of molecular O2 would predominate. It follows, therefore, that measurement of F2-IsoPs may not provide a sensitive and accurate index of lipid peroxidation severity in settings of high oxygen tension, such as hyperoxia-induced lung injury, retinopathy of prematurity, etc. We describe herein the discovery of products of lipid peroxidation that are formed via attack of the carbon-centered radical intermediate in the IsoP pathway by molecular O2 and demonstrate that, as anticipated, their formation is enhanced as oxygen tension increases.
Fig 1.
Pathway leading to the formation of IsoPs and other arachidonic acid-derived products of lipid peroxidation. Attack of the carbon-centered radical intermediate by oxygen prevents the formation of IsoPs while favoring the formation of other products.
Experimental Procedures
In Vitro Oxidation of Arachidonic Acid.
Arachidonic acid (Nu Chek Prep, Elysian, MN) was dissolved in ethanol and added to buffer (5 ml) at a final concentration of 20 mM. The oxidants used were 10 mM 2,2′-azobis(2-amidinopropane) hydrochloride (AAPH; Kodak) or an iron/ADP/ascorbate system, as described (4). PBS, pH 7.4, was used with AAPH, and phosphate buffer, pH 7.4 was used with the Fe/ADP/ascorbate system. Reactions were allowed to proceed at 37°C for 12–18 h, and aliquots were removed for analysis. For incubations examining the effects of MnCI2 and bicarbonate on product formation, buffers were washed over a Chelex-100 column before addition of 20 mM sodium bicarbonate and 1 mM MnCI2. Incubations were then carried out for 15 h with AAPH as the oxidant. For incubations in H218O (Cambridge Isotope Laboratories, Andover, MA), concentrations of all buffer salts, AAPH, and arachidonic acid were maintained, but total reaction volume was reduced to 500 μl because of the limited availability of H218O.
Modulation of O2 Tension.
For in vitro incubations, buffers were quantitatively degassed by using four to five cycles of a freeze-pump-thaw process after which a specified O2:N2 mixture, room air, or 100% O2 or 18O2 (Isotec, Miamisburg, OH) was introduced into the closed reaction vessel to a pressure of 1 atm, and the mixture was allowed to equilibrate overnight at 4°C. Oxidations were then carried out as described above.
MS Analyses.
Free and esterified F2-IsoPs and unknown compounds were analyzed and quantified by stable isotope dilution GC/negative ion chemical ionization MS as a pentafluorobenzyl ester, trimethylsilyl (TMS) ether derivative as described (5) by using [2H4] 15-F2t-IsoP as an internal standard (6). Compounds were also analyzed by GC/electron ionization MS as a methyl ester, TMS ether derivative.
Analysis of Liver from CCI4-Treated Rats.
Treatment of rats with CCI4 was used to induce an oxidant injury to the liver as described (7). Four hours after administration of CCl4, livers were removed and levels of compounds esterified in liver lipids were determined.
Analysis of Lungs Exposed to 100% O2.
For hyperoxia exposures, adult female C57/Bl6 mice were placed in a sealed plastic chamber with free access to food and water. Oxygen concentration within the chamber was measured by using an oxygen sensor (Pacific Analytical Technologies, Temecula, CA) and was maintained at >98% by a flow of O2 through the chamber of 3.5 liters/min. After 3 h of O2 exposure, animals were killed and the lungs were immediately perfused with cold PBS, homogenized, and analyzed for esterified compounds as described. A small aliquot of homogenate was used to determine total protein content by using the bicinchoninic acid assay (Pierce).
Analysis of Antioxidant Effects.
Male Sprague–Dawley rats weighing ≈300 g were administered either vehicle, 200 mg/kg N-acetylcysteine by i.p. injection twice daily for 3 days, or 1 g/liter α-lipoic acid dissolved in the drinking water (accomplished by addition of a minimum amount of NaOH and back titration to neutrality) for 3 days (≈50 mg/kg per day orally for 3 days). After administration, plasma was obtained from each animal and plasma lipids were assayed as described.
Statistical Analyses.
All statistical analyses were performed by using the GraphPad (San Diego) PRISM 3.0 software package. Means were compared by using either an unpaired Student's t test or univariate ANOVA with Bonferroni's correction for multiple comparisons. Unless otherwise stated, results are presented as mean ± SEM.
Results
Discovery of Products of Lipid Peroxidation.
During GC/negative ion chemical ionization MS, limited mass scanning from 500 to 700 Da for compounds formed during oxidation of arachidonic acid in vitro, a series of abundant compounds was detected at m/z 585, 16 Da higher than the M-⋅CH2C6F5 ion of F2-IsoPs (m/z 569). Analysis by selected ion monitoring of m/z 569 and m/z 585 revealed a series of peaks in the m/z 585 ion current chromatogram with a GC retention time slightly longer than the F2-IsoPs (Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org).
Because these compounds copurified with F2-IsoPs and had a similar GC retention time and a mass that was only 16 Da higher, we surmised that the unknown compounds and F2-IsoPs may share structural similarities. The presence of an epoxide or an additional carbonyl was initially considered to account for the additional 16 Da. An epoxide functionality was excluded when treatment with 1 M HCI or 50% acetic acid failed to eliminate the intensity of the m/z 585 peaks, nor was there an appearance of new compounds containing the expected m/z ratio for a chlorohydrin at m/z 693 or a diol at m/z 747. The presence of a carbonyl functionality was also excluded by using two approaches. First, treatment with NaBH4 failed to reduce the intensity of the m/z 585 peaks and failed to produce new peaks at the expected m/z ratio for the reduced carbonyl (m/z 647). Second, treatment with methoxyamine⋅HCl also failed to reduce the intensity of the m/z 585 peaks and failed to produce new peaks at the expected m/z ratio for an O-methyloxime derivative (m/z 614). We then investigated the functional groups present in these compounds more generally. Analysis of the compounds as a [2H9] TMS ether derivative resulted in an upward shift of all of the m/z 585 peaks 27 Da to m/z 612, indicating the presence of three hydroxyl groups, analogous to F2-IsoPs. Catalytic hydrogenation resulted in an upward shift of the m/z 585 peaks 4 Da to m/z 589, indicating the presence of two double bonds, analogous to F2-IsoPs.
In consideration of possible structures for these compounds, a number of constraints from the data obtained thus far had to be satisfied. First, the structural nature of the compounds must confer resistance to acid treatment. In addition, the compounds must have the same number and type of functional groups as F2-IsoPs. The dilemma remained, however, as to how to account for an additional 16 Da compared with F2-IsoPs. An attractive candidate that would satisfy all of these constraints would be a compound that contains a substituted tetrahydrofuran (THF) ring and the same number of hydroxyl groups and double bonds as F2-IsoPs. This was an attractive consideration in light of the fact that such compounds had been detected by other groups studying enzymatic arachidonic acid metabolism (8, 9), and because enzymes frequently only accelerate the rate of chemical reactions, it was reasonable to speculate that the formation of these compounds might also occur nonenzymatically.
We then sought to determine whether these compounds contained an atom of oxygen in addition to those contained in the three hydroxyl groups. We analyzed the compounds formed during oxidation of arachidonic acid in vitro under [18O2] gas. When analyzed by GC/MS, the m/z 585 peaks had disappeared, and new intense peaks were observed at both m/z 591 and m/z 593. This finding indicated that some compounds incorporated four [18O] atoms, as expected, but some had incorporated only three. Notably, the patterns of peaks in both ion current chromatograms were nearly identical, which could be explained by contamination of the 18O2 with air. Alternatively, two mechanisms may be involved in the formation of these compounds: (i) one that incorporates four oxygen atoms from molecular O2 and (ii) one that incorporates three oxygen atoms from molecular O2 and one from H2O. To explore this possibility, compounds were analyzed after oxidation of arachidonic acid in H218O. Under these conditions, peaks were observed both at m/z 585 and m/z 587, indicating that some compounds had incorporated one oxygen atom from H218O and three from molecular O2, and some had used molecular O2 as the sole source of incorporated oxygen atoms. The possibility of label incorporation caused by exchange of H218O into the final products was excluded by incubating unlabeled products with H218O under normal reaction conditions and observing that no appreciable labeling of products occurred. These data suggested that two mechanisms may be involved in the formation of these compounds. The integrated areas under the peaks in the m/z 587 ion current chromatogram were 5.5 ± 0.8-fold greater than the peaks in the m/z 585 ion current chromatogram. This finding suggests that the mechanism involving incorporation of an oxygen atom from water contributes to the formation of these compounds to a greater extent than the mechanism involving incorporation of all four oxygen atoms from molecular O2.
Based on these data, two plausible mechanisms for the formation of isofurans (IsoFs) are proposed: the cyclic peroxide cleavage pathway and the epoxide hydrolysis pathway (Fig. 7, which is published as supporting information on the PNAS web site). The last step in each pathway involves an epoxy alcohol rearrangement to the THF ring, a reaction for which there is precedence (10). These same epoxy alcohols can be formed by at least one other mechanism, but the formation of compounds by this pathway would be suppressed at high oxygen concentrations, which is incongruous with the data we have obtained (11) (see below). These two mechanisms together predict the formation of eight distinct regioisomers, each comprised of 16 racemic diastereomers for a total of 256 compounds (Fig. 8, which is published as supporting information on the PNAS web site). The epoxide hydrolysis pathway is predicted to contribute to the formation of all eight regioisomers, whereas the cyclic peroxide cleavage pathway is predicted to contribute to the formation of only four of the eight regioisomers (detailed mechanisms for regioisomer formation are shown in Figs. 9 and 10, which are published as supporting information on the PNAS web site). This is consistent with the finding in the 18O2 labeling experiment that the pattern of peaks representing compounds that had incorporated four [18O] atoms, formed by the cyclic peroxide cleavage pathway, was very similar to the pattern of peaks representing compounds that had incorporated only three [18O] atoms, formed by the epoxide hydrolysis pathway.
The epoxide hydrolysis pathway is thought to involve direct epoxidation of a double bond. This can occur by using a lipid hydroperoxide as the oxygen-donating species but this has not been thought to be a facile reaction unless catalyzed by group IVa, Va, or VIa transition metals such as Mo, V, and Cr (12). However, it has recently been demonstrated that direct epoxidation of a double bond can also be catalyzed by Mn in combination with bicarbonate in the presence of hydrogen peroxide (13). We therefore sought to obtain support for the epoxide hydrolysis mechanism by determining the effect of MnCI2 and NaHCO3 on the formation of these compounds. In these experiments, we compared the ratio of the amount of unknown compounds formed to that of F2-IsoPs, which internally controls for the extent of oxidation of arachidonic acid in different experiments. The addition of MnCI2 and bicarbonate significantly shifted the product ratio toward formation of the unknown compounds. In the absence of MnCI2 and NaHCO3 the ratio of unknown compounds/IsoPs was 5.6 ± 0.1, and in the presence of MnCI2 and NaHCO3, the ratio was 11.2 ± 2.3 (n = 3, P < 0.05). Although these data support the proposed epoxide hydrolysis mechanism, this does not imply that facilitation of lipid hydroperoxide epoxidation of double bonds by Mn and bicarbonate is the only mechanism whereby this occurs.
Analysis of the Unknown Compounds by MS.
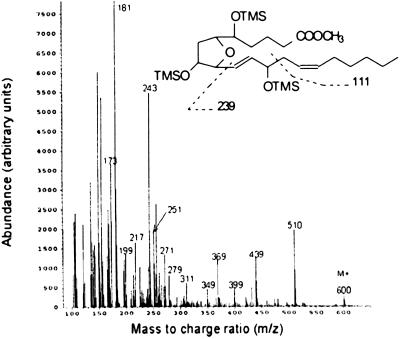
We then sought to obtain evidence for the proposed substituted THF ring structure and the proposed regioisomers by analyzing the compounds purified from in vitro incubations of oxidized arachidonic acid by GC/electron ionization MS as a methyl ester, TMS ether derivative. By GC/negative ion chemical ionization MS, multiple peaks representing the unknown compounds were detected during selected ion monitoring analysis, suggesting the presence of multiple isomers (Fig. 6). Consistent with this finding, multiple similar mass spectra were obtained by GC/electron ionization MS, eluting over an ≈30-s period that differed primarily in relative abundance of various ions. One of the mass spectra obtained is shown in Fig. 2. This mass spectrum was interpreted as a mixed mass spectrum with the major component being the regioisomer depicted. A prominent molecular ion [MH+] is present at m/z 600, representing the unfragmented methyl ester, TMS ether of the unknown compounds. Additional ions that can be assigned to the structure shown are m/z 510 (M-90, loss of Me3SiOH); m/z 399 (M-111–90), loss of ⋅CH2CH = CH(CH2)4CH3 + 90; m/z 271 (M-239–90), loss of ⋅CH = CHCH(OSiMe3)CH2CH = CH(CH2)4CH3 + 90; m/z 217 [M-203-(2 × 90)], loss of ⋅CH(OSiMe3)(CH2)3COOCH3 + (2 × 90); and m/z 181 (base) [M-239-(2 × 90)]. Several other prominent ions present could not be assigned to the structure depicted, but, as shown in Fig. 8, could be assigned to other coeluting regioisomers whose structures were predicted from the two pathways of formation. Importantly, the mass spectrometric data we obtained were entirely consistent with previously published mass spectra that were confirmed by analysis of a synthetically pure compound (9, 14). Because these data provide strong evidence that these compounds are substituted THF isomers, we propose to term these compounds IsoFs.
Fig 2.
Electron ionization mass spectrum of IsoFs isolated from an incubation after oxidation of arachidonic acid in vitro. Compounds were analyzed as a methyl ester, TMS ether derivative. In this mixed mass spectrum, the IsoF regioisomer shown appears to predominate this part of the spectrum, by way of assignment of the m/z 181 base ion. OTMS and TMSO indicate trimethylsilyl ether groups. The assignment of fragment ions to other regioisomers is outlined in Fig. 8.
Influence of Oxygen Tension on the Formation of IsoFs.
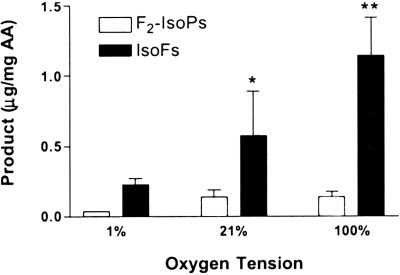
Unlike the mechanism of formation of IsoPs, neither the cyclic peroxide cleavage pathway nor the epoxide hydrolysis pathway involves a reaction that would be in direct competition with addition of molecular O2. Therefore, we predicted that the formation of IsoFs would be positively modulated by oxygen tension, whereas F2-IsoP formation would not. To test this hypothesis, the formation of IsoFs and F2-IsoPs was measured during oxidation of arachidonic acid in vitro in the presence of increasing concentrations of O2 (Fig. 3). Formation of both F2-IsoPs and IsoFs increases from 1% to 21% O2, as efficient oxidation requires the presence of O2. However, there is no further increase in F2-IsoP formation above 21% O2, whereas IsoF formation continues to increase significantly as oxygen tension is increased from 21% to 100% O2.
Fig 3.
Effect of oxygen tension on F2-IsoP and IsoF formation during oxidation of arachidonic acid in vitro. Each bar represents the mean ± SEM for three independent experiments. *, P < 0.05; **, P < 0.001.
We then explored the relative extent to which both pathways contributing to IsoF formation are favored by increasing O2 tension. In these experiments, arachidonic acid was oxidized in H218O under an atmosphere of 21% or 100% O2. At 21% O2, the ratio of 18O-labeled compounds to unlabeled compounds was ≈6:1, and this ratio persisted at 100% O2. This finding suggests that both pathways are favored by increased oxygen tension to approximately the same extent. This notion is consistent with oxygen attack of the carbon-centered radical in the cyclic peroxide cleavage pathway and enhanced lipid hydroperoxide formation, facilitating product formation, via the epoxide hydrolysis pathway.
Formation of IsoFs in Vivo: Relationship to the Level of Tissue Oxygenation and the Effect of Antioxidants.
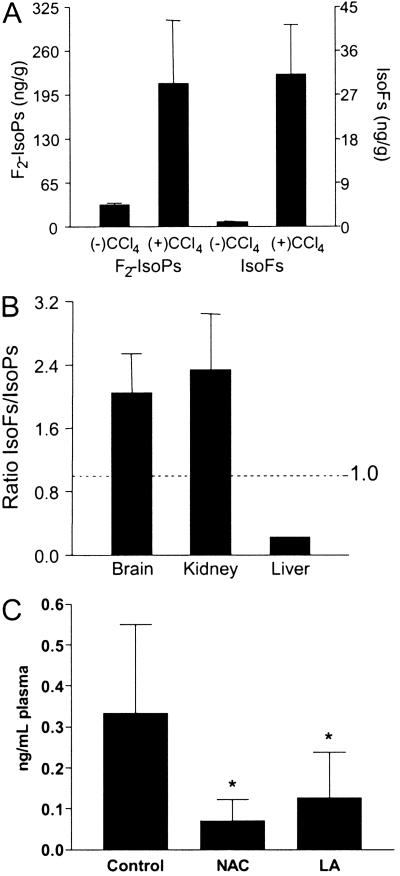
We next sought to explore whether IsoFs are formed in vivo. For this purpose, we used CCl4-treated rats, an established animal model of oxidant injury to the liver. IsoFs would be expected to form initially esterified in tissue phospholipids, as occurs with IsoPs (15). Previously, we have shown that levels of F2-IsoPs esterified in liver phospholipids increase markedly after administration of CCI4 (15). Livers from untreated and treated animals collected 4 h after administration of CCI4 were analyzed for esterified IsoFs and F2-IsoPs (Fig. 4A). Levels of both IsoFs and F2-IsoPs increased markedly after administration of CCl4. Note, however, that the levels of IsoFs in livers from both control and CCl4-treated animals were much lower than levels of F2-IsoPs. This finding contrasts with what was observed during oxidation of arachidonic acid in vitro, where the amount of IsoFs formed greatly exceeded the amount of IsoPs formed (Fig. 3). A plausible explanation for this difference is the fact that the liver is a poorly oxygenated organ, the majority of its blood supply being venous blood supplied by the portal vein. If this explanation were true, then the ratio of IsoFs to IsoPs in various tissues might vary in accordance with the level of tissue oxygenation in vivo. To further explore this hypothesis, we compared levels of IsoFs and F2-IsoPs in two highly oxygenated organs, the kidney and brain hippocampus from normal rats. As shown in Fig. 4B, the ratio of IsoFs to IsoPs in these highly oxygenated organs was the inverse of that in the liver, with levels of IsoFs exceeding those of IsoPs by ≈2.0- to 2.3-fold. These data suggest that the ratio of levels of esterified IsoFs and F2-IsoPs in various organs may be able to serve as an index of tissue oxygenation.
Fig 4.
IsoF and F2-IsoP levels in normal rat organs and liver from rats treated with CCl4. (A) Levels of IsoFs and F2-IsoPs esterified in liver 4 h after administration of CCl4 and in untreated animals. Results are the mean ± SEM for three animals each in the untreated and treated groups. (B) Ratios of IsoF/F2-IsoP levels measured in rat brain (n = 4), kidney (n = 6), and liver (n = 3). Brain represents rat brain hippocampus, one of the most highly oxygenated regions of the brain. Results are shown as the mean ± SEM. (C) Effect of antioxidants on IsoF formation. Results are shown as the mean ± SEM. Control are normal rats (n = 8), NAC are rats treated with N-acetylcysteine (n = 4), and LA are rats treated with α-lipoic acid (n = 6). *, P < 0.05 vs. control.
We then explored whether IsoFs are also present at detectable levels in normal biological fluids. The levels of IsoFs detected in normal biological fluids are as follows: rat urine 3.3 ± 0.3 ng/ml (n = 8), rat plasma 334 ± 80 pg/ml (n = 8), mouse bronchoalveolar lavage fluid (210 ± 30 pg/ml, n = 2), human plasma (71 ± 10 pg/ml, n = 2), and human urine (5.8 ± 1.0 ng/ml, n = 5).
Although it is often assumed that there is a normal “oxidant tone” in vivo, as suggested by detection of F2-IsoPs in fluids and tissues even under basal conditions, the existence of IsoFs under basal conditions in vivo could potentially be explained by the action of one or a number of enzymatic systems instead of nonenzymatic free radical processes. To explore this possibility, normal rats were treated with either N-acetylcysteine or α-lipoic acid, two commonly used antioxidants. In both groups, levels of total IsoFs in the plasma dropped by >50% compared with untreated controls (Fig. 4C). This finding demonstrates that even under basal conditions any potential enzymatic contribution to IsoF formation in vivo is negligible.
IsoFs and IsoPs in Normobaric Oxygen Toxicity.
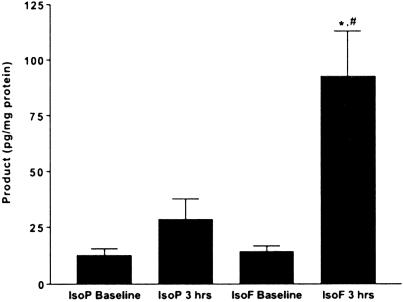
We next sought to establish definitive in vivo relevance for the formation and quantification of IsoFs by exploring the phenomenon of hyperoxia-induced lung injury, or so-called normobaric oxygen toxicity. Oxygen toxicity involves an injury to the lungs of an animal breathing high concentrations of oxygen for an extended period. Alveolar fluid accumulation, hypoxemia, pulmonary fibrosis, and cell death characterize the injury (16). Oxygen toxicity has long been assumed to be an oxidative injury, and although there is much indirect evidence to support this notion (17–20), the direct evidence for oxidant injury to the lung is equivocal. Indeed, F2-IsoPs have failed to show any changes in an animal model of oxygen toxicity (L.J.R. and J. H. Newman, unpublished results). Given that oxygen toxicity may involve an oxidant injury in a setting of elevated O2 tension, we measured both F2-IsoPs and IsoFs in the lungs of mice exposed to 100% O2 (Fig. 5). After just 3 h of hyperoxia, F2-IsoPs esterified in the lung tissue of treated animals are not significantly different from controls. In striking contrast, IsoFs in the lung increase more than 5-fold after a 3-h exposure to 100% O2. This finding demonstrates that oxygen toxicity not only involves an oxidant insult, but that damage begins very early, before any apparent respiratory distress. Also of note is the fact that the IsoF/F2-IsoP ratio at baseline is ≈1:1, which is reflective of the fact that while the lungs are exposed to a higher O2 concentration than most organs, they are also perfused by more deoxygenated blood than any other organ in the body, as the entirety of cardiac output in deoxygenated blood is supplied to the lungs via the pulmonary arteries and comprises the majority of total blood flow through the lungs.
Fig 5.
IsoF and F2-IsoP levels esterified in lung tissue at baseline and after 3 h of hyperoxia. Results are shown as mean ± SEM. *, P < 0.001 vs. IsoF baseline; #, P < 0.01 vs. IsoP 3 h, n = 8 in each group.
Discussion
We report the discovery of a pathway of lipid peroxidation that forms a series of isomeric compounds containing a substituted THF ring (IsoFs). IsoFs are stable compounds that are present at readily detectable levels in normal tissues and biological fluids, and levels increase markedly in a well-established animal model of oxidant injury. These are precisely the same attributes that led to the recognition that measurement of F2-IsoPs is one of the most valuable approaches to assess oxidative stress in vivo (2, 3). Importantly, however, as discussed below, measurement of IsoFs provides unique information that complements measurement of F2-IsoPs as an index of lipid peroxidation.
We have proposed two distinct mechanisms for the formation of IsoFs and have provided supporting evidence for each. Although the mechanisms proposed are consistent with all of the data obtained thus far, it should be noted that, in the absence of isolated intermediates, the mechanisms are speculative, and ongoing experiments could necessitate future modification of the mechanisms as they are written. Nonetheless, the insights gained from these proposed mechanisms led not only to the elucidation of the full panel of IsoF regioisomers but also to the prediction of how their formation would be modulated by oxygen tension. One of the most interesting aspects of the discovery of IsoFs is that their formation is modulated in a positive fashion by increasing oxygen tension, unlike the effects of elevated oxygen tension on IsoP formation. We demonstrated that the formation of IsoFs increases in what appears to be a linear fashion from 1% to 100% O2, whereas the formation of IsoPs plateaus at 21% O2. The plateau effect for F2-lsoPs replicates the findings of earlier investigations in our laboratory (4, 21). These disparate effects of oxygen concentration on the formation of IsoFs and IsoPs can be explained by the fact that oxygen concentration is the critical determinant for the rates of mutually exclusive competing reactions of a common intermediate involved in the mechanisms of IsoP and IsoF formation.
These data suggest that measurement of IsoFs can provide unique information about the severity of lipid peroxidation under settings of elevated oxygen tension. Whereas measurement of F2-IsoPs would have limited value as an index of oxidant injury in settings of high oxygen tension, measurement of IsoFs should provide a more sensitive and robust indicator of oxidant injury under these conditions. In support of this idea, we have demonstrated that measurement of IsoFs, but not F2-IsoPs, reveals oxidant damage in the lung in normobaric oxygen toxicity. Not only is this an important observation for establishing the in vivo modulation of IsoF formation by oxygen tension, this observation is a major step toward understanding the basic processes underlying oxygen toxicity. We have provided direct evidence for oxidative injury in oxygen toxicity and have shown that this injury begins very early in the disease process, before any respiratory distress is apparent. Quantification of IsoFs in further studies of oxygen toxicity might prove useful not only in understanding the molecular mechanisms underlying cellular damage but also in evaluating potential interventions to prevent injury in the earliest stages. These types of studies have until now been difficult or impossible because no reliable biomarker for the injury that precedes clinically apparent oxygen toxicity has been identified in the past.
We envision a potential utility for measuring IsoFs in a wide variety of other settings as diverse as oxidative damage to transplant organs during storage in ambient air (22, 23), retinopathy of prematurity (24), and the sequelae of hyperbaric oxygen exposure (25). Additionally, mitochondrial dysfunction can be a source of free radical generation and is a feature of a number of disorders including neurodegenerative diseases such as Parkinson's disease (26, 27). Mitochondrial dysfunction theoretically could also lead to increased cellular O2 concentration due to impaired mitochondrial O2 utilization. Interestingly in that regard, we recently found that levels of F2-IsoPs in the substantia nigra from patients with Parkinson's disease were no different from levels in age-matched controls, whereas IsoF levels were significantly increased (unpublished work).
It should be mentioned that IsoFs are not the only products of lipid peroxidation whose formation ought to be favored by high oxygen tension. For example, the formation of lipid hydroperoxides should increase as well. While measurement of such products may provide a reliable measure of lipid peroxidation in vitro, there are severe limitations associated with measurement of such products as an index of lipid peroxidation in vivo. For example, after administration of CCl4 to rats, plasma levels of F2-IsoPs increase profoundly whereas lipid hydroperoxides could not be detected in the circulation even under this unusually severe setting of oxidant injury.∥
In addition, the data obtained suggest that the IsoF/F2-IsoP ratio can serve as a biochemical index of tissue oxygenation, acting as an in vivo “oxygen sensor.” The approach of assessing IsoF/F2-IsoP ratios as a indicator of tissue oxygenation could be used to obtain inimitable information in a variety of situations. These might include assessment of the efficacy of synthetic oxygen carriers (so-called “blood substitutes”), evaluation of the state of tissue oxygenation in disease states, measurement of the severity of ischemia in vascular diseases, etc. The sensitivity of the GC/MS assay for F2-IsoPs and IsoFs allows for quantification from human tissue biopsies, which would also allow an assessment of the ratio of IsoFs/F2-IsoPs as an index of tissue oxygenation before and after therapeutic interventions.
Measurement of IsoFs appears to be a valuable adjunct to established approaches for assessing oxidative stress in vivo. When measurements of IsoFs are combined with measurements of F2-IsoPs, more information can be obtained than with either measurement in isolation. Importantly, it should be pointed out that IsoFs and F2-IsoPs are quantified in the same GC/MS assay using a single purification protocol, allowing for maximum analytical simplicity. Finally, the possibility exists that IsoFs, like IsoPs, may be found to exert biological activity. However, this possibility cannot be explored until IsoFs become available to us in synthetic form, which we are currently undertaking with the help of published synthesis schemes (14, 28).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants GM42056, GM15431, DK22657, and CA68485 and National Institutes of Health Medical Scientist Training Program Grant 5-T32GM07437-22 (to J.P.F.). J.P.F. is the recipient of a PhRMA Foundation Medical Student Research Fellowship. L.J.R. is the recipient of a MERIT Award from the National Institutes of Health.
Abbreviations
IsoP, isoprostane
IsoF, isofuran
THF, tetrahydrofuran
TMS, trimethylsilyl
Mathews, W. R., McKenna, R., Guido, D. M., Petre, T. W., Jolly, R. A., Morrow, J. D. & Roberts, L. J., II, Proceedings of the 41st ASMS Conference on Mass Spectrometry and Allied Topics, May 30–June 4, 1993, San Francisco, 865A–865B.
References
- 1.Morrow J. D., Hill, K. E., Burk, R. F., Nammour, T. M., Badr, K. F. & Roberts, L. J., II (1990) Proc. Natl. Acad. Sci. USA 87, 9383-9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts L. J., II & Morrow, J. D. (2000) Free Radical Biol. Med. 28, 505-513. [DOI] [PubMed] [Google Scholar]
- 3.Pryor W. (2000) Free Radical Biol. Med. 28, 503-504. [DOI] [PubMed] [Google Scholar]
- 4.Longmire A. J., Swift, L. L., Roberts, L. J., II, Awad, J. A., Burk, R. F. & Morrow, J. D. (1994) Biochem. Pharmacol. 47, 1173-1177. [DOI] [PubMed] [Google Scholar]
- 5.Morrow J. D. & Roberts, L. J., II (1998) Methods Enzymol. 300, 3-12. [DOI] [PubMed] [Google Scholar]
- 6.Taber D. F., Morrow, J. D. & Roberts, L. J., II (1997) Prostaglandins 53, 63-67. [DOI] [PubMed] [Google Scholar]
- 7.Burk R. F. & Lane, J. M. (1979) Toxicol. Appl. Pharmacol. 50, 467-478. [DOI] [PubMed] [Google Scholar]
- 8.Moghaddam M., Motoba, K., Borhan, B., Pinot, F. & Hammock, B. D. (1996) Biochim. Biophys. Acta 1290, 327-339. [DOI] [PubMed] [Google Scholar]
- 9.Pace-Asciak C. (1971) Biochemistry 10, 3664-3669. [DOI] [PubMed] [Google Scholar]
- 10.Nixon J. R., Cudd, M. A. & Porter, N. A. (1978) J. Org. Chem. 43, 4048-4052. [Google Scholar]
- 11.Porter N. A., Zuraw, P. J. & Sullivan, J. A. (1984) Tetrahedron Lett. 25, 807-810. [Google Scholar]
- 12.Gardner H. W. (1989) Free Radical Biol. Med. 7, 65-86. [DOI] [PubMed] [Google Scholar]
- 13.Lane B. S. & Burgess, K. (2001) J. Am. Chem. Soc. 123, 2933-2934. [DOI] [PubMed] [Google Scholar]
- 14.Just G. & Oh, H. (1981) Can. J. Chem. 59, 2729-2736. [Google Scholar]
- 15.Morrow J. D., Awad, J. A., Boss, H. J., Blair, I. A. & Roberts, L. J., II (1992) Proc. Natl. Acad. Sci. USA 89, 10721-10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Limper A. H. & Rosenow, E. C., II (2000) in Textbook of Respiratory Medicine, eds. Murray, J. F. & Nadel, J. A. (Saunders, Philadelphia), pp. 1987.
- 17.O'Donovan D. J. & Fernandes, C. J. (2000) Mol. Genet. Metab. 71, 352-358. [DOI] [PubMed] [Google Scholar]
- 18.Freeman B. A. & Crapo, J. D. (1981) J. Biol. Chem. 256, 10986-10992. [PubMed] [Google Scholar]
- 19.Ilizarov A. M., Koo, H., Kazzaz, J. A., Mantell, L. L., Li, Y., Bhapat, R., Pollack, S., Horowitz, S. & Davis, J. M. (2001) J. Respir. Cell Mol. Biol. 24, 436-441. [DOI] [PubMed] [Google Scholar]
- 20.Brown L. A., Perez, J. A., Harris, F. L. & Clark, R. H. (1996) Am. J. Physiol. 270, L446-L451. [DOI] [PubMed] [Google Scholar]
- 21.Morrow J. D., Roberts, L. J., II, Daniel, V. C., Awad, J. A., Mirochnitchenko, O., Swift, L. L. & Burk, R. F. (1998) Arch. Biochem. Biophys. 353, 160-171. [DOI] [PubMed] [Google Scholar]
- 22.Salahudeen A., Nawaz, M., Poovala, V., Kanji, V., Wang, C., Morrow, J. & Roberts, J., II (1999) Kidney Int. 55, 1759-1762. [DOI] [PubMed] [Google Scholar]
- 23.Cracowski J. L., Souvignet, C., Quirin, N., Grosbois, X., Bayle, F., Stanke-Labesque, F., Vialtel, P. & Bessard, G. (2001) Clin. Transplant. 15, 58-62. [DOI] [PubMed] [Google Scholar]
- 24.Hardy P., Dumont, I., Bhattacharya, M., Hou, X., Lachapelle, P., Varma, D. R. & Chemtob, S. (2000) Cardiovasc. Res. 47, 489-509. [DOI] [PubMed] [Google Scholar]
- 25.Huang K. L., Wu, J. N., Lin, H. C., Mao, S. P., Kang, B. & Wan, F. J. (2000) Neurosci. Lett. 293, 159-162. [DOI] [PubMed] [Google Scholar]
- 26.Reichmann H. & Janetzky, B. (2000) J. Neurol. 247, Suppl. 2, II63-II68. [DOI] [PubMed] [Google Scholar]
- 27.Simonian N. A. & Coyle, J. T. (1996) Annu. Rev. Pharmacol. Toxicol. 36, 83-106. [DOI] [PubMed] [Google Scholar]
- 28.Narayan R. S., Sivakumar, M., Bouhlel, E. & Borhan, B. (2001) Org. Lett. 3, 2489-2492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.