Abstract
Conformational movement of the C-terminal α7 helix in the integrin inserted (I) domain, a major ligand-binding domain that adopts an α/β Rossmann fold, has been proposed to allosterically regulate ligand-binding activity. Disulfide bonds were engineered here to reversibly lock the position of the α7 helix in one of two alternative conformations seen in crystal structures, termed open and closed. Our results show that pairs of residues with Cβ atoms farther apart than optimal for disulfide bond stereochemistry can be successfully replaced by cysteine, suggesting that backbone movement accommodates disulfide formation. We also find more success with substituting partially exposed than buried residues. Disulfides stabilizing the open conformation resulted in constitutively active αMβ2 heterodimers and isolated αM inserted domains, which were reverted to an inactive form by dithiothreitol reduction. By contrast, a disulfide stabilizing the closed conformation resulted in inactive αMβ2 that was resistant to activation but became activatable after dithiothreitol treatment.
Keywords: cell adhesion, protein design, CD11b, Mac-1, VWA domain
Nine of 18 integrin α subunits in vertebrates contain an inserted (I) domain of ≈200 residues that is implicated in binding to protein ligands (1–3). The I domain adopts a VWA domain α/β fold with a central hydrophobic β-sheet surrounded by α-helices. The “top” face of the domain is implicated in ligand binding and contains a metal ion-dependent adhesion site (MIDAS) with a bound Mg2+ ion. I domain structures have revealed two different conformations, the liganded open conformation and the unliganded closed conformation (4, 5). Ligation of the MIDAS metal ion by ligand or a ligand-mimetic lattice contact is associated with a rearrangement of metal ion coordination at the MIDAS and a linked 10-Å movement of the C-terminal helix down the body of the domain (5, 6). The transition from the closed to the open conformation upon ligand binding is thought to convey outside-in signaling from the I domain to the rest of the receptor by means of the downward shift of the C-terminal α7 helix of the I domain.
The transition from the closed to the open conformation has been shown to regulate affinity for ligand and suggested to function in inside-out signaling to the I domain from the rest of the receptor. Structure-oriented mutagenesis approaches have been taken to stabilize one conformation over another and to address the functional significance of alternative conformations of the αM I domain (7, 8). One approach used the computational algorithm ORBIT to stabilize αM I domains in open or closed conformations by finding mutations in the hydrophobic core that minimize the energy of either the open or closed conformation (7). The designed open I domains, which contained 8–13 mutations in the hydrophobic core, showed increased binding to ligand both in intact αMβ2 and when expressed as isolated I domains on the cell surface. By contrast, the designed closed I domain was inactive. Another study mutated Ile-316, which is in the last one-half of the C-terminal α7 helix of the αM I domain and is packed in a hydrophobic pocket at the bottom of the I domain in the closed but not in the open conformation (8). Packing of Ile-316 in the pocket was predicted to favor the closed conformation. Mutation of the isoleucine to glycine resulted in increased ligand-binding affinity.
Here we test the effect on ligand-binding activity of reversibly locking the αM I domain in alternative conformations with disulfide bonds. Furthermore, we compare residues mutated to cysteine for side-chain burial and pairwise distance to determine the factors that influence the ability to successfully engineer conformation-specific disulfide bonds. Previously, disulfide bonds were engineered that lock the αL I domain in either the open or the closed conformation (9, 10). Only one disulfide bond was tested to stabilize each conformation. Each pair of residues mutated to cysteine had Cβ atom distances ideal for disulfide formation. Each mutation stabilized the desired conformational state; however, whereas the activating effect of the open mutant was reversible with reduction (10), the deactivating effect of the closed mutant was not (unpublished data). We find that introduction of a pair of cysteines equivalent to those used in the open mutant of αL is disruptive and does not activate the αM I domain. Nonetheless, we are able to find other pairs of activating and deactivating cysteine substitutions and provide evidence that their functional effects are reversed by disulfide reduction. Our results show that the search space for finding conformation-specific disulfide bonds can be expanded markedly beyond optimal Cβ–Cβ atom distances into a range in which backbone movement is required to accommodate disulfide bond formation. Our results also suggest that boundary residues are favored over buried residues for disulfide-forming cysteine substitutions that have predictable effects on affinity. Furthermore, we engineer disulfide bonds that bridge not only from α7 to β6 but also from α7 to α1. We provide a demonstration of reversibly locking an I domain in the closed conformation and a demonstration that the αM I domain can be reversibly locked in the open conformation. The results generalize and markedly expand the scope for engineering conformation-specific disulfide bonds.
Methods
cDNA Construction and Expression.
Overlap-extension PCR (11) was used to generate cysteine-substitution mutations in the αM I domain. Wild-type human intact αM (12) and wild-type αM I domain platelet-derived growth factor receptor cDNA constructs (7) in vector pcDNA 3.1/hygro were used as templates. Transient transfection of 293T and stable transfection of K562 cells were as described (7, 13).
Flow Cytometry and Adhesion Assay.
Immunofluorescence flow cytometry using indirect staining with fluoresceinated anti-mouse IgG was as described (7, 13). Cell adhesion to immobilized iC3b, a cleaved form of the complement component C3, in flat 96-well plates was as described (7, 13).
Results
Prediction of Disulfide Bonds to Stabilize the αM I Domain in Either the Open or the Closed Conformation.
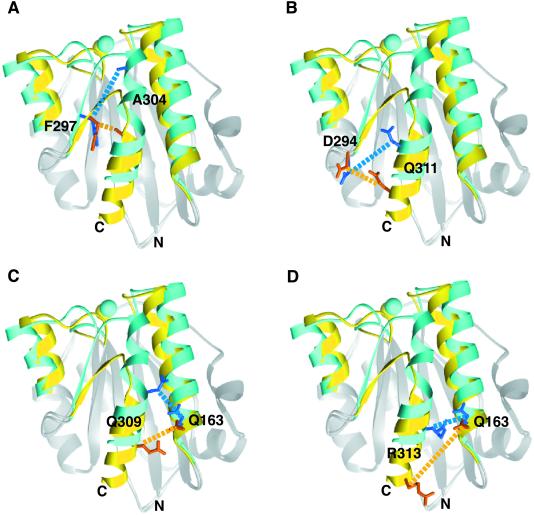
Regions of the αM I domain that differ between the open and closed conformations (4, 5) were visually inspected by using molecular graphics for positions in which pairs of cysteines could be introduced that would form disulfides that would favor one conformation over the other. Thus, the region of the conformationally mobile C-terminal α7 helix and the preceding loop were examined for positions in which one cysteine could be introduced, and structurally adjacent regions were searched for positions where a second cysteine could be introduced that would form a disulfide. Pairs of residues whose side chains face toward one another were chosen. The distance between the Cβ atoms of each of these residue pairs was measured in both the open and the closed conformation (Table 1). We found three pairs of cysteine substitutions where the Cβ–Cβ distances were more favorable for disulfide formation in the open conformation than in the closed conformation: F297C/A304C, Q163C/Q309C, and D294C/Q311C (Table 1 and Fig. 1 A–C), and we found one pair of cysteine substitutions where the Cβ–Cβ distances were more favorable for disulfide formation in the closed conformation than in the open conformation: Q163C/R313C (Table 1 and Fig. 1D). Notably, all substitutions except F297C/A304C involved Cβ–Cβ distances larger than the optimal distance of 3.4–4.2 Å (14) (Table 1) and thus would require backbone readjustments to accommodate disulfide formation.
Table 1.
Cβ distances and solvent-accessible surface of mutated residues in alternative conformations
| Mutations
|
Cβ distance, Å | Residue
|
Accessibility, % | ||
|---|---|---|---|---|---|
| Open | Closed | Open | Closed | ||
| Locked open | |||||
| Q163C/Q309C | 6.4 | 7.2 | Q163 | 50 | 41 |
| Q309 | 18 | 16 | |||
| D294C/Q311C | 7.1 | 10.0 | D294 | 40 | 40 |
| Q311 | 26 | 54 | |||
| F297C/A304C | 3.8 | 10.2 | F297 | 10 | 8 |
| A304 | 0 | 23 | |||
| Locked closed | |||||
| Q163C/R313C | 13.3 | 5.2 | Q163 | 50 | 41 |
| R313 | 86 | 20 | |||
Fig 1.
Spatial relationships of residues substituted for cysteine in the open and closed conformations of the αM I domain. Superimposed structures of open (PDB ID code , ref. 4) and closed (PDB ID code , ref. 5) αM I domains are shown as ribbon diagrams. Backbone regions where conformational changes are significant are shown in yellow (open) and blue (closed). Other backbone regions are gray. MIDAS metal ions are shown as spheres (open, yellow; closed, blue). Side chains of the residues mutated to cysteines are in orange (open) and dark blue (closed). Cβ–Cβ distances are shown as dashed lines in orange (open) and blue (closed). The structures were superimposed by using residues 132–141, 166–206, 211–241, 246–270, and 287–294.
Functional Analysis of Locked Open Mutants in Intact αMβ2 Heterodimers.
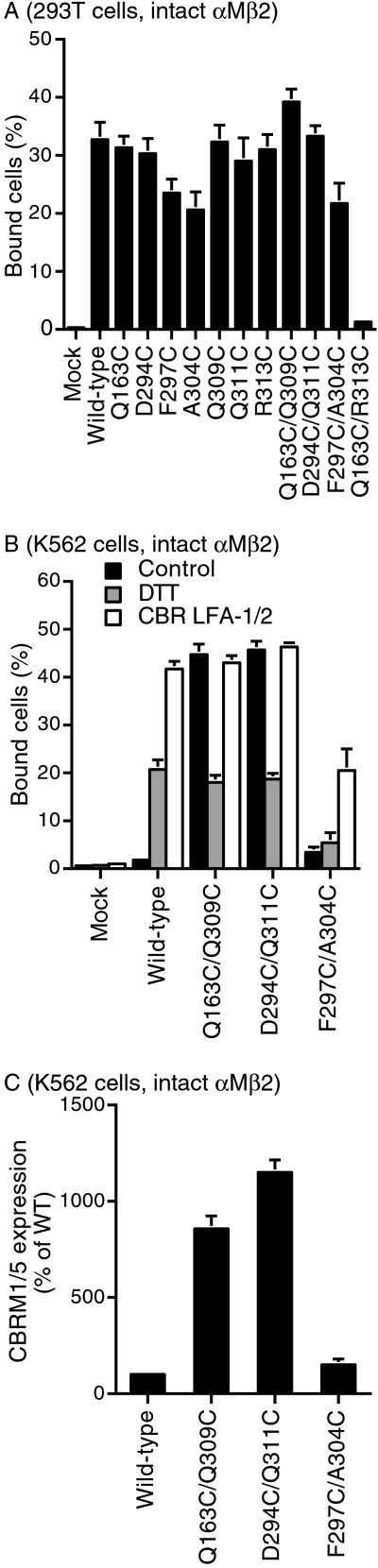
αMβ2 heterodimers containing wild-type or mutant I domains were transiently expressed in 293T cells, in which even wild-type αMβ2 has basal activity for ligand binding (7, 13). Transfectants were tested for adhesion to iC3b-coated substrates. All but two of the single-cysteine substitutions bound ligand as well as did wild type. The exceptions were mutations F297C and A304C, which partially impaired ligand binding (Fig. 2A). Of the three double mutations designed to stabilize the open conformation of the I domain, Q163C/Q309C and D294C/Q311C bound ligand as well as did wild type (Fig. 2A). By contrast, F297C/A304C was less active than wild type (Fig. 2A).
Fig 2.
Ligand binding and expression of activation epitopes by αMβ2 cysteine-substitution mutants. (A) Binding of 293T transient transfectants to immobilized iC3b. Mock, transfected with empty vector. (B) Binding of K562 stable transfectants to immobilized iC3b. Binding of the transfectants was determined in L15/FBS that contained Mg2+ and Ca2+ in the presence of 10 μg/ml of the activating mAb CBR LFA-1/2, control nonbinding IgG X63, or 10 mM DTT as indicated. (C) Expression of CBRM1/5 activation epitope by K562 transfectants. Binding of the CBRM1/5 mAb was determined by flow cytometry as specific mean fluorescence intensity and is expressed as a percentage of wild type. All results are mean ± SEM of three independent experiments with duplicate samples.
To further characterize the mutants, stable transfectants were made in K562 cells, in which wild-type αMβ2 shows little basal ligand-binding activity (7, 13). αMβ2 containing the wild-type I domain showed little binding to ligand (Fig. 2B) and little expression of the CBRM1/5 activation epitope (15) (Fig. 2C). By contrast, αMβ2 containing open mutations Q163C/Q309C and D294C/Q311C showed strong binding to ligand and marked expression of the CBRM1/5 epitope (Fig. 2 B and C). By contrast, the F297C/A304C mutant was inactive, correlating with the decreased activity of the individual F297C and A304C and double F297C/A304C mutations in 293T cells.
Reduction with DTT was used to reverse the engineered disulfide bonds. DTT generally augments adhesiveness by wild-type integrins, probably by reducing certain disulfide bonds that help restrain integrins in a resting conformation (10, 16, 17). Adhesion through wild-type αMβ2 was increased by DTT (Fig. 2B). However, DTT treatment did not increase adhesion through αMβ2 to the same level as achieved by activation with CBR LFA-1/2 mAb, or by the Q163C/Q309C or D294C/Q311C mutations in the absence of DTT (Fig. 2B). DTT treatment of the open Q163C/Q309C and D294C/Q311C mutants reduced adhesion, to the same level as DTT-treated wild-type αMβ2 transfectants. This result provides functional evidence that reversal of the introduced disulfide bond in these mutants abolishes its enhancement of adhesiveness.
Functional Analysis of Locked Closed I Domains in Intact αMβ2 Heterodimers.
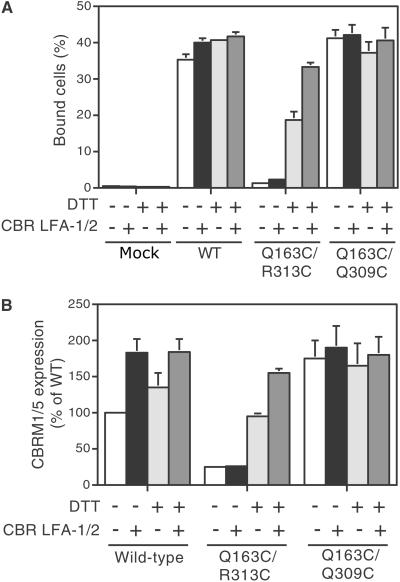
αMβ2 containing the closed Q163C/R313C mutation did not bind to ligand, whereas wild type as well as the corresponding Q163C and R313C single-cysteine substitutions bound ligand strongly in 293T transfectants (Fig. 2A). Even after activation by CBR LFA-1/2 mAb, αMβ2 containing the closed Q163C/R313C mutation failed to bind to ligand (Fig. 3A). By contrast, DTT treatment of Q163C/R313C αMβ2 transfectants restored ligand-binding activity, and this mutant became susceptible to activation by CBR LFA-1/2 mAb (Fig. 3A). All mutants studied here bound CBR LFA-1/2 mAb similarly to wild type, whether or not treated with DTT (not shown). This result provides functional evidence that a disulfide formed in the Q163/R313C mutant restrained αMβ2 in an inactive conformation.
Fig 3.
Ligand binding and expression of activation epitope by 293T transfectants expressing mutant αMβ2. (A) Binding of transfectants was determined in L15/FBS medium that contains Mg2+ and Ca2+ in the presence of 10 μg/ml of the activating mAb CBR LFA-1/2 or control nonbinding IgG X63, and in the presence or absence of 10 mM DTT as indicated. (B) Expression of the CBRM1/5 activation epitope. Binding of CBRM1/5 mAb was determined by flow cytometry. Cells were stained with phycoerythrin-labeled CBRM1/5 mAb or phycoerythrin-labeled control IgG at 37°C in the presence of CBR LFA-1/2 mAb (+) or X63 control IgG1 (−), and in the presence or absence of 10 mM DTT as indicated. Values are specific mean fluorescence intensity and are expressed as a percentage of wild type (WT). Results are mean ± SEM of three independent experiments with duplicate samples.
Consistent with these results, the expression of the CBRM1/5 activation epitope in αMβ2 containing the closed Q163C/R313C mutation was lower than that in wild-type αMβ2 and was not increased by activation with CBR LFA-1/2 mAb (Fig. 3B). However, after DTT treatment, CBRM1/5 epitope expression was increased and was further enhanced by CBR LFA-1/2 mAb (Fig. 3B).
Functional Analysis of Mutant I Domains in Isolation.
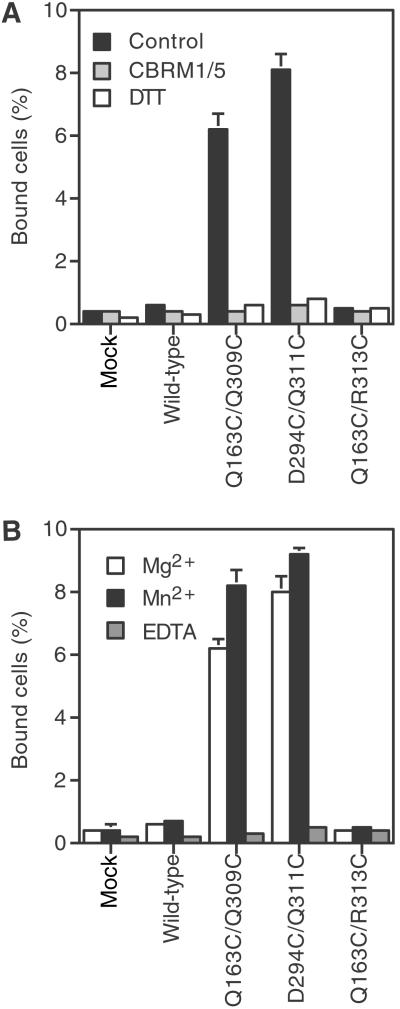
To examine ligand binding by the mutant I domains in isolation from other integrin domains, αM I domains containing the open Q163C/Q309C, open D294C/Q311C, and closed Q163C/R313C mutations were expressed on K562 cells after fusion to an N-terminal signal sequence and a C-terminal platelet-derived growth factor receptor transmembrane domain (7). Wild-type and closed mutant Q163C/R313C isolated I domains showed essentially no binding to iC3b (Fig. 4). By contrast, open mutant Q163/Q309C and D294C/Q311C isolated I domains expressed at the same level on the cell surface gave robust binding to iC3b (Fig. 4). Binding of the open Q163C/Q309C and D294C/Q311C isolated αM I domain mutants was equivalent to that of the isolated αM I domain mutant ido1r, which is stabilized in the open conformation by hydrophobic core substitutions (7) (data not shown). Complete inhibition by CBRM1/5 (Fig. 4A) or EDTA (Fig. 4B) shows that ligand binding is specific to the αM I domain MIDAS. Binding was equivalent in Mg2+ and Mn2+ (Fig. 4B).
Fig 4.
Ligand binding by the isolated cell surface αM I domains. (A) Effects of inhibitory mAb and disulfide reduction by DTT. Binding of K562 stable transfectants expressing mutant or wild-type isolated I domains was examined in L15/FBS containing Mg2+ and Ca2+ in the presence of the inhibitory I domain mAb CBRM1/5 (10 μg/ml), the control nonbinding IgG X63 (control), or 10 mM DTT. (B) Effect of divalent cations. Binding was performed in Hepes/NaCl/glucose supplemented with 1 mM MgCl2, 1 mM MnCl2, or 2 mM EDTA. All results are mean ± SEM of three independent experiments with duplicate samples.
DTT treatment was used to confirm that a disulfide bond was required for the ligand-binding activity of the open mutant isolated I domains. Binding of open mutant Q163C/Q309C and D294C/Q311C isolated I domains to iC3b was abolished after DTT treatment (Fig. 4A). The wild-type isolated I domain, which contains no disulfide bond, failed to bind to ligand regardless of DTT treatment. These data functionally confirmed that the pairs of cysteines introduced into the Q163C/Q309C and D294C/Q311 mutants formed disulfide bridges that constrained the I domains in the open, high-affinity, conformation and that after reduction the conformation shifted to the closed, low-affinity state.
Discussion
We have demonstrated that the αM I domain can be reversibly stabilized in either the open or the closed conformation by engineered disulfide bonds that link the α7 helix to neighboring regions in the I domain structure. Two of three mutants designed to stabilize the open, high-affinity conformation of the αM I domain were successful. They greatly increased ligand-binding activity in both αMβ2 heterodimers and isolated αM I domains expressed on the cell surface. Furthermore, disulfide reduction decreased ligand binding to wild-type levels. These data are consistent with previous findings that for the wild-type αM I domain the closed conformation is energetically favored over the open conformation (7, 8). Contrasting results were obtained with a mutation designed to introduce a disulfide that would stabilize the αM I domain in the closed conformation. This mutant αMβ2 was constitutively inactive in 293T cells, in which wild-type αMβ2 is constitutively active. αMβ2 containing this locked closed I domain was resistant to activation, but after DTT reduction became competent for ligand binding and became susceptible to further activation with an activating mAb. These results suggest that activation signals to the I domain MIDAS are transmitted by pull-down of the C-terminal α7 helix, which was blocked by constraint with the disulfide bridge in the locked closed I domain, and that communication through this helix was restored after release of the constraint by DTT. Thus, mutations that reversibly inactivate I domains have been demonstrated. Interestingly, small molecule antagonists of αLβ2 have been described that have a similar mechanism of action. These bind in a pocket beneath the α7 helix, interfere with its downward movement, and allosterically block activation of ligand binding (18–20).
Comparisons among the different double-cysteine mutations tested here reveal some principles that may be generally useful in future designs of conformation-specific disulfide bonds. The ideal separation for cysteine Cβ carbons for formation of a disulfide bond is reported to be 3.41–4.25 Å (14). Nonetheless, the two successful open mutants, Q163C/Q309C and D294C/Q311C, spanned larger Cβ distances (6.4 and 7.1 Å), as did the closed mutant Q163C/R313C (5.2 Å) (Table 1). Backbone movement must therefore have occurred to accommodate disulfide formation. The residues 309, 311, and 313 are in the most C-terminal half of the α7 helix. Residue 163 is the last residue in the α1 helix, and residue 294 is in a short α-helical turn preceding the β6 strand. These α-helical segments would be more permissive than β-strands for rigid body movements as well as localized backbone deformation. Because some backbone movements appear to have occurred, it is impressive that the mutants favored the predicted conformations, especially Q163C/Q309C, in which the two Cβ atoms were only 0.8 Å less distant in the open conformation than in the closed conformation (Table 1).
We believe that the failure of the F297C/A304C mutation to stabilize the open conformation is due to disruption of the hydrophobic core. All of the successful designs involved mutations of residues that are more exposed than either F297 or A304 (Table 1). The individual substitutions F297C and A304C were deleterious for ligand binding, in contrast to all of the other individual substitutions. This result suggests that they were individually destabilizing, and the results with the double substitution suggest that in combination they are even more destabilizing. Treatment with DTT did not reverse destabilization by the F297C/A304C mutation, suggesting the deleterious effect is due to disruption of packing rather than disulfide bond formation. Previous work has shown that despite some notable successes, it is difficult to engineer in disulfide bonds that increase the stability of proteins because of disruption of packing interactions and the stereospecific requirements of disulfide bonds (21–23). In αL, the residues equivalent to F297 and A304 are each lysine, implying a markedly different packing environment. This implication is consistent with the finding that replacement of these lysines by cysteines in αL results in a disulfide bond that dramatically stabilizes the open, ligand-binding, conformation (9, 10).
The residues with which design was successful are all boundary residues, i.e., intermediate between hydrophobic core and fully surface-exposed residues. The solvent accessibility of these residues ranges from 18% to 50% (mean = 32%) in the conformation favored by the design, in contrast to Phe-304 and Ala-297, which are 10% and 0% exposed. Boundary residues appear favorable for mutation to disulfide-forming cysteines because their more loosely packed environment can adjust to the stereochemical requirements of the disulfide-bridged cysteine side chains, while at the same time providing partial burial of these largely hydrophobic moieties.
Disulfide bonds have previously been used to study conformational movements within proteins or between subunits, e.g., see refs. 24–26. Current programs used to find pairs of residues that can be mutated to cysteine to form disulfide bonds, e.g. ref. 14, select only those residues with optimal Cα and Cβ distances and stereochemistry. Ironically, none of the successful designs here meet these criteria, whereas the one unsuccessful one does. Our results thus show that the search space for conformation-specific disulfide bond-forming mutations can be expanded to pairs of residues with Cβ atom distances of up to at least 7.1 Å and at the same time suggest that partially buried residues with nonoptimal Cβ atom distances may yield better success than fully buried residues with optimal Cβ distances. The same principles have been used in parallel to successfully introduce multiple conformation-specific disulfide bonds in the αL I domain (27). Therefore, it seems highly likely that stabilizing the open, high-affinity, conformation with disulfide bonds can be extended to all nine vertebrate integrin α subunits that contain I domains, and possibly to the I-like domain present in all integrin β subunits as well. Furthermore, extension to other allosterically regulated proteins that can be expressed in nonreducing environments seems promising.
Acknowledgments
We thank Alison McCormack and Mazen Ferzly for technical assistance and Drs. M. L. Dustin, U. Hommel, and J.-h. Wang for reviewing the manuscript. This work was supported by National Institutes of Health Grant CA31799.
Abbreviations
I, inserted
MIDAS, metal ion-dependent adhesion site
References
- 1.Dickeson S. K. & Santoro, S. A. (1998) Cell. Mol. Life Sci. 54, 556-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humphries M. J. (2000) Biochem. Soc. Trans. 28, 311-339. [PubMed] [Google Scholar]
- 3.Shimaoka M., Takagi, J. & Springer, T. A. (2002) Annu. Rev. Biophys. Biomol. Struct. 31, 485-516. [DOI] [PubMed] [Google Scholar]
- 4.Lee J.-O., Rieu, P., Arnaout, M. A. & Liddington, R. (1995) Cell 80, 631-638. [DOI] [PubMed] [Google Scholar]
- 5.Lee J.-O., Bankston, L. A., Arnaout, M. A. & Liddington, R. C. (1995) Structure (London) 3, 1333-1340. [DOI] [PubMed] [Google Scholar]
- 6.Emsley J., Knight, C. G., Farndale, R. W., Barnes, M. J. & Liddington, R. C. (2000) Cell 101, 47-56. [DOI] [PubMed] [Google Scholar]
- 7.Shimaoka M., Shifman, J. M., Jing, H., Takagi, J., Mayo, S. L. & Springer, T. A. (2000) Nat. Struct. Biol. 7, 674-678. [DOI] [PubMed] [Google Scholar]
- 8.Xiong J.-P., Li, R., Essafi, M., Stehle, T. & Arnaout, M. A. (2000) J. Biol. Chem. 275, 38762-38767. [DOI] [PubMed] [Google Scholar]
- 9.Shimaoka M., Lu, C., Palframan, R., von Andrian, U. H., Takagi, J. & Springer, T. A. (2001) Proc. Natl. Acad. Sci. USA 98, 6009-6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu C., Shimaoka, M., Ferzly, M., Oxvig, C., Takagi, J. & Springer, T. A. (2001) Proc. Natl. Acad. Sci. USA 98, 2387-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho S. N., Hunt, H. D., Horton, R. M., Pullen, J. K. & Pease, L. R. (1989) Gene 77, 51-59. [DOI] [PubMed] [Google Scholar]
- 12.Oxvig C., Lu, C. & Springer, T. A. (1999) Proc. Natl. Acad. Sci. USA 96, 2215-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu C. & Springer, T. A. (1997) J. Immunol. 159, 268-278. [PubMed] [Google Scholar]
- 14.Hazes B. & Dijkstra, B. W. (1988) Protein Eng. 2, 119-125. [DOI] [PubMed] [Google Scholar]
- 15.Diamond M. S. & Springer, T. A. (1993) J. Cell Biol. 120, 545-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis G. E. & Camarillo, C. W. (1993) J. Immunol. 151, 7138-7150. [PubMed] [Google Scholar]
- 17.Lynam E. B., Rogelj, S., Edwards, B. S. & Sklar, L. A. (1996) J. Leukocyte Biol. 60, 356-364. [DOI] [PubMed] [Google Scholar]
- 18.Kallen J., Welzenbach, K., Ramage, P., Geyl, D., Kriwacki, R., Legge, G., Cottens, S., Weitz-Schmidt, G. & Hommel, U. (1999) J. Mol. Biol. 292, 1-9. [DOI] [PubMed] [Google Scholar]
- 19.Last-Barney K., Davidson, W., Cardozo, M., Frye, L. L., Grygon, C. A., Hopkins, J. L., Jeanfavre, D. D., Pav, S., Qian, C., Stevenson, J. M., et al. (2001) J. Am. Chem. Soc. 123, 5643-5650. [DOI] [PubMed] [Google Scholar]
- 20.Liu G., Huth, J. R., Olejniczak, E. T., Mendoza, R., DeVries, P., Leitza, S., Reilly, E. B., Okasinski, G. F., Fesik, S. W. & von Geldern, T. W. (2001) J. Med. Chem. 44, 1202-1210. [DOI] [PubMed] [Google Scholar]
- 21.Clarke J., Henrick, K. & Fersht, A. R. (1995) J. Mol. Biol. 253, 493-504. [DOI] [PubMed] [Google Scholar]
- 22.Hinck A. P., Truckses, M. & Markley, J. L. (1996) Biochemistry 35, 10328-10338. [DOI] [PubMed] [Google Scholar]
- 23.Mansfeld J., Vriend, G., Dijkstra, B. W., Veltman, O. R., Van den Burg, B., Venema, G., Ulbrich-Hofmann, R. & Eijsink, V. G. H. (1997) J. Biol. Chem. 272, 11152-11156. [DOI] [PubMed] [Google Scholar]
- 24.Falke J. J. & Koshland, D. E., Jr. (1987) Science 237, 1596-1600. [DOI] [PubMed] [Google Scholar]
- 25.Yu H., Kono, M. & Oprian, D. D. (1999) Biochemistry 38, 12028-12032. [DOI] [PubMed] [Google Scholar]
- 26.Tomishige M. & Vale, R. D. (2000) J. Cell Biol. 151, 1081-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimaoka, M., Xiao, T., Liu, J.-H., Yang, Y., Dong, Y., Jun, C.-D., McCormack, A., Zhang, R., Joachimiak, A., Takagi, J., Wang, J.-h. & Springer, T. A. (2002) Cell, in press. [DOI] [PMC free article] [PubMed]
- 28.Mizuguchi K., Deane, C. M., Blundell, T. L., Johnson, M. S. & Overington, J. P. (1998) Bioinformatics 14, 617-623. [DOI] [PubMed] [Google Scholar]