Abstract
Accurate positioning of the division septum at the equator of Escherichia coli cells requires a rapid oscillation of MinD ATPase between the polar halves of the cell membrane, together with the division inhibitor MinC, under MinE control. The mechanism underlying MinD oscillation remains poorly understood. Here, we demonstrate that purified MinD assembles into protein filaments in the presence of ATP. Incubation with phospholipid vesicles further stimulates MinD polymerization. Addition of purified MinE in the presence of lipids promotes bundling of MinD filaments as well as their disassembly through activation of MinD ATPase. MinE thus provokes a net decay in the steady-state MinD polymer mass. Taken together, our results suggest that reversible MinD assembly modulated by MinE underlies the dynamic processing of positional information in E. coli to identify precisely the nascent site for cell division.
Cell division in Escherichia coli and other rod-shaped bacteria depends on the precise placement of a division septum at the cell center, a process initiated by the assembly of an equatorial ring (Z-ring) of the tubulin-like FtsZ GTPase on the cytoplasmic membrane (1, 2). The Z-ring assembly is spatially restricted to midcell by nucleoid occlusion (3, 4) and by the MinCDE system (5–7). The first mechanism ensures that Z-rings form only in cellular space devoid of nucleoid mass, while the Min system prevents Z-ring assembly at the cell poles.
Although the basis of nucleoid-mediated inhibition of Z-ring assembly remains unclear, MinC is a division inhibitor that has been shown to block Z-ring assembly in vivo (8, 9) and FtsZ polymerization in vitro (10). MinC is recruited to the membrane by MinD ATPase and, in the absence of MinE, the MinCD complex is dispersed throughout the membrane, blocking cell division at all sites in the cell (11–14). In the presence of MinE, however, the midcell site is relieved of MinCD inhibition, allowing normal equatorial septation. The biochemical mechanism underlying such topological regulation remains poorly understood.
Cytological studies have shed light on subcellular localization and dynamics of the Min proteins in E. coli. MinD fused to the GFP localizes into a horseshoe structure on the polar membrane and undergoes a rapid (periodicity ≈50 s) pole-to-pole oscillation in live E. coli cells in a MinE-dependent manner (15, 16). MinC also colocalizes and cooscillates with the MinD horseshoe when MinE is present (12, 13), so that the time-averaged concentration of MinC is lowest at midcell to allow medial division.
Interestingly, MinE localizes both as a ring structure (E-ring) at the edge of the MinCD horseshoe as well as a polar zone that extends along the horseshoe arms (17, 18). The E-ring, together with the MinE polar zone, moves to the nearest cell pole, causing rapid shrinkage of the MinCD horseshoe and forcing coupled oscillation of MinCD and MinE to the opposite cell pole (17, 18). Recently, MinE was shown to stimulate MinD ATPase activity in the presence of phospholipids, and the level of stimulation correlated with the period of MinD oscillation (19).
In this study, we demonstrate that purified MinD polymerizes into protein fibers upon incubation with ATP and phospholipids. Adding purified MinE to the assembly reaction promotes both bundling of MinD filaments as well as their depolymerization. The resultant diminution in the MinD polymer mass ensues from MinE-mediated stimulation of MinD ATPase in the presence of lipids. These results suggest that dynamic MinD assembly regulated by MinE is the biochemical basis for MinD oscillation in E. coli and underscore that protein assembly and protein motion, powered by ATP binding and hydrolysis, evolved in primitive cells as a mechanism for searching and measuring cellular space.
Materials and Methods
Protein Expression and Purification.
His6-tagged Pyrococcus furiosus MinD1 was expressed from pET-28a containing minD1 in BL21(λDE3) cells and purified by using Ni2+- chelate affinity chromatography essentially as described (20). His6-tagged E. coli MinD was expressed from pEMDwt (20) in BL21ΔminCDE:aph(λDE3), and untagged E. coli MinE was expressed from pSLR1 (21) in HMS174(λDE3) cells. A detailed description of protein purification can be found in Supporting Text, which is published as supporting information on the PNAS web site, www.pnas.org.
Preparation of Large Unilamellar Vesicles (LUVs) and Membrane Vesicles.
LUVs were prepared by suspending E. coli phospholipids (Avanti Polar Lipids) in an aqueous buffer (10 mM Tris⋅HCl, pH 7.5/150 mM NaCl/5 mM DTT) with vigorous vortex mixing, followed by multiple freeze–thaw cycles and filtration through stacked polycarbonate filters, essentially as described (22). LUVs (5 mg/ml) were stored at −70°C and warmed to 37°C immediately before use. Membrane vesicles were prepared from E. coli BL21(λDE3) cells by lysozyme treatment and sonication, as described (23).
Polymerization Assay.
MinD protein stocks were centrifuged (25°C) in a TLA 100.2 rotor (Beckman Coulter) at 80,000 rpm for 15 min to remove any preexisting aggregate. Clarified MinD was incubated in 30 μl of buffer F (25 mM Tris⋅HCl, pH 7.5/50 mM KCl/5 mM MgCl2) containing 1 mM of an appropriate nucleotide. Buffered NTP stocks (pH ≈7.0) were used in all assays. When necessary, LUVs (0.5 mg/ml) and purified MinE were included in the reactions. MinD assembly was initiated at 37°C by adding nucleotides, and the reactions were centrifuged exactly as above to sediment the polymers. The supernatants were aspirated carefully, and the pellets were dissolved in 30 μl of buffer F containing 0.025% SDS. The MinD levels in the supernatant and the pellet samples were analyzed by SDS/12% PAGE and Coomassie blue staining. Digital images of the gels were analyzed by using IMAGEQUANT V.1.2 software (Amersham Biosciences) to quantify MinD levels in the bands.
To analyze cosedimentation of MinE with the MinD polymers, the supernatant and the pellet samples were electrophoresed on a 16.5% Tris-Tricine-SDS gel and either blotted onto a poly(vinylidene difluoride) membrane (Bio-Rad Immun-Blot, 0.2 μm) or Coomassie blue stained. The blot was immunostained with rabbit antibody directed against the MinE2–19 peptide (21), and the MinE bands were visualized with goat anti-rabbit secondary antibody-horseradish peroxidase conjugate (Zymed) and enhanced chemiluminescence substrates (Amersham Pharmacia).
ATPase Assay.
MinD ATPase activity was assayed by the malachite green–phosphomolybdate method for determination of inorganic phosphate (24). MinD was incubated at 37°C in buffer F containing LUVs and ATP, and the level of ATPase stimulation was assessed at different MinD:MinE ratios (see Fig. 4, where exact conditions are described in the legend). Reaction aliquots (10 μl) were withdrawn at intervals and quenched with 25 mM EDTA, and the amounts of phosphate resulting from ATP hydrolysis were measured.
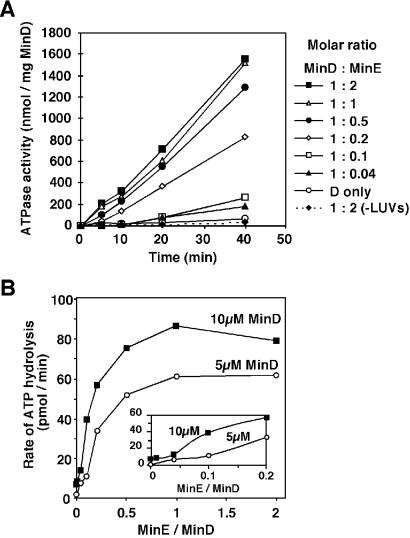
Fig 4.
Stimulation of MinD ATPase activity by MinE. The assembly reactions containing different MinD:MinE molar ratios were set up as in Fig. 3A. At 0 time, ATP was added, and the ATPase activity was determined by measuring the release of inorganic phosphate. The controls were MinD incubated either with LUVs only or with MinE in the absence of LUVs.
Electron Microscopy.
MinD assembly reactions (10 μl) in buffer F (refer to legends in Figs. 2 and 5 for exact conditions) were applied to the carbon surface of ultrathin or regular carbon-coated copper grids (300–400 mesh size, type A; Ted Pella, Inc., Redding, CA). After 10 sec, the drops were blotted off and the grids were stained with 1% uranyl acetate (pH unadjusted) and dried. In some cases, the grids were washed with a drop of water after staining. The grids were examined under a Philips CM-10 electron microscope and photographed at ×11,500–145,000 magnification. Images were digitized on a scanner (Expression 636, EPSON, Long Beach, CA) and processed by using Adobe Systems PHOTOSHOP V.5.5 (San Jose, CA) and Deneba CANVAS V.7 (Miami).
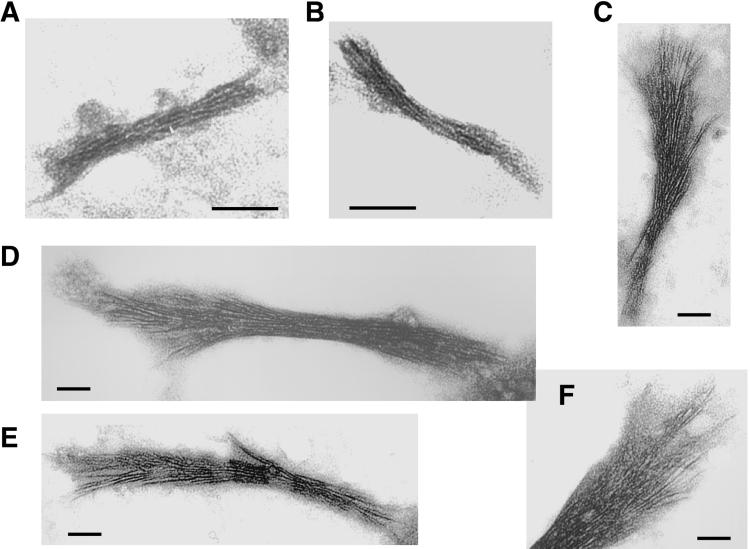
Fig 2.
Analysis of MinD polymers by electron microscopy. (A–E) Polymers of E. coli MinD (5 μM) assembled at 37°C after a 10-min incubation in 20 μl of buffer F. (A and B) Short, thin MinD filaments (arrows) formed with 1 mM ATP. Filaments shown in A and B were photographed at ×21,000 and ×73,000 magnification, respectively. MinD filaments appear two-stranded in B. (C) MinD bundles that assembled in the presence of LUVs (0.5 mg/ml) and 1 mM ATP. The arrows indicate MinD bundles, and the arrowheads show LUVs associated with the bundles. (D and E) Thick MinD bundles (arrow in E) formed in the presence of 1 mM GTP with (E) or without (D) LUVs (arrowhead in E). (F) Polymers of P. furiosus MinD1 (10 μM) assembled in 10 μl of buffer F supplemented with 1 mM ATP, 10 mM CaCl2, E. coli membrane vesicles (350 μg of protein per ml), and 5% glycerol. Incubation was for 5 min at 25°C. The arrow shows a MinD1 filament connected to membrane vesicles (arrowheads). (G) Shape deformation of a membrane vesicle by MinD1 filaments wrapping around it. The image was acquired at ×145,000 magnification. (Bar = 100 nm.)
Fig 5.
Ultrastructure of MinD polymers in the presence of MinE. (A and B) Electron micrographs of MinD polymers formed after a 20-min incubation of E. coli MinD (5 μM) with LUVs and 1 mM ATP at 37°C. (C–F) MinD bundles assembled as in A and B, but with 5 μM MinE present during incubation. All images were acquired at ×73,000 magnification. (Bar = 50 nm.)
Results
MinD Self-Assembly.
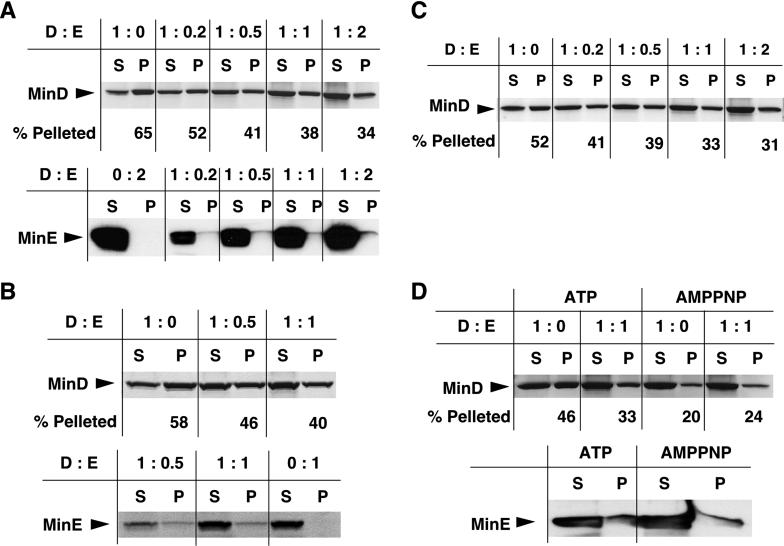
To investigate whether the MinD horseshoe may represent a polymeric structure, we purified MinD from E. coli and assayed in vitro polymerization by high-speed sedimentation. Fig. 1A shows a representative sedimentation profile of MinD under various assembly conditions. E. coli MinD (3.6 μM) sedimented inefficiently in the presence of ATP (lane 1), whereas MinD (in the ADP-bound form; see Materials and Methods and ref. 20) showed a significantly higher sedimentation in the presence of LUVs (lane 2). Inclusion of both LUVs and ATP in the reaction further stimulated MinD sedimentation, with more than two-thirds of the protein being in the pellet fraction (Fig. 1A, lane 3). Because LUVs promoted MinD assembly, all subsequent reactions shown in Fig. 1A contained lipid vesicles. Addition of excess EDTA to a reaction containing ATP reduced MinD assembly by one-third (lane 4), suggesting that chelation of Mg2+ weakens ATP binding by MinD, thus resulting in reduced assembly. The amount of MinD pelleted in the presence of the nonhydrolyzable ATP analog, adenosine 5′-[β,γ-imido]triphosphate (AMPPNP; Fig. 1A, lane 6), was half of that sedimentable with ATP. This may be caused by a lower binding affinity of AMPPNP for MinD. Surprisingly, the amount of MinD pelleted in the presence of GTP (lane 7) was similar to that seen with ATP (lane 2), indicating that nucleotide recognition by MinD has a relaxed specificity.
Fig 1.
MinD polymerization assays. MinD assembly reactions were incubated at 37°C for 10 min, and the polymers were pelleted by high-speed centrifugation (see Materials and Methods). S, supernatant; P, solubilized pellet; M, molecular mass standards. Protein samples were separated by SDS/12% PAGE, and the gels were stained with Coomassie blue. (A) Effect of LUVs, nucleotides, and EDTA (25 mM) on E. coli MinD (3.6 μM) assembly. (B) Effect of different concentrations of LUVs on E. coli MinD (10 μM) assembly. (C) Effect of LUVs and ATP on P. furiosus MinD1 (3.3 and 10 μM) assembly. % Pelleted refers to % MinD in the P fraction of total MinD (S + P) in each sample.
To determine the concentration of LUVs for optimum MinD sedimentation, we carried out a MinD pelleting assay with 10 μM protein and various concentrations of LUVs. As shown in Fig. 1B, the amounts of MinD pelleted rose with increasing phospholipid concentration, with the pelleting efficiency nearly reaching a plateau at 1 mg/ml LUVs. Therefore, our choice of 0.5 mg/ml LUVs for MinD assembly reactions represents an optimum lipid concentration.
To examine whether a distant MinD ortholog from the hyperthermophilic archaeon P. furiosus could also polymerize in vitro, we purified the P. furiosus MinD1 protein whose atomic structure has been solved (ref. 20; P. furiosus contains three MinD paralogs). MinD1 displayed negligible sedimentation in the presence of ATP (Fig. 1C, lanes 1 and 3). In contrast, addition of LUVs to reactions containing 3.3 or 10 μM MinD1 caused ≈7% or ≈26% of the protein to accumulate in the pellet fractions, respectively (Fig. 1C, lanes 2 and 4). These results indicate that the interaction of MinD with lipids may induce a conformational change in the protein that is conducive for nucleation and growth of MinD polymers.
Ultrastructure of MinD Polymers.
The nature of MinD polymers forming under a variety of conditions was investigated by negative-stain electron microscopy. E. coli MinD assembled into short, thin filaments (12–110 nm long, 5–7 nm wide) when incubated with ATP in a simple buffer (Fig. 2 A and B). These short filaments failed to pellet under our centrifugation conditions (Fig. 1A, lane 1). A close inspection of the MinD polymers in Fig. 2B (×73,000 magnification) reveals mostly paired protofilaments. We assume that E. coli MinD has dimensions similar to those of P. furiosus MinD1 (monomer size, 5 × 3.7 × 3 nm; ref. 20). The average width (6 nm) of MinD polymers assembled with ATP (Fig. 2 A and B) suggests that the MinD subunits in the paired protofilaments are laterally bonded across the narrow (3 nm) profile. In that case, the longitudinal subunit spacing along MinD protofilaments is likely to be 5 nm.
Addition of LUVs to the assembly reaction caused MinD to grow into longer and thicker bundles (100–300 nm long, ≈15–21 nm wide) that were associated with lipid vesicles (Fig. 2C). Higher magnification images of two such MinD bundles (width along the bundles ranged between 15 and 21 nm; see Fig. 4 A and B) show approximately five protofilaments across the narrow profile of each bundle. The polymers shown in Fig. 2 A–C were obtained after a 10-min assembly. Identical MinD polymers also formed after a 30-sec incubation (not shown), attesting to a fast in vitro polymerization in tune with the rapid MinD horseshoe assembly seen in vivo (15, 16). This observation suggests that in vitro MinD assembly in the presence of ATP and lipids likely recapitulates its in vivo assembly on the polar cell membrane.
Replacing ATP with GTP allowed MinD assembly. Surprisingly, in contrast to ATP, GTP caused MinD to assemble into bundles (average 35 nm wide) in the absence of lipid vesicles (Fig. 2D). Addition of LUVs led to thicker and ordered MinD bundles (170–300 nm long, average 50 nm wide) emanating radially from the surface of the vesicles (Fig. 2E), confirming that the substantial level of MinD sedimentation in the presence of GTP and LUVs (Fig. 1A, lane 7) indeed represented MinD polymerization.
We also analyzed P. furiosus MinD1 assembly in the presence of E. coli membrane vesicles. Fig. 2F shows bundles of long MinD1 filaments forming in the presence of ATP, with polymers growing on and around membrane vesicles. Fig. 2G shows a membrane vesicle in a state of rectangular deformation caused by MinD1 filaments wrapping around it. These results strongly suggest that lipid-stimulated MinD assembly not only may be relevant for its function in the bacterial cell, but also may be likely to be important for archaeal organisms and for plant chloroplasts (25) because MinD is widely conserved among prokaryotes and plastids (www.ncbi.nlm.nih.gov).
MinE Promotes Disassembly of MinD Filaments.
MinE is essential for both assembly and oscillation of the MinD horseshoe in E. coli (15, 16, 19). Therefore, by using the pelleting assay and different MinD:MinE molar ratios, we investigated whether purified MinE affects MinD assembly in vitro. We first carried out the experiment by preincubating MinD with MinE in the presence of LUVs and then adding ATP to initiate protein assembly. At the MinD:MinE ratio of 1:0.2, there was an ≈20% decrease in the amount of E. coli MinD pelleted compared with the control (Fig. 3A Upper, lanes 1 and 2), whereas the decrease ranged between ≈37% and 52% when the MinD:MinE stoichiometry in the reactions was increased to physiological levels (1:0.5–1:1) or higher (1:2) (Fig. 3A Upper, lanes 3–5). This result shows that MinE can cause a significant reduction in the steady-state MinD polymer mass in vitro. The physiological ratio of MinD:MinE is ≈1:0.7, based on the recent report that the concentrations of MinD and MinE are ≈2,000 and 1,400 molecules per E. coli cell, respectively (26).
Fig 3.
Effect of MinE and AMPPNP on MinD assembly. (A Upper) E. coli MinD (10 μM) was incubated with or without MinE for 5 min, at different molar ratios, in buffer F containing LUVs. Assembly was initiated by adding 1 mM ATP, and the reactions were incubated for 10 min at 37°C. The reactions were centrifuged at high speed for 15 min to pellet the MinD polymers (see Materials and Methods). The supernatant (S) and pellet (P) fractions were analyzed by SDS/12% PAGE and Coomassie blue staining. D and E refer to MinD and MinE, respectively. (A Lower) Aliquots of S and P fractions from the experiment shown in Upper were electrophoresed on a 16.5% Tris-Tricine-SDS gel and Western blotted (lanes 2–5). MinE (20 μM) was incubated in buffer F with LUVs and ATP but without MinD, and the S and P fractions were Western blotted (lane 1). The MinE bands were immunostained with anti-MinE antibody. (B) MinD assembly performed as in A, except that the assembly reactions were incubated for 3 min at 37°C with 200 μM thiol-cleavable crosslinker dithiobis(succinimidyl propionate) (DSP, Pierce) before ultracentrifugation. The S and P fractions were heated at 95°C for 10 min in gel-loading buffer containing 5% 2-mercaptoethanol to reverse the crosslinks before SDS/PAGE. MinD (Upper) and MinE (Lower) bands were visualized by Coomassie blue staining. (C) MinD (10 μM) was incubated in buffer F containing LUVs and ATP for 2 min at 37°C to allow assembly. MinE was then added at different molar ratios, and the reactions were incubated for 10 min at 37°C. The S and P fractions were analyzed as in A Upper. (D) MinD assembly reactions with or without MinE were set up as in A Upper. Assembly was initiated with either ATP or AMPPNP (each at 1 mM). The S and P fractions were analyzed for MinD by Coomassie staining (Upper) and for MinE by immunostaining (Lower).
To examine whether MinE cosedimented with MinD polymers, we performed Western blot analysis with the supernatant and pellet samples from the MinD assembly experiment shown in Fig. 3A. Upon coassembly of MinD and MinE at different protein ratios, a very small fraction of MinE appeared to be associated with the MinD pellets (Fig. 3A Lower, lanes 2–5). Note, however, that when 20 μM MinE was incubated under identical assembly conditions in the absence of MinD, there was no trace of a band in the pellet fraction (Fig. 3A Lower, lane 1). To assess whether the meager amounts of MinE in the pellets could be caused by loss of MinE during high-speed centrifugation, we added the thiol-cleavable crosslinker dithiobis(succinimidyl propionate) (DSP; molecular span = 1.2 nm) to the assembly reactions before centrifugation to preserve the putative MinE contacts with the MinD filaments and reversed the crosslinks before analyzing the supernatant and the pellet fractions. This procedure allowed significantly higher amounts of MinE, cosedimenting with the MinD filaments, to be visible by Coomassie staining (Fig. 3B Lower, lanes 1 and 2), whereas MinE failed to sediment in the absence of MinD (Fig. 3B Lower, lane 3). These results demonstrate that MinE associates physically with the MinD polymers.
To rule out that the diminution in MinD polymer mass, shown in Fig. 3 A and B, resulted from sequestration of MinD monomers by MinE during preincubation, we allowed MinD to assemble first in the presence of ATP and then added MinE to the reactions (Fig. 3C). The levels of MinD polymer decay at MinD:MinE ratios of 1:0.2–1:2 were between ≈20% and 40% (Fig. 3C), indicating that MinE stimulates MinD filament disassembly and is unlikely to block polymerization by sequestering MinD monomers.
MinE-Stimulated ATP Hydrolysis Causes MinD Polymer Disassembly.
To assess whether ATP hydrolysis triggers depolymerization of MinD filaments, we used the nonhydrolyzable analog AMPPNP for MinD assembly. As seen in Fig. 1A, AMPPNP was less proficient than ATP in supporting MinD assembly (Fig. 3D Upper, lane 1 vs. lane 3). In contrast to MinD polymers assembled with ATP, an equimolar amount of MinE did not cause any reduction in the MinD polymer mass assembled with AMPPNP (Fig. 3D Upper, compare lanes 2 and 4). The amount of MinE cosedimenting with the MinD:AMPPNP polymers was similar to that associated with the MinD:ATP filaments (Fig. 3D Lower), indicating that the lack of turnover of MinD:AMPPNP polymers is not caused by their inability to associate with MinE but results from abrogation of ATP hydrolysis.
Cooperative Stimulation of MinD ATPase by MinE.
We examined the kinetics of MinD ATPase activity at three different MinD concentrations over a wide range of MinD:MinE molar ratios. Fig. 4A shows a representative ATPase time-course when 5 μM MinD was allowed to coassemble with MinE (MinD:MinE stoichiometries ranging from 1:0.04 to 1:2), with or without LUVs. Consistent with another report (19), stimulation of MinD ATPase activity by MinE required the presence of phospholipids (Fig. 4A). There was a substantial lag (10 min or more) in ATP hydrolysis at MinD:MinE ratios of 1:0.04 and 1:0.1, but this lag diminished significantly as the ratio was increased to 1:0.2 and higher (Fig. 4A). Essentially similar kinetics of ATP hydrolysis were observed at 2.5 and 10 μM MinD concentrations, or when MinE was added to reactions after initiation of MinD assembly with ATP (data not shown).
Half-maximal stimulation of ATP hydrolysis occurred at a MinD:MinE ratio of 1:0.12 when 10 μM MinD was present in the reaction, whereas this ratio was 1:0.175 for both 5 and 2.5 μM MinD (Fig. 4B; 2.5 μM data not shown). Maximal ATPase stimulation occurred at the MinD:MinE ratio of 1:1 (Fig. 4B). Because of the pronounced lag in MinD ATPase activity at low MinE concentrations and a sharp upward transition in activity as the MinD:MinE ratio was increased from 1:0.1 to 1:0.2 (Fig. 4A), it appears that MinE may cooperatively stimulate ATP hydrolysis. A sigmoidal relationship between ATPase rates and MinE concentrations is revealed in Fig. 4B Inset, which was drawn by using an expanded scale at lower MinD:MinE stoichiometries. This result suggests MinE cooperativity in the activation of MinD ATPase.
MinE Reorganizes MinD Assembly into Large Bundles Frayed at One End.
To gain an insight into how MinE affects MinD assembly, we examined by electron microscopy MinD polymers that formed in the presence of MinE. Fig. 5 A and B depict two control MinD bundles (150–170 nm long, 15–21 nm wide) that assembled in the presence of ATP and LUVs. Upon incubation with an equimolar amount of MinE, MinD typically assembled into longer (420–580 nm) and thicker (25–80 nm in the compact areas) bundles (Fig. 5 C–F). Strikingly, after incubation with MinE, one end of the MinD bundles appeared markedly frayed, with the protofilaments peeling apart, whereas the distal segments mostly retained their structural integrity (Fig. 5 C–F). Such an “old broom”-like appearance of the MinD bundles indicates that MinD filaments have polarity. It is noteworthy that MinE causes MinD bundles to be significantly longer and thicker than the control polymers (Fig. 5). This finding indicates that MinE not only promotes depolymerization of MinD filaments but also reorganizes/stabilizes MinD assembly. These seemingly contradictory in vitro effects of MinE presumably reflect the in vivo requirements of MinE for both assembly and oscillation of the MinD horseshoe (15, 16).
Discussion
MinD Assembly Promoted by ATP and Phospholipids: In Vivo Implications.
We have shown that MinD assembles into short, paired filaments (median length, ≈50 nm; width, ≈6 nm) in the presence of ATP, and that addition of phospholipid vesicles stimulates assembly into longer and thicker bundles (100–300 nm long, 15–21 nm wide; Figs. 2 and 5). If MinD were to assemble into bundles in vivo, ≈2,000–3,000 molecules of MinD in an E. coli cell (11, 26) would be insufficient to cover the polar half of the cell membrane to form a horseshoe. A possible in vivo arrangement could be MinD:ATP assembly into ≈100–150 two-stranded filaments, each ≈50 nm long, that are dispersed over one-half of the cell membrane (one-half cell length, ≈1 μm; cell width, ≈0.6 μm). Such a scattered arrangement of MinD filaments on the polar membrane could give the appearance of a horseshoe, as seen by fluorescence microscopy (15, 16).
Modulation of MinD Assembly by MinE.
Our results reveal that MinE affects MinD in three ways: (i) it reorganizes MinD filaments into longer and thicker bundles with a characteristic fraying at one end (Fig. 5); (ii) it causes a net diminution in the steady-state MinD polymer mass (Fig. 3); and (iii) it cooperatively stimulates MinD ATPase in the presence of lipids (Fig. 4), which likely reflects a recycling of MinD. MinD recycling would contribute to reorganization and disassembly of the polymers. It is tempting to speculate that the frayed and splayed appearance of one end of MinD bundles formed in the presence of MinE is caused by a preferential activation of ATP hydrolysis at the decaying end. ATP hydrolysis could continually displace MinE along the shrinking MinD filaments, which explains the coupled dissolution of the MinD horseshoe and the poleward MinE movement seen in vivo (17, 18). The in vitro association of MinE with MinD polymers (Fig. 3) suggests that direct binding of MinE to the edge of the MinD horseshoe could be sufficient for E-ring assembly, as hinted at in ref. 17, without the need for another topological determinant.
The enhanced MinD bundling induced by MinE (Fig. 5) suggests that a fraction of MinE also may decorate the filaments away from the ends, and such end-distal binding may underlie the formation of the MinE polar zone along the MinD horseshoe (17, 18). MinE is a bifunctional protein with the N-terminal anti-MinCD domain (residues 1–32; refs. 27 and 28) being involved in stimulating MinD ATPase (19), whereas the C-terminal domain (residues 32–88) is critical for forming an antiparallel homodimer (28, 29) and for E-ring assembly (30). The antiparallel arrangement of the putative N-terminal helices in a MinE dimer (29) could allow the end-distal MinE molecules to noncovalently crosslink the MinD filaments to promote bundling.
MinE Cooperativity in Stimulation of MinD ATPase.
The lag in MinD ATPase activity at low MinE concentrations and the cooperative increase in ATPase rates at higher MinE concentrations (Fig. 4) suggest a rate-limiting step, possibly MinE self-association, that regulates ATP hydrolysis. Equilibrium sedimentation performed to study MinE self-association in vitro had yielded a dissociation constant of 0.6 μM for the dimer–monomer equilibrium (21). MinE is present at ≈1.4 μM per E. coli cell (26). Because all of the cellular MinE is recruited to the membrane by MinD (17, 18, 30), its local concentration in the membrane is likely to be ≈20- to 50-fold higher. Therefore, MinE would be predominantly dimeric on the membrane surface, and this dimerization step may be rate-limiting for stimulation of MinD ATPase activity and hence for MinD oscillation.
MinD-ParA Superfamily.
A member of the MinD-ParA superfamily and a ParA homolog, the Soj protein of Bacillus subtilis, is involved in chromosome segregation and is known to oscillate in the cell (31, 32). Recently, a plasmid-encoded ParA protein was also shown to oscillate in E. coli (33). It remains to be seen whether the Soj/ParA chromosome/plasmid-partitioning proteins and the MinD homologs in chloroplasts (25), yeast (34), and humans (35) may also function as polymerization-based engines.
While this paper was under review, Hu et al. (36) reported ATP-dependent MinD polymerization regulated by MinE. A notable difference between the two studies is the Hu et al. finding that MinD assembles as tubes on lipid vesicles (36), as opposed to our observation that MinD assembles predominantly into filament bundles in the presence of lipids and MinE. In contrast to untagged MinD used in the Hu et al. study, we have used hexahistidine-tagged MinD that was previously shown to be biologically active in E. coli (20) and is biochemically responsive to stimulation of ATP hydrolysis by MinE (Fig. 4). Our MinD preparation displays an ≈3-fold higher ATPase activity than that reported by Hu et al. (36). The preparation of lipid vesicles is also different in these studies. It is unclear at present if these differences may account for the varying nature of the MinD polymers.
Supplementary Material
Acknowledgments
We thank G. F. King, K. Morikawa, T. Oyama, L. I. Rothfield, and S. L. Rowland for reagents; C. Linsenmayer for micrograph prints; D. Dawson, E. Goldberg, and I. Hayashi for discussions; J. T. Park for comments on the manuscript; anonymous reviewers for helpful suggestions; and M. W. Kirschner and A. Wright for encouragement. This work was supported in part by the Center for Gastroenterology Research on Absorptive and Secretory Processes at Tufts–New England Medical Center (NIDDK, P30 DK-34928). D.R.C. also thanks the U.S. Defense Advanced Research Projects Agency for support.
Abbreviations
AMPPNP, adenosine 5′-[β,γ-imido]triphosphate
LUV, large unilamellar vesicle
References
- 1.Lutkenhaus J. & Addinall, S. G. (1997) Annu. Rev. Biochem. 66, 93-116. [DOI] [PubMed] [Google Scholar]
- 2.Rothfield L., Justice, S. & Garcia-Lara, J. (1999) Annu. Rev. Genet. 33, 423-448. [DOI] [PubMed] [Google Scholar]
- 3.Woldringh C. L., Mulder, E., Huls, P. G. & Vischer, N. (1991) Res. Microbiol. 142, 309-320. [DOI] [PubMed] [Google Scholar]
- 4.Yu X. C. & Margolin, W. (1999) Mol. Microbiol. 32, 315-326. [DOI] [PubMed] [Google Scholar]
- 5.de Boer P. A., Crossley, R. E. & Rothfield, L. I. (1989) Cell 56, 641-649. [DOI] [PubMed] [Google Scholar]
- 6.RayChaudhuri D., Gordon, G. S. & Wright, A. (2000) Nat. Struct. Biol. 7, 997-999. [DOI] [PubMed] [Google Scholar]
- 7.Rothfield L. I., Shih, Y. L. & King, G. (2001) Cell 106, 13-16. [DOI] [PubMed] [Google Scholar]
- 8.de Boer P. A., Crossley, R. E. & Rothfield, L. I. (1990) Proc. Natl. Acad. Sci. USA 87, 1129-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pichoff S. & Lutkenhaus, J. (2001) J. Bacteriol. 183, 6630-6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Z., Mukherjee, A., Pichoff, S. & Lutkenhaus, J. (1999) Proc. Natl. Acad. Sci. USA 96, 14819-14824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Boer P. A., Crossley, R. E., Hand, A. R. & Rothfield, L. I. (1991) EMBO J. 10, 4371-4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raskin D. M. & de Boer, P. A. (1999) J. Bacteriol. 181, 6419-6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu Z. & Lutkenhaus, J. (1999) Mol. Microbiol. 34, 82-90. [DOI] [PubMed] [Google Scholar]
- 14.Marston A. L. & Errington, J. (1999) Mol. Microbiol. 33, 84-96. [DOI] [PubMed] [Google Scholar]
- 15.Raskin D. M. & de Boer, P. A. (1999) Proc. Natl. Acad. Sci. USA 96, 4971-4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowland S. L., Fu, X., Sayed, M. A., Zhang, Y., Cook, W. R. & Rothfield, L. I. (2000) J. Bacteriol. 182, 613-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu X., Shih, Y. L., Zhang, Y. & Rothfield, L. I. (2001) Proc. Natl. Acad. Sci. USA 98, 1332-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hale C. A., Meinhardt, H. & de Boer, P. A. (2001) EMBO J. 20, 1563-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Z. & Lutkenhaus, J. (2001) Mol. Cell 7, 1337-1343. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi I., Oyama, T. & Morikawa, K. (2001) EMBO J. 20, 1819-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y., Rowland, S., King, G., Braswell, E. & Rothfield, L. I. (1998) Mol. Microbiol. 30, 265-273. [DOI] [PubMed] [Google Scholar]
- 22.Mayer L. D., Hope, M. J. & Cullis, P. R. (1986) Biochim. Biophys. Acta 858, 161-168. [DOI] [PubMed] [Google Scholar]
- 23.RayChaudhuri D. & Park, J. T. (1992) Nature 359, 251-254. [DOI] [PubMed] [Google Scholar]
- 24.Akiyama Y., Kihara, A., Tokuda, H. & Ito, K. (1996) J. Biol. Chem. 271, 31196-31201. [DOI] [PubMed] [Google Scholar]
- 25.Colletti K. S., Tattersall, E. A., Pyke, K. A., Froelich, J. E., Stokes, K. D. & Osteryoung, K. W. (2000) Curr. Biol. 10, 507-516. [DOI] [PubMed] [Google Scholar]
- 26.Shih Y. L., Fu, X., King, G. F., Le, T. & Rothfield, L. (2002) EMBO J. 21, 3347-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao C.-R., de Boer, P. A. & Rothfield, L. I. (1995) Proc. Natl. Acad. Sci. USA 92, 4313-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pichoff S., Vollrath, B., Touriol, C. & Bouche, J. P. (1995) Mol. Microbiol. 18, 321-329. [DOI] [PubMed] [Google Scholar]
- 29.King G., Shih, Y.-l., Maciejewski, M., Bains, N., Pan, B., Rowland, S. L., Mullen, G. & Rothfield, L. I. (2000) Nat. Struct. Biol. 7, 1013-1017. [DOI] [PubMed] [Google Scholar]
- 30.Raskin D. M. & de Boer, P. A. (1997) Cell 91, 685-694. [DOI] [PubMed] [Google Scholar]
- 31.Quisel J. D., Lin, D. C. & Grossman, A. D. (1999) Mol. Cell 4, 665-672. [DOI] [PubMed] [Google Scholar]
- 32.Marston A. L. & Errington, J. (1999) Mol. Cell 4, 673-682. [DOI] [PubMed] [Google Scholar]
- 33.Ebersbach G. & Gerdes, K. (2001) Proc. Natl. Acad. Sci. USA 98, 15078-15083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vitale G., Fabre, E. & Hurt, E. C. (1996) Gene 178, 97-106. [DOI] [PubMed] [Google Scholar]
- 35.Shahrestanifar M., Saha, D. P., Scala, L. A., Basu, A. & Howells, R. D. (1994) Gene 147, 281-285. [DOI] [PubMed] [Google Scholar]
- 36.Hu Z., Gogol, E. P. & Lutkenhaus, J. (2002) Proc. Natl. Acad. Sci. USA 99, 6761-6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.