Abstract
To define the topology of the skeletal muscle ryanodine receptor (RyR1), enhanced GFP (EGFP) was fused in-frame to the C terminus of RyR1, replacing a series of C-terminal deletions that started near the beginning or the end of predicted transmembrane helices M1–M10. The constructs were expressed in HEK-293 (human embryonic kidney cell line 293) or mouse embryonic fibroblast (MEF) cells, and confocal microscopy of intact and saponin-permeabilized cells was used to determine the subcellular location of the truncated fusion proteins. The fusion protein truncated after M3 exhibited uniform cytoplasmic fluorescence, which was lost after permeabilization, indicating that proposed M′, M′′, M1, M2, and M3 sequences are not membrane-associated. The fusion protein truncated at the end of the M4–M5 loop and containing M4 was membrane-associated. All longer truncated fusion proteins were also associated with intracellular membranes. Mapping by protease digestion and extraction of isolated microsomes demonstrated that EGFP positioned after either M5, the N-terminal half of M7 (M7a), or M8 was located in the lumen, and that EGFP positioned after either M4, M6, the C-terminal half of M7 (M7b), or M10 was located in the cytoplasm. These results indicate that RyR1 contains eight transmembrane helices, organized as four hairpin loops. The first hairpin is likely to be made up of M4a–M4b. However, it could be made up from M3–M4, which might form a hairpin loop even though M3 alone is not membrane-associated. The other three hairpin loops are formed from M5–M6, M7a–M7b, and M8–M10. M9 is not a transmembrane helix, but it might form a selectivity filter between M8 and M10.
Skeletal muscle contraction is initiated by activation of a Ca2+ release channel (ryanodine receptor isoform 1 or RyR1) located in the junctional terminal cisternae of the sarcoplasmic reticulum. Activation occurs through physical interaction with the dihydropyridine receptor, located in the transverse tubular membrane, where it is directly apposed to the ryanodine receptor (1, 2). Four RyR monomers, each of 565 kDa, assemble as a homotetrameric complex and transmembrane sequences from each of the four monomers interact to form the ion-conducting pore (3). Hydropathy profiles of RyR1 (4, 5), based on criteria defined by Kyte and Doolittle (6), indicate that the bulk of the RyR1 molecule is cytoplasmic and that 4–12 transmembrane sequences lie within the C-terminal one-tenth to one-fifth of the molecule. Among the predicted transmembrane sequences, four potential transmembrane sequences are clearly more hydrophobic than others, with hydropathy indices between 2.0 and 2.9. These four sequences, amino acids Phe-4564–Tyr-4580, Pro-4641–Leu-4664, Gln-4836–Phe-4859, and Ile-4918–Ile-4937, were designated M1–M4 and were proposed to form two hairpin loops in the topological model proposed by Takeshima et al. (4). In a second model, proposed by Zorzato et al. (5), eight additional hydrophobic sequences were identified, with hydropathy indices ranging from 0.8 to 1.6, and proposed to form four additional hairpin loops. The first two sequences in the Zorzato model, M′ (Gly-3124–Phe-3144) and M′′ (Pro-3188–Leu-3206), were considered to be tentative; the others were M1 (Leu-3985–Ala-4004), M2 (Met-4023–Ala-4041), M3 (Gly-4277–Ala-4300), M4 (Ala-4342–Phe-4362), M5 (Phe-4559–Tyr-4580), M6 (Leu-4648–Phe-4671), M7 (Phe-4789–Val-4820), M8 (Leu-4837–Phe-4856), M9 (Met-4879–Gly-4898), and M10 (Val-4914–Ile-4937). M1–M4 in the Takeshima model correspond to M5, M6, M8, and M10 in the Zorzato model. Brandt et al. (7) proposed an additional transmembrane segment in the middle of the molecule. In this paper, designations of transmembrane helices are those used in the more complicated Zorzato model.
Earlier attempts have been made to elucidate the structure and topology of RyR. Proteolytic digestion of RyR1 yielded several major fragments (8–10), identified by sequencing (8) or by immunoblotting with a series of seven antibodies (9). Five major tryptic fragments are estimated to span the molecule starting from the N terminus (9, 10). Their masses and approximate amino acid sequence composition (9, 10) are 135 or 150 kDa (amino acids 1–1397 or 1508), 100 kDa (1398 or 1509–2401), 50 kDa (2402–2840), 160 kDa (3119–4475), and 76 kDa (4476–5037). The more N-terminal 150-, 50-, and 100-kDa tryptic fragments and their subfragments could be extracted by Na2CO3, indicating that they did not contain transmembrane sequences (9). The more C-terminal 160-kDa fragment reacted with an antibody against an epitope contained within amino acids 4382–4417 and was resistant to Na2CO3 extraction (9), implying that transmembrane sequences exist in the sequence between amino acids 3119 and 4476. This sequence would include M′, M′′, M1, M2, M3, and M4 from the Zorzato model. The 76-kDa C-terminal fragment (4476–5037) could not be extracted into Na2CO3, indicating that it was membrane-associated (9). This fragment would include M5–M10 from the Zorzato model and M1–M4 from the Takeshima model.
Several studies have provided evidence that the N and C termini are located in the cytoplasm (11–13), indicating that an even number of sequences traverse the membrane. Amino acids 4581–4640 between M5 and M6 and 4860–4886 between M8 and M9 have been shown to reside in the lumen (11) and amino acids 4676–4699 between M6 and M7 in RyR2 to reside in the cytoplasm (14).
Three-dimensional reconstruction of RyR1 at 25–30 Å has been carried out using averaged electron microscopic images of large numbers of individual molecules (15–17). From these studies, it has been possible to estimate the mass of protein contained in the transmembrane domains, based on the total volume of the transmembrane domain and the volume occupied by a transmembrane helix (18, 19). The intramembrane domain could accommodate 24–32 transmembrane helices (18), suggesting that the most likely number of transmembrane helices per monomer is between 6 and 8.
In this study, we addressed questions of the number of transmembrane helices and their orientations by creating a progressive series of C-terminal sequence deletions, which started near the beginning or the end of proposed transmembrane sequences in RyR1. In each deletion construct, the enhanced GFP (EGFP) was fused in-frame to form a new C terminus. Our observations using confocal microscopy, alkaline extraction, and proteolysis rule out M′, M′′, M1, M2, and M3 alone as transmembrane sequences. A hairpin loop that includes M4 is formed either as M4a–M4b or, possibly, as M3–M4, which might form a hairpin loop even though M3 alone is not membrane-associated. M5–M6, M7a–M7b, and M8–M10 form transmembrane hairpin loops, with M9 possibly inserted between M8 and M10 to form a selectivity filter.
Experimental Procedures
Materials.
DNA restriction enzymes and modifying enzymes were purchased from New England Biolabs, Fermentas (Hanover, MD), Amersham Pharmacia Biotech, and Roche Molecular Biochemicals. Plasmid DNA for transfection was purified using Qiagen (Valencia, CA) columns. The plasmid EGFP (pEGFP) vectors were from CLONTECH. The expression vector pcDNA 3.1(−) was from Invitrogen. The monoclonal antibody against GFP was from CLONTECH. All other reagents were of reagent grade or highest grade available.
Vector Construction.
Full-length RyR1 and C-terminal deletion mutants were inserted into the pEGFP_N series vectors to form fusion proteins with EGFP at the C terminus (Fig. 1). A HindIII site was created by mutating the last amino acid and the stop codon in RYR1 cDNA. Full-length RYR1 cDNA was then excised with XbaI and HindIII, treated to form a blunt end and ligated in-frame into the SmaI site of pEGFP-N1 to form construct PostM10. Fragments of RYR1 encoding amino acids 1–4888, 1–4771, 1–4628, 1–4556, 1–4186, and 1–3224 were excised with XbaI plus SnaBI, XbaI plus CalI, XbaI plus Bst1107I, XbaI plus NruI, XbaI plus NheI, and XbaI plus AvrII, respectively. These fragments were also blunt ended and ligated in-frame into the SmaI site of pEGFP_N series vectors to form constructs PostM8, PostM6, PostM5, PostM4, PostM2, and PreM1. The insertion of NheI (20), AvrII (20), and Nru I (21) restriction enzyme sites into RyR1 was described earlier. A SnaB I site was created by silent mutagenesis of amino acids Tyr-4888 and Val-4889. Construct PostM3 was obtained by the three-piece ligation of the PmeI [in multiple cloning site (MCS)]–NheI fragment from RYR1 in pcDNA 3.1(−), which encodes amino acids 1–4186; the NheI–SmaI fragment from C10 (21), which encodes amino acids 4187–4302; and pEGFP, which had been linearized by cleavage at the SmaI site. PostM7a was constructed by ligation of the PmeI (MCS)–ClaI fragment from RYR1, which encodes amino acids 1–4771; the ClaI–XmnI fragment from C11 (21), which encodes amino acids 4772–4807; and pEGFP, which had been linearized by cleavage at the SmaI site. The XmnI site in C11 (21) was obtained by mutagenesis of Asn-4805 to Gln to delete the coding sequence from Phe-4807 onward (22). PostM7b was constructed by ligation of the Pme I (MCS)–ClaI fragment from RYR1, which encodes amino acids 1–4771; the ClaI–PvuII fragment from C11 (21), which encodes amino acids 4772–4836; and pEGFP, which had been linearized by cleavage at the SmaI site.
Fig 1.
Prediction of transmembrane sequences of RyR1 and illustration of constructs. (A) The amino acid sequence of rabbit skeletal muscle RyR1 was analyzed by MACVECTOR 6.5.3 (Accelrys, Burlington, MA) with the Argos transmembrane algorithm (28) in the default setting. (Upper) The analysis of the full-length sequence of RyR1. (Lower) The expanded C-terminal region, including 10 potential transmembrane sequences corresponding to those identified in the Zorzato model (5). (B) Scheme for construction of a series of RyR1–EGFP fusion vectors. The white bar represents RyR1 and the black bar represents EGFP. (C) The boundaries of the transmembrane sequences tested in this study are shown and the relative positions for each truncation of RyR1 in the Zorzato model are indicated by X. The construction of these vectors is described in Experimental Procedures.
Expression in HEK-293 Cells and Microsome Preparation.
Fusion proteins were expressed in HEK-293 cells (human embryonic kidney cell line 293) by transfection with 20 μg of each cDNA construct per 10-cm plate, using the standard calcium phosphate transfection protocol (23). After 36–48 h, the cells were harvested and microsomes were prepared as described (24).
Confocal Microscopy.
Mouse embryonic fibroblast (MEF) cells (a kind gift from T. Mak, Ontario Cancer Institute, University of Toronto) or HEK-293 cells were cultured and transfected with the above constructs in 6-well plates (1–4 μg of DNA per well) in which square coverslips were placed. Within 24–36 h after transfection, the wells were washed twice with PBS. Cells on the coverslips were fixed directly with 4% paraformaldehyde for 30 min or permeabilized with 100–200 μg/ml saponin for 30 min, followed by removal of saponin, and continued fixation with 4% paraformaldehyde for 30 min. The coverslips were washed twice with PBS, mounted onto glass microscope slides with 80% (vol/vol) glycerol and 20% PBS, and sealed to the slides with molten agarose. Laser scanning confocal microscopy was performed using a Leica DM IRBE inverted microscope equipped with a Leica TCS SP confocal system. EGFP signals were visualized and recorded through a ×100 oil immersion objective.
Alkaline Extraction.
To determine whether the expressed fusion proteins were associated peripherally or integrally with the membrane, Na2CO3 was used to extract secretory proteins and peripheral membrane proteins without solubilizing membrane proteins (25). Microsomal fractions (40–80 μl) were pelleted with a Beckman 70.1 Ti rotor at 45,000 rpm (186,000 × g) for 30 min; the pellets were resuspended in 200–400 μl of 0.1 M Na2CO3 (pH 11.5) and incubated on ice for 1 h. After centrifugation at 45,000 rpm for 30 min, the pellets were suspended in 1× loading buffer. Supernatants were precipitated with 6% trichloroacetic acid (TCA), and the pellets were suspended in 1× loading buffer.
Protease Digestion.
To determine whether EGFP at the C terminus of truncated fusion proteins was located on the luminal or cytosolic surface, 60–200 μg of the microsomal proteins were treated with trypsin at a protein:trypsin ratio of 6:20. The samples were incubated at 37°C for 1 h before trypsin inhibitor and aprotinin were added at a concentration 10-fold over the trypsin concentration.
After tryptic digestion, insoluble membrane fractions were precipitated by centrifugation at 14,000 × g for 15 min at room temperature. Supernatants were collected, and pellets were washed once with PBS and suspended in 1× loading buffer with 10 μg/ml trypsin inhibitor. The samples were subjected to SDS/PAGE and immunoblotting with an antibody.
Sodium Dodecyl Sulfate PAGE and Immunoblotting.
Samples were separated by 6% or 12% SDS/PAGE according to standard protocols (26). Proteins were detected by a standard Western blotting assay with a primary antibody against EGFP.
Results
Prediction of Transmembrane Helices and Strategy for Designing Constructs.
We analyzed the rabbit skeletal muscle RyR1 amino acid sequence, deduced from its cDNA, using the Argos algorithm (27, 28) for prediction of transmembrane domains (Fig. 1A). Stretches of hydrophobicity, compatible with transmembrane sequences, were found mainly in the last 1,000 aa, although two sequences with relative strong hydrophobicity were found in the central region of the protein. If these sequences formed a hairpin loop, a long sequence would lie in the lumen. No such mass has been observed in the structure of RyR1 (18, 19). In the C-terminal end of RyR1, stretches of amino acids for which the average index for each amino acid is >1 include Glu-4275–Ala-4300 (average index 1.137), Thr-4323–Gly-4363 (1.197), Arg-4557–Ile-4576 (1.241), Gly-4637–Asn-4662 (1.170), Gln-4776–Thr-4825 (1.092), Gly-4834–Val-4854 (1.201), and Leu-4911–Leu-4935 (1.253). These sequences correspond closely to M3, M4, M5, M6, M7, M8, and M10 in the Zorzato model. M1, M2, and M9 are only weakly hydrophobic. With the Argos algorithm, the hydrophobic regions of M4 and M7 are ≈40 and ≈50 aa long, respectively; both sequences are long enough to form a transmembrane hairpin loop.
C-terminal-truncated RyR1–EGFP fusion constructs were designed to analyze membrane sequences in RyR1 (Fig. 1 B and C) as follows: EGFP was attached after the proposed M′–M′′ hairpin loop sequence (PreM1), M1 and M2 (PostM2), M3 (PostM3), M4 (PostM4), M5 (PostM5), M6 (PostM6), M7 (PostM7b), M8 (PostM8), and M10 (PostM10). We also investigated the possibility that amino acids Gln-4776–Thr-4825 (M7) form an independent hairpin loop by placing EGFP after amino acid 4806 in the middle of M7 (PostM7a). We anticipated that EGFP after transmembrane helices would be translated and translocated to the side of the membrane corresponding to the native orientation of the transmembrane sequence, unless the deleted C-terminal sequence had an impact on the topogenesis of the preceding transmembrane sequence.
Determination of the Association of RyR1 Fusion Proteins with Subcellular Membranes.
It has been well documented that full-length RyR1 and its N-terminal RyR1–EGFP fusion proteins are localized to the endoplasmic reticulum (ER) membrane (29, 30). The insertion of our fusion proteins into this organellar membrane was tested first by confocal microscopy of transfected MEF or HEK-293 cells expressing individual fusion proteins. Cells on parallel slides were treated with saponin before fixation to wash out soluble proteins and leave behind those proteins that were integrally associated with membranes or were intrinsically insoluble because they formed cytoskeletal structures or recombinant protein aggregates.
Cells expressing EGFP alone showed a diffuse fluorescence pattern with uniform intensity throughout the cell (Fig. 2A), indicating that EGFP is soluble and is distributed throughout the cytosol and the nucleus. After treatment of the cells with saponin, EGFP fluorescence disappeared, indicating that the fluorescent protein was washed out of transfected cells. In some cells, fluorescence from small aggregates could be visualized (Fig. 2B). In contrast, fluorescence in cells expressing the PostM10 fusion protein was localized to the perinuclear region of the cell, forming a typical ER-like network (Fig. 2C), similar to the pattern of immunostaining observed by staining of expressed RyR1 with antibody 34C against RyR1 (not shown). The fluorescence pattern was unchanged after saponin treatment (Fig. 2D). In some cells, perinuclear fluorescence was in sharper contrast and part of the fluorescence outside of the nucleus disappeared, possibly because of some solubilization of the ER by saponin. These results clearly demonstrate that RyR1 protein is retained in nonnuclear organellar compartments that include the ER membrane.
Fig 2.
Confocal microscopy of MEF cells expressing EGFP and RyR–EGFP fusion proteins. (Left) Transfected MEF cells before treatment with saponin. (Right) Cells after treatment with saponin, which permeabilizes the cell membrane so that soluble components can leave the cell (e.g., B, F, H, and J), leaving aggregated (F, H, and J) and membrane proteins in situ (D, L, and N). The designation for each construct is on the left side of the image.
The fluorescence patterns for PreM1 (Fig. 2 E and F), PostM2 (Fig. 2 G and H), and PostM3 (Fig. 2 I and J) were different from that for PostM10 and resembled that observed for EGFP alone, except that there was no obvious association of fluorescence with the nucleus. The subcellular distribution of fluorescence for fusion proteins PostM4 (Fig. 2 K and L) and PostM5 (Fig. 2 M and N) was very similar to that for PostM10, in the absence and presence of saponin. Similar data were obtained for PostM6, PostM7a, PostM7b, and PostM8, but are not shown. These data demonstrate that the entire sequence upstream of amino acid 4302, containing M′, M′′, M1, M2, and M3 is expressed as a soluble protein, whereas those expressed sequences that contain any combination of M4–M10 are targeted to the ER membrane.
Further analysis involved determination of whether these fusion proteins were associated with microsomal fractions. HEK-293 cell homogenates were centrifuged at 8,000 × g, made 0.6 M in KCl, and centrifuged at 186,000 × g for 1 h. The proportion of the fusion protein in the high-speed pellet, representing the microsomal fraction, and in the supernatant, representing the soluble fraction, was determined by SDS/PAGE and Western blotting with EGFP antibody. Each of the fusion proteins was associated with the microsomal fractions (Fig. 3A, lanes 1–7) and with some of the supernatant fractions. However, no protein was found in the supernatant for PostM10 (lane 8), PostM6 (lane 9), or PostM5 (lane 10), or for fusion proteins PostM8, PostM7b, and PostM7a (not shown), even when the blots were probed with another high-affinity antibody 34C (not shown). Less than 5% of the total protein for PostM4 (lane 11) was present in the supernatant. By contrast, >50% of the total protein for PostM3, PostM2, and PreM1 remained in the supernatant. The appearance of PostM3, PostM2, and PreM1 proteins in the membrane fraction (Fig. 3A, lane 5–7) is probably due to the insoluble, intracellular aggregates that can be seen to remain in the cell after saponin permeabilization (Fig. 2), but that are not found in trypsin-digested membrane fragments (Fig. 4B).
Fig 3.
Immunoblotting of RyR1–EGFP fusion proteins. (A) Samples of microsomal preparations from transfected HEK-293 cells were probed with anti-EGFP antibody, as described in Experimental Procedures. Postmitochondrial supernatants from cell homogenates were made 0.6 M in KCl and centrifuged at 186,000 × g to obtain pellet and supernatant fractions. Aliquots of 10 μl from a total of 200 μl of microsomes was dissolved in loading buffer, whereas 100 μl from a total of 5 ml of supernatants was precipitated by 6% trichloroacetic acid (TCA) and resuspended in loading buffer for SDS/PAGE. (B) The microsomes isolated from the transfected HEK-293 cells, described in A, were subjected to extraction with 0.1 M Na2CO3, pH 11.0. Lanes 1–7 show the proteins remaining in the microsomal pellet and lanes 8–14 show proteins in the alkaline extract of the corresponding samples. For the pellet, one-fifth of the sample was used for SDS/PAGE, and for the supernatant, one-half of the sample was precipitated by 6% TCA, resuspended in loading buffer, and used for SDS/PAGE.
Fig 4.
Localization of EGFP in HEK-293 cells transfected with RyR1 fusion protein constructs. Microsomes from transfected HEK-293 cells were digested with trypsin and centrifuged to separate membrane-associated proteins from soluble digests. Proteins in the supernatant (A) and microsomes (B) were subjected to 12% SDS/PAGE and immunoblotted with anti-EGFP antibody. The cartoon on the left in A indicates that EGFP will be released to the cytosol with a normal mass, after tryptic digestion, if it lies in a helical hairpin loop facing the cytosolic side. The cartoon on the left in B indicates that EGFP will remain membrane-bound with an increased mass, after tryptic digestion, if it lies at the luminal end of a single transmembrane helix. In PostM8, PostM7a, and PostM5, EGFP remained mainly in membranes, with only trace amounts in the supernatants. The mass of the EGFP-containing fragment in the pellet was increased in proportion to the mass of the transmembrane and luminal sequence attached. EGFP from other fusion proteins was exclusively in the supernatant. EGFP and EGFP after tryptic digestion are included in the 12th and 11th lanes of A, respectively.
Extraction of proteins with Na2CO3 was also performed (Fig. 3B). Following treatment with 0.1 M Na2CO3, pH 11.5, no protein was extracted into solution for PostM10 (lanes 1 and 8), PostM6 (lanes 2 and 9), or PostM5 (lanes 3 and 10), or for PostM8, PostM7b, and PostM7a (not shown). Less than 5% extraction was observed for PostM4 (lanes 4 and 11), but >50% extraction was observed for PostM3 (lanes 5 and 12), PostM2 (lanes 6 and 13), and PreM1 (lanes 7 and 14).
Topology of EGFP in RyR1 Fusion Proteins in Microsomal Membranes.
All RyR1 fusion proteins were tested for location of EGFP by measurement of their susceptibility to proteolytic digestion in microsomal fractions, as described in Experimental Procedures. EGFP is intrinsically resistant to digestion by proteases (31) and, if translocated to the cytoplasmic side of the membrane, can be detected with a normal mass in the supernatant. If translocated to the lumen, it is protected from digestion and its mass is increased because of its fusion to the protected transmembrane sequence and to any protected linking luminal sequence.
EGFP migrated as a 28-kDa protein (Fig. 4A, lanes 11 and 12). EGFP from PostM5, PostM7a, and PostM8 remained largely in the membrane after trypsin digestion and with molecular mass increased in proportion to the mass calculated for each transmembrane domain and/or linker (7–8 kDa for PostM5 and PostM8, and 2–3 kDa for PostM7a). If the microsomes were treated with Triton X-100 during digestion, there was no increase in molecular mass (not shown). These results indicate that EGFP in these three proteins was located on the luminal side of the membrane, demonstrating that the orientations for M5, M7a, and M8 are from cytoplasm to lumen. EGFP from all of the other fusion proteins was present in the supernatant and had a molecular mass similar to that of wild-type EGFP. These results suggest that EGFP from PreM1, PostM2, and PostM3 is located in the cytosol, because it forms part of a long soluble sequence, whereas EGFP fused to PostM4, PostM6, PostM7b, and PostM10 lies in the cytosol because it is located at the C terminus of a hairpin loop. Thus, the orientations of M4, M6, M7b, and M10 are from lumen to cytoplasm.
Discussion
The ryanodine receptor is an integral and polytopic membrane protein located in the sarco(endo)plasmic reticulum. The synthesis of ER membrane proteins is initiated at the N terminus and the protein is translocated cotranslationally, to the point where the membrane protein becomes integrated into the ER membrane, by machinery shared with secretory proteins (32, 33). In the present study, each nascent protein contained the N-terminal sequence of RyR1, but C-terminal sequences were deleted incrementally and the product was tagged with EGFP. This strategy allowed us not only to examine RyR1 synthesis in stepwise fashion, but also to determine the topology of RyR1 by examining the location of the C-terminal EGFP tag.
We found that RyR1 fusion proteins truncated after amino acid 4302 at the end of M3 were soluble, and that EGFP attached at the C terminus of these fusion proteins remained in the cytosol. These results eliminate M′, M′′, M1, M2, and M3 alone as transmembrane helices, but an ambiguity with respect to M3 will be discussed below. Although M2 is not a transmembrane sequence, it contains amino acids important for channel function (22, 34), indicating that these amino acids must form a part of an important regulatory domain.
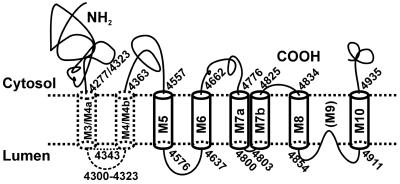
The fusion protein that contained the sequence between amino acids 4302 and 4556, which included M4 and the M4–M5 loop (L45), was membrane associated, as were all longer fusion proteins. We also found that EGFP located after M5, M7a, and M8 was located in the lumen, whereas EGFP located after M4, M6, M7b, and M10 was located on the cytosolic side. These results establish the orientation and the topology of the transmembrane sequences. On the basis of these observations, we propose that RyR1 contains eight transmembrane helices, formed into four transmembrane helical hairpin loops. The composition of the last three hairpin loops is unambiguous; they are formed from M5–M6, M7a–M7b, and M8–M10, as depicted in Fig. 5. The composition of the first hairpin loop is ambiguous, however, because it might be formed either from M4a–M4b or, possibly, from M3–M4.
Fig 5.
Proposal for the transmembrane topology of rabbit skeletal muscle RyR1. Possible boundaries for eight transmembrane sequences with cytosolic N and C termini are shown. The first two cylinders with dashed lines indicate the tentative nature of the composition of the first predicted helical hairpin loop (M3–M4 or M4a–M4b) in the transmembrane sector of RyR1. The numbers, M3–M10, inside each transmembrane sequence are those proposed in the Zorzato model (5). The long M7 sequence is now designated as M7a and M7b. The proposed selectivity filter between M8 and M10 is designated as “(M9),” even though it is clearly not a transmembrane sequence.
We have shown that M5 is a membrane sequence with an N-terminal-cytoplasmic to C-terminal-luminal orientation (Fig. 4B). We have also shown that EGFP in PostM4, truncated just before M5, is associated with organellar membranes (Fig. 2 K and L) and is located on the cytosolic side of the membrane (Fig. 4A). Thus, any transmembrane sequences upstream of M5 must exist as hairpin loops. M4 is predicted as a potential transmembrane sequence of ≈40 aa, long enough to form a hairpin loop. Because the cDNA encoding the M3–M4 sequence is so GC-rich, we were foiled in our attempts to introduce a truncation and a tag near amino acid 4343, which might be predicted as the C-terminal end of M4a. In the absence of the critical information that would have been gained from such an experiment, we cannot rule out the possibility that M3, which does not act as a signal anchor sequence by itself, may act as a signal anchor when paired with M4 as a stop transfer sequence.
It is well documented that some individual transmembrane sequences in membrane proteins have only weak topogenic function and require neighboring transmembrane sequences to achieve both integration into the membrane and proper orientation (35, 36). Comparable observations to those that we have made concerning the insertion of the first hairpin were made in a topological study of the inositol 1,4,5-trisphosphate receptor (IP3R) (37). If the protein was truncated from the C terminus, it became soluble before deletion of a sequence later deduced to be the first transmembrane sequence. Provided that the truncated protein contained both the first and the second transmembrane sequences, it was targeted to the ER membrane (37).
The first hairpin loop in RyR1, whether it consists of M3–M4 or M4a–M4b, does not appear to be critical for Ca2+ release channel function. Deletion of amino acids 4274–4535, containing M3 and M4, did not disrupt channel function, measured in terms of [3H]ryanodine binding and caffeine-induced Ca2+ release in intact HEK-293 cells (21). That study implies that the hairpin loop does not form either the core of the channel conduction pore or a vital regulatory domain. M3 is very poorly conserved among RyR isoforms and among species. Indeed, it is very short in RyR3 and in RyR from Drosophila. M4 is conserved as a long hydrophobic sequence, but not in terms of amino acid identity.
In our model of the topology of the transmembrane sequences in RyR1 (Fig. 5), we have included a hairpin loop in the M3–M4 region, but it is dashed to indicate the ambiguity of its composition as M3–M4 or M4a–M4b. The topological data for the three other proposed transmembrane hairpin loops are much less ambiguous, and the sequences of the six other transmembrane sequences that make up these hairpins are highly conserved.
Between amino acids 4628 and 4771, only M6 has a long enough stretch of hydrophobic amino acids to form a transmembrane helix. Thus, our finding that EGFP was located in the lumen after M5, and in the cytoplasm both before M5 and after M6, clearly indicates that M5 and M6 are transmembrane helices that form a helical loop, with M5 running from the cytoplasm to the lumen and M6 running from the lumen to the cytoplasm.
Although it has been questioned whether M7 is a transmembrane helix (11), it graphs as a long hydrophobic sequence, with a dip near its center (Fig. 1). Although the excessive length of M7 was noted earlier (5), inclusion of M9 in the original prediction and the constraints imposed by an even number of transmembrane helices led to the prediction of M7 as a single transmembrane sequence. However, with a 50-aa hydrophobic span, it could easily traverse the membrane twice.
In this study, we have provided evidence not only that M7 is a transmembrane sequence, but also that it forms a helical hairpin loop. In PostM7a, a construct only 35 aa longer than PostM6, EGFP was translocated to the lumen, preventing its proteolysis by trypsin. In PostM7b, only 30 aa longer than PostM7a, EGFP was dissociated from the membrane after digestion by trypsin, indicating that it was located on the cytoplasmic side. On the basis of the Argos algorithm (Fig. 1) and our experimental evidence, we predict that the 50-aa sequence, Gln-4776–Thr-4825, forms two transmembrane helices with a relatively short luminal loop (Fig. 5). Sequence M7b, like sequences M2 and M10 sequences (11), contains at least two residues, Asn-4806 and Asp-4815, that are critical to channel function.
The sequence corresponding to the C terminus of M7a and all of M7b in RyR1 is conserved among RyR and IP3R molecules, but in IP3R, it is designated as M4. According to the current six-helix topology model of IP3R, M4 in IP3R forms only a single helix, because a tag positioned before this sequence in a biosynthetic experiment was located in the lumen, whereas a tag positioned after this sequence was located in the cytoplasm (37).
The M8–M10 region is of great interest because it may form a structure that corresponds to the structure of a tetrameric bacterial K+ channel (38). The basic K+ channel pore contains eight transmembrane helices in the form of a homotetramer of hairpin loops. The sequence that lies between the transmembrane helices that form the hairpin loops lines the inner half of the channel and has two functional components: a short, oriented helix that provides a dielectric field for cation stabilization in the inner vestibule, and an extended structure that acts as a selectivity filter. In tetrameric RyR1, M8 and M10 may form the helical hairpin loops and M9 may provide the pore lining for the corresponding Ca2+ release channel.
Our observations are fully consistent with the correspondence between the M8–M9–M10 sequence and the sequence of the K+ channel monomer. In our experiments, EGFP was located in the cytoplasm just before M8 and after M10, but it was located in the lumen after M8, suggesting that M9 is not a transmembrane helix and that M8–M10 constitute a helical hairpin loop, in which M9 may act as a pore-forming sequence. In support of the view that M9 is, indeed, the ion-conducting pore, mutations of many of the highly conserved amino acids in the short sequence GVRAGGIGD4831 in the M9 region of RyR2 (39, 40) and in the corresponding region of RyR1 (41) had diverse effects on such channel functions as caffeine-induced Ca2+ release, ryanodine binding, single channel conductance and modulation, and cation selectivity.
Acknowledgments
We thank Dr. Tak Mak for the kind gift of MEF cell lines and Dr. N. Michael Green (Medical Research Council, Mill Hill, U.K.) for critical reading of the manuscript. This work was supported by Canadian Institutes of Health Research Grant MT-3399 (to D.H.M.).
Abbreviations
RyR, ryanodine receptor
RyR1, RyR2, and RyR3, RyR isoforms 1, 2, and 3, respectively
IP3R, inositol 1,4,5-trisphosphate receptor
HEK-293 cells, human embryonic kidney cell line 293
MEF, mouse embryonic fibroblast
EGFP, enhanced GFP
pEGFP, plasmid EGFP
ER, endoplasmic reticulum
References
- 1.Fleischer S. & Inui, M. (1989) Annu. Rev. Biophys. Biophys. Chem. 18, 333-364. [DOI] [PubMed] [Google Scholar]
- 2.Franzini-Armstrong C. & Protasi, F. (1997) Physiol. Rev. 77, 699-729. [DOI] [PubMed] [Google Scholar]
- 3.Lai F. A., Misra, M., Xu, L., Smith, H. A. & Meissner, G. (1989) J. Biol. Chem. 264, 16776-16785. [PubMed] [Google Scholar]
- 4.Takeshima H., Nishimura, S., Matsumoto, T., Ishida, H., Kangawa, K., Minamino, N., Matsuo, H., Ueda, M., Hanaoka, M., Hirose, T. & Numa, S. (1989) Nature 339, 439-445. [DOI] [PubMed] [Google Scholar]
- 5.Zorzato F., Fujii, J., Otsu, K., Phillips, M., Green, N. M., Lai, F. A., Meissner, G. & MacLennan, D. H. (1990) J. Biol. Chem. 265, 2244-2256. [PubMed] [Google Scholar]
- 6.Kyte J. & Doolittle, R. F. (1982) J. Mol. Biol. 157, 105-132. [DOI] [PubMed] [Google Scholar]
- 7.Brandt N. R., Caswell, A. H., Brandt, T., Brew, K. & Mellgren, R. L. (1992) J. Membr. Biol. 127, 35-47. [DOI] [PubMed] [Google Scholar]
- 8.Marks A. R., Fleischer, S. & Tempst, P. (1990) J. Biol. Chem. 265, 13143-13149. [PubMed] [Google Scholar]
- 9.Chen S. R., Airey, J. A. & MacLennan, D. H. (1993) J. Biol. Chem. 268, 22642-22649. [PubMed] [Google Scholar]
- 10.Callaway C., Seryshev, A., Wang, J. P., Slavik, K. J., Needleman, D. H., Cantu, C., III, Wu, Y., Jayaraman, T., Marks, A. R. & Hamilton, S. L. (1994) J. Biol. Chem. 269, 15876-15884. [PubMed] [Google Scholar]
- 11.Grunwald R. & Meissner, G. (1995) J. Biol. Chem. 270, 11338-11347. [DOI] [PubMed] [Google Scholar]
- 12.Marty I., Villaz, M., Arlaud, G., Bally, I. & Ronjat, M. (1994) Biochem. J. 298, 743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z., Zhang, J., Sharma, M. R., Li, P., Chen, S. R. & Wagenknecht, T. (2001) Proc. Natl. Acad. Sci. USA 98, 6104-6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tunwell R. E., Wickenden, C., Bertrand, B. M., Shevchenko, V. I., Walsh, M. B., Allen, P. D. & Lai, F. A. (1996) Biochem. J. 318, 477-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagenknecht T., Grassucci, R., Frank, J., Saito, A., Inui, M. & Fleischer, S. (1989) Nature 338, 167-170. [DOI] [PubMed] [Google Scholar]
- 16.Radermacher M., Rao, V., Grassucci, R., Frank, J., Timerman, A. P., Fleischer, S. & Wagenknecht, T. (1994) J. Cell Biol. 127, 411-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serysheva I. I., Orlova, E. V., Chiu, W., Sherman, M. B., Hamilton, S. L. & van Heel, M. (1995) Nat. Struct. Biol. 2, 18-24. [DOI] [PubMed] [Google Scholar]
- 18.Wagenknecht T. & Radermacher, M. (1995) FEBS Lett. 369, 43-46. [DOI] [PubMed] [Google Scholar]
- 19.Stokes D. L. & Wagenknecht, T. (2000) Eur. J. Biochem. 267, 5274-5279. [DOI] [PubMed] [Google Scholar]
- 20.Tong J., Oyamada, H., Demaurex, N., Grinstein, S., McCarthy, T. V. & MacLennan, D. H. (1997) J. Biol. Chem. 272, 26332-26339. [DOI] [PubMed] [Google Scholar]
- 21.Du G. G., Khanna, V. K. & MacLennan, D. H. (2000) J. Biol. Chem. 275, 11778-11783. [DOI] [PubMed] [Google Scholar]
- 22.Du G. G. & MacLennan, D. H. (1998) J. Biol. Chem. 273, 31867-31872. [DOI] [PubMed] [Google Scholar]
- 23.Chen C. & Okayama, H. (1987) Mol. Cell. Biol. 7, 2745-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maruyama K. & MacLennan, D. H. (1988) Proc. Natl. Acad. Sci. USA 85, 3314-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steck T. L. & Yu, J. (1973) J. Supramol. Struct. 1, 220-232. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli U. K. (1970) Nature 227, 680-685. [DOI] [PubMed] [Google Scholar]
- 27.Mohana Rao J. K. & Argos, P. (1986) Biochim. Biophys. Acta 869, 197-214. [DOI] [PubMed] [Google Scholar]
- 28.Persson B. & Argos, P. (1994) J. Mol. Biol. 237, 182-192. [DOI] [PubMed] [Google Scholar]
- 29.Treves S., Pouliquin, R., Moccagatta, L. & Zorzato, F. (2002) Cell Calcium 31, 1-12. [DOI] [PubMed] [Google Scholar]
- 30.Bhat M. B. & Ma, J. (2002) J. Biol. Chem. 277, 8597-8601. [DOI] [PubMed] [Google Scholar]
- 31.Bokman S. H. & Ward, W. W. (1981) Biochem. Biophys. Res. Commun. 101, 1372-1380. [DOI] [PubMed] [Google Scholar]
- 32.Blobel G. (1980) Proc. Natl. Acad. Sci. USA 77, 1496-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hegde R. S. & Lingappa, V. R. (1997) Cell 91, 575-582. [DOI] [PubMed] [Google Scholar]
- 34.Chen S. R., Ebisawa, K., Li, X. & Zhang, L. (1998) J. Biol. Chem. 273, 14675-14678. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J. T. (1996) Mol. Biol. Cell 7, 1709-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ota K., Sakaguchi, M., Hamasaki, N. & Mihara, K. (1998) J. Biol. Chem. 273, 28286-28291. [DOI] [PubMed] [Google Scholar]
- 37.Galvan D. L., Borrego-Diaz, E., Perez, P. J. & Mignery, G. A. (1999) J. Biol. Chem. 274, 29483-29492. [DOI] [PubMed] [Google Scholar]
- 38.Doyle D. A., Morais Cabral, J., Pfuetzner, R. A., Kuo, A., Gulbis, J. M., Cohen, S. L., Chait, B. T. & MacKinnon, R. (1998) Science 280, 69-77. [DOI] [PubMed] [Google Scholar]
- 39.Du G. G., Guo, X., Khanna, V. K. & MacLennan, D. H. (2001) J. Biol. Chem. 276, 31760-31771. [DOI] [PubMed] [Google Scholar]
- 40.Zhao M., Li, P., Li, X., Zhang, L., Winkfein, R. J. & Chen, S. R. (1999) J. Biol. Chem. 274, 25971-25974. [DOI] [PubMed] [Google Scholar]
- 41.Gao L., Balshaw, D., Xu, L., Tripathy, A., Xin, C. & Meissner, G. (2000) Biophys. J. 79, 828-840. [DOI] [PMC free article] [PubMed] [Google Scholar]