Abstract
Hypertonicity-induced increase in activity of the transcription factor tonicity-responsive enhancer/osmotic response element-binding protein (TonEBP/OREBP) protects renal cells by increasing transcription of genes, including those involved in increased accumulation of organic osmolytes. We previously showed that hypertonicity increases transactivating activity of TonEBP/OREBP. Assay with a binary GAL4 transactivation system showed that the 984 C-terminal amino acids of TonEBP/OREBP (amino acids 548–1531) contain a tonicity-dependent transactivation domain (TAD). Also, amino acids 548–1531 undergo tonicity-dependent phosphorylation, and some inhibitors of protein kinases reduce the tonicity-dependent transactivation. In the present studies we examined the role of protein kinase A (PKA). Results: (i) An inhibitor of PKA (H89) reduces tonicity-dependent increases in transactivation, ORE/TonE reporter activity, and induction of aldose reductase and betaine transporter mRNAs. (ii) Overexpression of the catalytic subunit of PKA (PKAc) increases transactivation activity of amino acids 548–1531 and activity of an ORE/TonE reporter. The increases are much greater under isotonic than under hypertonic conditions. (iii) A dominant-negative PKAc reduces activity of an ORE/TonE reporter. (iv) PKAc activity increases with tonicity but cAMP does not. (v) TonEBP/OREBP and PKAc coimmunoprecipitate. (vi) amino acids 872–1271, including N– and C-terminal polyglutamine stretches, demonstrate tonicity-dependent transactivation, albeit less than amino acids 548–1531, and a similar role for PKA. Conclusions: (i) PKA plays an important role in TonEBP/OREBP activation of tonicity-dependent gene expression; (ii) PKA activation of TonEBP/OREBP appears to be cAMP-independent; and (iii) amino acids 872–1271 are sufficient for tonicity-dependent transactivation of TonEBP/OREBP.
TonEBP/OREBP (tonicity-responsive enhancer/osmotic response element-binding protein) (1, 2) participates in osmotic regulation of renal medullary cells by initiating adaptive accumulation of compatible organic osmolytes. In response to increased tonicity, TonEBP/OREBP binds to the enhancer element, ORE/TonE, which regulates expression of several genes, including aldose reductase (AR), the betaine/γ-aminobutyric acid transporter (BGT1), and the sodium–myo-inositol cotransporter (SMIT) (1–3). AR catalyzes the enzymatic conversion of glucose to the organic osmolyte sorbitol, whereas the transporters BGT1 and SMIT mediate the cellular accumulation of glycine betaine and myo-inositol, respectively. In addition to its role in the kidney medulla, TonEBP/OREBP is expressed in many other tissues (4) and is involved in osmotic stimulation of cytokine gene transcription (5) and integrin-mediated carcinoma metastasis (6).
Tonicity affects TonEBP/OREBP in several ways, including tonicity-dependent transactivation (7). Increased osmolality causes increased phosphorylation of serine and tyrosine residues in native TonEBP/OREBP (8). Tonicity-dependent phosphorylation within amino acids 548–1531 coincides with enhanced transactivation (7). Previously, using a binary GAL4 transactivation domain (TAD) reporter assay, we found that serine/threonine kinase and tyrosine kinase inhibitors each decrease transactivation activity of TonEBP/OREBP amino acids 548–1531 at high NaCl concentrations (7). The same kinase inhibitors decrease tonicity-dependent transcriptional activity of native TonEBP/OREBP, measured with an ORE/TonE-driven reporter (7). Thus, in common with many transcription factors, phosphorylation is involved in the regulation of TonEBP/OREBP. Osmolality-dependent phosphorylation could regulate TonEBP/OREBP by direct phosphorylation or by phosphorylation of an associated protein or upstream signaling molecule.
TonEBP/OREBP has several serines in two putative protein kinase A (PKA) consensus phosphorylation sites: one N-terminal and one C-terminal. Because PKA is a well known activator of many transcription factors, e.g., CREB (9), we investigated the possible role of PKA in osmotic regulation of TonEBP/OREBP. Herein, we find that PKA plays an important role in TonEBP/OREBP activation of tonicity-dependent gene expression, PKA activation of TonEBP/OREBP appears to be cAMP-independent, and amino acids 872–1271 are sufficient for tonicity-dependent transactivation of TonEBP/OREBP.
Materials and Methods
Cell Culture and Treatment.
HepG2 cells (passages 51–59) and HEK293 cells (passages 36–39) were cultured in isotonic medium (300 mosmol/kg) according to American Type Culture Collection instructions. At experiment-specific time points, medium was replaced with medium that was isotonic, hypotonic (Biofluids NaCl-free medium, adjusted to 200 mosmol/kg by adding NaCl), or hypertonic (adjusted to 500 mosmol/kg by adding NaCl).
N-{2-[(p-bromocinnamyl)amino]ethyl}-5-isoquinolinesulfonamide⋅2HCl (H89) is a PKA inhibitor, forskolin is an adenylate cyclase activator, dibutyryl-cAMP is a cAMP analog, and 3-isobutyl-1-methylxanthine (IBMX) is an inhibitor of cAMP phosphodiesterase. Compounds were obtained from Calbiochem and solubilized with Me2SO (DMSO); the same concentration of DMSO (<0.25%) was added to controls.
PKA Activity and cAMP Assays.
PKA activity was measured on HepG2 whole-cell extracts by using a fluorescent kemptide assay (PepTag, Promega) according to the supplier's instructions. Kemptide is a specific substrate of PKA. As kemptide is phosphorylated, it becomes negatively charged and migrates toward the anode on an agarose gel run at neutral pH. Images were captured and densitometry was performed by using a Molecular Imager FX/PC (Bio-Rad Laboratories).
cAMP was measured in HepG2 whole-cell extracts by using a cAMP Direct Immunoassay kit (Calbiochem) according to the supplier's instructions. cAMP (nmol/μg of protein) is expressed relative to isotonic control (300 mosmol/kg).
RNA Isolation and cDNA Preparation.
HepG2 cells were harvested 16 h after NaCl concentration was altered. Total RNA was isolated (RNeasy, Qiagen), and cDNA was prepared (TaqMan reverse transcription kit, Applied Biosystems) according to the supplier's instructions.
Plasmids.
ORE-Luc contains base pairs −3429 to +27 of the rabbit AR gene upstream of the Photinus pyralis luciferase gene (described in ref. 10 as ARLuc9). The sequence −3429 to +27 includes the AR promoter and three ORE/TonEs in native gene context (GenBank accession no. U12317).
Human TonEBP/OREBP cDNA clone KIAA0827 was a gift from Takahiro Nagase of the Kazusa DNA Research Institute, Chiba, Japan. Sequence coding for amino acids 77–1531 of KIAA0827 was cloned into expression vector pcDNA6V5-His (Invitrogen) to generate 77-1531V5-His. Dominant-negative PKAα catalytic subunit (PKAc DN) (11) was a gift from Sankar Ghosh, Yale University School of Medicine, New Haven, CT.
The binary GAL4 reporter system has been described previously (7). In brief, plasmid pFR-Luc (Stratagene) contains the yeast GAL4-binding site (upstream activating sequence, UAS) upstream of a minimal promoter and the P. pyralis luciferase gene. Expression plasmid pFA-CMV (Stratagene) contains sequence coding for the yeast GAL4 DNA-binding domain (dbd) under control of a cytomegalovirus promoter. Fusion proteins were generated by in-frame insertion of the sequence coding for amino acids 548–1531 or 872–1271 of clone KIAA0827 into pFA-CMV to generate GAL4dbd-548-1531 or GAL4dbd-872-1271, respectively. GAL4dbd contains no TAD but expresses the GAL4dbd (pFC2-dbd, Stratagene). PKAc contains amino acids 1–331 of the catalytic subunit of PKAα cloned into the vector pScript (pFC-PKA, Stratagene).
Transfection and Luciferase Assays.
HepG2 cells were grown in isotonic medium (300 mosmol/kg) in six-well plates and transfected by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. For the GAL4 binary assay, cells were cotransfected with 1 μg of pFR-Luc and 30 ng of GAL4dbd, GAL4dbd-548-1531, or GAL4dbd-872-1271 with or without 30 ng of pFC-PKAc or empty vector (pScript). For ORE/TonE reporter assays, cells were transfected with 5 μg of ORE-Luc alone, with 30 ng of pFC-PKAc or GAL4dbd, or with 2 μg of PKAc DN or empty vector (pcDNA3.1).
Twenty-four hours after transfection at 300 mosmol/kg, fresh medium was substituted that was isotonic, hypotonic (200 mosmol/kg), or hypertonic (500 mosmol/kg). Cells were treated with inhibitors or other pharmacological agents in isotonic medium beginning 1 hr before NaCl concentration was altered. Sixteen hours after NaCl concentration was altered, cells were harvested in 150 μl of passive lysis buffer (Dual Luciferase Reporter assay system; Promega). Total protein was measured (Bio-Rad protein assay kit), and P. pyralis luciferase activity was determined on duplicate aliquots (Dual Luciferase Reporter assay system). P. pyralis luciferase activity was expressed in relative light units (RLU) per μg of total cell protein.
Immunoprecipitation (IP).
HEK293 cells were grown at 300 mosmol/kg in 10-cm dishes and transfected by using Effectene (Qiagen) according to the manufacturer's instructions. Cells were transfected with 77-1531V5-His. Twenty-four hours after transfection at 300 mosmol/kg, fresh medium was substituted that was either isotonic or hypertonic (500 mosmol/kg). Six hours after NaCl concentration was altered, cells were trypsinized and pelleted by centrifugation. The pellet from one 10-cm dish was extracted with 1 ml of lysis buffer [(PBS/0.5% Triton X-100/protease inhibitor mixture (Roche Diagnostics)] and centrifuged (15,000 × g, 10 min). The supernatant was mixed overnight with 1 mg of Dynabeads (M-280, Dynal Biotech) preabsorbed with 4 μg of mouse anti-His-biotin conjugate (H-3 B, Santa Cruz Biotechnology) and washed five times with lysis buffer. The beads were resuspended in Laemmli sample buffer, and 30% of the volume was loaded on a Tris⋅HCl/4–15% polyacrylamide gel (Bio-Rad). After electrophoresis, the gel was transferred to a poly(vinylidene difluoride) (PVDF) membrane, blocked with 5% nonfat milk powder, and cut into two parts. The top part was incubated at 4°C overnight with mouse anti-V5 (Invitrogen); the bottom, with rabbit anti-PKAcα (C-20, Santa Cruz Biotechnology). Blots were detected by ECL (Amersham Pharmacia Bioscience). Two negative controls were used. Mouse IgG-biotin conjugate (sc-2762, Santa Cruz Biotechnology) was substituted for anti-His-biotin conjugate. For the second, cells were transfected with empty pcDNA6-V5His and IP was performed with anti-His as above.
The reciprocal IP was performed by using the same protocol on the same 77-1531V5-His transfected cells with the following substitution. The supernatant was mixed with rabbit anti-PKAcα agarose conjugate (sc-903 AC, Santa Cruz Biotechnology). As a negative control, IP was performed with mouse IgG agarose conjugate (sc-2343, Santa Cruz Biotechnology).
Real-Time PCR.
PCR was performed on 8- and 80-ng cDNA samples in 20-μl reaction mixtures in triplicate (n = 1) (TaqMan PCR master mix, Applied Biosystems). Amplicons were detected with an ABI Prism 7900HT sequence detection system (Applied Biosystems). Primers directed against the sequence of human AR were 5′-ATCGCAGCCAAGCACAATAA-3′ and 5′-AGCAATGCGTTCTGGTGTCA-3′; the 6-carboxyfluorescein (6FAM)-labeled probe was 5′-CAGCCCAGGTCCTGATCCGGTTC-3′. The primers for human BGT1 were 5′-CCCGAGGAGGGAGAGAAGTT-3′ and 5′-TCCATCTTGTTGGTCCATTGG-3′. The 6FAM-labeled probe was 5′-AAAGACGAGGACCAGGTGAAGGATCGG-3′.
Calculation of Relative mRNA Abundance from the Real-Time PCR Data.
The detection system records the number of PCR cycles (Ct) required to produce an amount of product equal to a threshold value, which is a constant. From the Ct values we calculated the mRNA abundance in each experimental condition, relative to that of control cells at 300 mosmol/kg (taken as 100%), using the following principles. (i) By definition, the number of specific cDNA molecules at the threshold (NCt) is constant for a given cDNA, independent of the number of cycles that it takes to reach it. (ii) For a specific cDNA, the ratio N(exp)i/N(cont)i is independent of i, assuming only that the efficiency (E) of PCR for a specific template is constant and the same for samples from experimental and control cells, where i is the cycle number, and N(X)i is the number of specific cDNA molecules in a sample (X = control or experimental) at cycle i. (iii) The ratio of the number of specific cDNA molecules at a cycle, Ct, to the number at another cycle, i, is Ni/NCt = 1/E(Ct−i).
To normalize the comparison between control and experimental results, we compared all results to the number of specific molecules at an arbitrary cycle, I, chosen for convenience to be the largest whole number that is less than any of the experimental values of Ct. Then, we calculated N(X)I/NCt for each sample. Each result from an experimental condition was normalized to the control value by dividing each value of N(X)I/NCt by the value at 300 mosmol/kg in that experiment.
Statistical Analysis.
Data were compared by a one-way ANOVA followed by Dunnett's multiple comparison test for separation of significant means. Normalized data were log-transformed before ANOVA. Results are expressed as mean ± SEM (n ≥ 3). Differences were considered significant for P ≤ 0.05.
Results
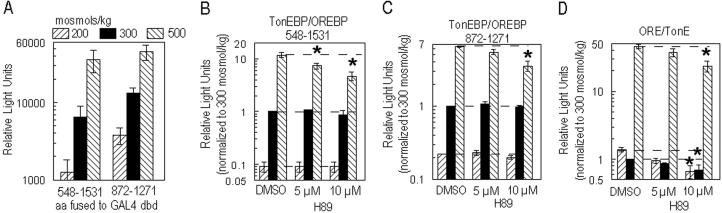
By using a binary GAL4 transactivation system, it was previously demonstrated that C-terminal amino acids 548–1531 of TonEBP/OREBP contain a functional transactivation domain (5, 7). The C terminus of TonEBP/OREBP contains two homopolymeric glutamine stretches that are characteristic of transactivation domains (12–15). To determine whether the regions encompassed by the glutamine stretches were sufficient for transactivation, we cloned amino acids 872–1271, including N- and C-terminal polyglutamine stretches, into a GAL4 expression vector to generate the chimera GAL4dbd-872-1271. GAL4dbd-872-1271 was cotransfected with a reporter construct containing the GAL4 upstream activating sequence of a luciferase gene. In this system, generation of luciferase depends on the presence of a functional TAD fused to the GAL4dbd. Our results demonstrate that amino acids 872–1271 contain a transactivation domain that is functional in isotonic medium (300 mosmol/kg) (Fig. 1A).
Fig 1.
(A) Amino acids 872–1271 of TonEBP/OREBP are sufficient for tonicity-dependent transactivation. HepG2 cells were cotransfected with pFR-Luc, a GAL4 upstream activating sequence reporter plasmid, and a GAL4dbd expression vector to generate either GAL4dbd-548-1531 or GAL4dbd-872-1271. Amino acids 548–1531 of TonEBP/OREBP contain a tonicity-dependent TAD (7); amino acids 872–1271 contain the C-terminal region bounded by polyglutamine stretches. Twenty-four hours after transfection at 300 mosmol/kg, medium was replaced with medium that was isotonic, hypotonic (NaCl-free medium, adjusted to 200 mosmol/kg by adding NaCl), or hypertonic (500 mosmol/kg by adding NaCl). Sixteen hours after NaCl concentration had been altered, cells were harvested and analyzed for P. pyralis luciferase activity (RLU/μg of total cell protein). Mean ± SEM; n ≥ 3. For each construct, the results at each osmolality differ significantly from those at the other osmolalities (P ≤ 0.05). (B–D) H89, a PKA inhibitor, decreases TonEBP/OREBP TAD activity of amino acids 548–1531 (B) and amino acids 872–1271 (C) as well as native TonEBP/OREBP-driven reporter gene expression (D). HepG2 cells were cotransfected with pFR-Luc and an expression vector to generate GAL4dbd-548-1531 or GAL4dbd-872-1271. Other HepG2 cells were transfected with ORE-Luc containing base pairs −3429 to +27 of the rabbit AR gene upstream of the P. pyralis luciferase gene. The sequence −3429 to +27 includes the AR promoter and three ORE/TonEs in native gene context. Twenty-four hours after transfection at 300 mosmol/kg, medium was replaced with medium that was isotonic (300 mosmol/kg), hypotonic (200 mosmol/kg), or hypertonic (500 mosmol/kg). Cells were exposed to DMSO or H89 in isotonic medium beginning 1 hr before NaCl was altered. Sixteen hours after NaCl concentration had been altered, cells were harvested and analyzed for P. pyralis luciferase activity (RLU/μg of total cell protein). Values are normalized to cells at 300 mosmol/kg treated with DMSO. Mean ± SEM; n ≥ 3. *, P ≤ 0.05.
Previously, we also showed that the transactivation activity of amino acids 548–1531 is osmotically regulated (7). In a similar manner, we examined the effect of different tonicities on the activity of amino acids 872–1271. The transactivating activity of amino acids 872–1271 increases (3.5×) in medium made hypertonic with NaCl (500 mosmol/kg) and decreases (3.5×) when the medium is hypotonic (200 mosmol/kg) (Fig. 1A), demonstrating that this C-terminal region is sufficient for tonicity-dependent transactivation.
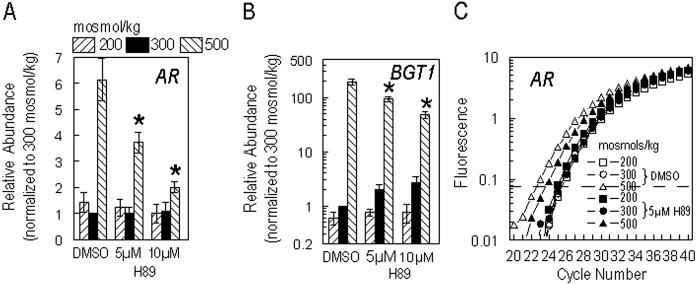
Phosphorylation is a common modulator of transcription factor activity (16). We know that the chimera GAL4dbd-548-1531 undergoes tonicity-dependent phosphorylation (7) and that the native protein shows increased serine phosphorylation with increased tonicity (17). TonEBP/OREBP contains several serines (amino acids 404, 408, 618, and 619) within two putative PKA sites, which suggested to us that PKA activity could be involved in tonicity-dependent increase in transactivating activity. Therefore, we examined the effect of a PKA inhibitor, H89. H89 significantly reduces the transactivation activity of both amino acids 548–1531 (by 60%) and amino acids 872–1271 (46%) at 500 mosmol/kg (Fig. 1 B and C) in transfected HepG2 cells. Inhibition of TAD activity should, in turn, reduce transcriptional activity of native TonEBP/OREBP, which acts through binding to the cognate DNA elements, ORE/TonEs. Thus, we tested H89 on HepG2 cells transfected with an ORE/TonE-driven reporter construct, ORELuc. H89 reduces luciferase activity (49%) at 500 mosmol/kg (Fig. 1D). Inhibition at 200 (52%) and 300 (31%) mosmol/kg is attributable to the presence of partial activation at all tonicities, as previously demonstrated (7, 17). The tonicity-dependent transcription of several genes, including AR and BGT1, is controlled by ORE/TonEs. Therefore, we tested whether H89 affects the mRNA abundance of the AR and BGT1 genes. As measured by quantitative real-time PCR, H89 inhibits hypertonic induction of both AR (67%) and BGT1 (75%) genes (Fig. 2 A and B). A representative amplification plot is shown in Fig. 2C.
Fig 2.
H89, a PKA inhibitor, depresses ORE/TonE-dependent AR (A) and BGT1 (B) gene expression at a high concentration of extracellular NaCl. HepG2 cells were grown at 300 mosmol/kg. Medium was replaced with medium that was isotonic, hypotonic (200 mosmol/kg), or hypertonic (500 mosmol/kg). Cells were exposed to DMSO or H89 in isotonic medium beginning 1 hr before NaCl was altered. Total RNA was isolated 16 hr after alteration of NaCl concentration and was reverse transcribed. Real-time PCR was used to determine cDNA abundance. Values are normalized to cells at 300 mosmol/kg treated with DMSO. Mean ± SEM; n ≥ 3. *, P ≤ 0.05. (C) Representative amplification curve showing position of amplicon threshold (broken line).
Transactivation activity, as measured in the GAL4 binary system, can be regulated by factors that are cotransfected. When constitutively active recombinant PKAc is cotransfected into HepG2 cells, transactivation activity of amino acids 548–1531 and 872–1271 increases (Fig. 3A). For amino acids 548–1531 and 872–1271, the increase at 300 mosmol/kg (9.5× and 4.4×, respectively) is much greater than at 500 mosmol/kg (2× and 1.7×, respectively).
Fig 3.
PKAc expression increases tonicity-dependent transactivation (A) and ORE/TonE reporter activity (B) much more at 300 mosmol/kg than at 500 mosmol/kg. (A) HepG2 cells were cotransfected with pFR-Luc, an expression vector to generate GAL4dbd-548-1531 or GAL4dbd-872-1271 and either pFC-PKAc, to generate a constitutively active catalytic subunit of PKA, or, as controls, pFC-dbd to generate the GAL4dbd or empty vector (pScript). (B) HepG2 cells were transfected with pFR-Luc or ORE-Luc, containing base pairs −3429 to +27 of the rabbit AR gene upstream of the P. pyralis luciferase gene. Cotransfectants were either pFC-PKAc, to generate a constitutively active catalytic subunit of PKA, or, as a control, pFC-dbd to generate the GAL4dbd. Twenty-four hours after transfection at 300 mosmol/kg, medium was replaced with medium that was isotonic, hypotonic (200 mosmol/kg), or hypertonic (500 mosmol/kg). Sixteen hours after NaCl concentration was altered, cells were harvested and analyzed for P. pyralis luciferase activity (RLU/μg of total cell protein). (C) Dominant-negative PKAc decreases ORE/TonE reporter activity. HepG2 cells were cotransfected with ORE-Luc (described for B) and control vector or PKAc DN, a constitutively expressed dominant-negative mutant of PKAα catalytic subunit. All values are normalized to cells at 300 mosmol/kg transfected with control vector. Mean ± SEM; n ≥ 3. *, P ≤ 0.05.
Because PKAc stimulates the transactivation of TonEBP/OREBP, one would expect native TonEBP/OREBP activity to increase. Therefore, we examined the affect of cotransfection of the PKAc recombinant on the ORE/TonE-driven luciferase expression of ORELuc. In the presence of PKAc, similar to TAD activity, ORE/TonE-driven transcription increases (12×) at 300 mosmol/kg (Fig. 3B). Conversely, when a constitutively active PKAc dominant-negative is cotransfected with ORELuc, luciferase activity decreases (80%) at both 300 and 500 mosmol/kg (Fig. 3C).
Since PKAc stimulates transactivation of TonEBP/OREBP, we next asked whether hypertonicity increases PKAc activity. We examined the effect of NaCl concentration on PKAc activity in HepG2 cells by using the fluorescent kemptide assay. PKAc activity increases significantly (33 ± 7%, n = 3, P ≤ 0.05) within 15 min after increasing from 300 to 500 mosmol/kg with NaCl.
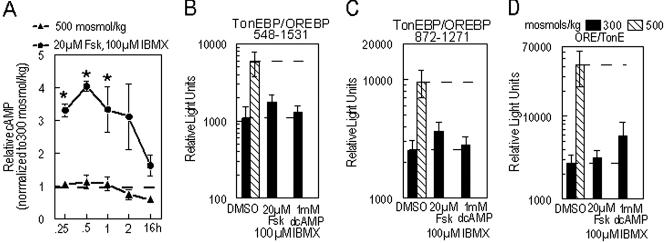
PKA is also known as cAMP-dependent kinase. To determine whether the observed increase in PKAc activity depended on increased levels of cAMP, we examined the effect of increased NaCl concentration on cAMP levels in HepG2 cells. Elevation of NaCl concentration (500 mosmol/kg) has no significant effect on cellular cAMP levels (Fig. 4A). As a positive control, we used forskolin, which generates increased PKA activity through elevation of cellular cAMP. Forskolin (20 μM) plus IBMX (100 μM) increases cAMP levels in HepG2 cells ≈3- to 4-fold within 15–30 min (Fig. 4A).
Fig 4.
(A) High extracellular NaCl concentration does not increase cAMP. HepG2 cells were grown at 300 mosmol/kg. Fresh medium was substituted that either had the same osmolality or was hypertonic (500 mosmol/kg). Media contained either DMSO or forskolin (Fsk) plus IBMX. cAMP (nmol/μg of protein) is expressed relative to isotonic control (300 mosmol/kg). Mean ± SEM; n ≥ 3. *, P ≤ 0.05. (B–D) Agents that activate or mimic cAMP do not increase TonEBP/OREBP TAD activity of amino acids 548–1531 (B), of amino acids 872–1271 (C), or native TonEBP/OREBP-driven reporter gene expression (D). HepG2 cells were cotransfected with pFR-Luc and an expression vector to generate GAL4dbd-548-1531 or GAL4dbd-872-1271. Other HepG2 cells were transfected with ORE-Luc containing base pairs −3429 to +27 of the rabbit AR gene upstream of the P. pyralis luciferase gene. Twenty-four hours after transfection at 300 mosmol/kg, medium was replaced with medium that was isotonic, hypotonic (200 mosmol/kg), or hypertonic (500 mosmol/kg). Cells were exposed to DMSO, forskolin (Fsk, an adenylate cyclase activator), or dibutyryl-cAMP (dcAMP, a cAMP analog), and IBMX (an inhibitor of cAMP phosphodiesterase) in isotonic medium beginning 1 hr before NaCl concentration was altered. Sixteen hours after NaCl concentration had been altered, cells were harvested and analyzed for P. pyralis luciferase activity (RLU/μg of total cell protein). Mean ± SEM; n ≥ 3.
We also asked whether agents that would increase or mimic cellular cAMP would have an effect on TonEBP/OREBP function. We used concentrations of forskolin plus IBMX demonstrated to increase cAMP levels in HepG2 cells (Fig. 4A). To mimic cAMP, we used dibutyryl-cAMP plus IBMX. Transactivating activity of amino acids 548–1531 is not significantly affected by forskolin plus IBMX or dibutyryl-cAMP plus IBMX (Fig. 4B). Similarly, IBMX plus either forskolin or dibutyryl-cAMP does not significantly affect transactivating activity of amino acids 872–1271 (Fig. 4C) or ORE/TonE-driven transcription of ORELuc (Fig. 4D).
PKAc could exert its effect on TonEBP/OREBP transactivation in several ways, including a direct effect on TonEBP/OREBP or on a coactivator. To determine whether PKAc is part of a transcription complex in association with TonEBP/OREBP, we transfected HEK293 cells with a construct that would generate a fusion of amino acids 77–1531 of TonEBP/OREBP and V5-His epitopes. When His antibody was used to immunoprecipitate TonEBP/OREBP from cells in isotonic (300 mosmol/kg) or hypertonic (500 mosmol/kg) medium, Western transfer showed that PKAc coimmunoprecipitates with TonEBP/OREBP (Fig. 5A). The amount of coimmunoprecipitated PKAc does not differ significantly between cells examined at 300 and 500 mosmol/kg. Two types of control IPs are negative for the presence of TonEBP/OREBP and PKAc. In the first control, the extracts were immunoprecipitated with a nonspecific IgG antibody. In the second, HEK293 cells were transfected with the empty vector pcDNA6V5-His and the IP was performed with an anti-His antibody. The IgG heavy chain (≈55 kDa) was visible in longer exposures of negative controls but TonEBP/OREBP and PKAc were not.
Fig 5.
TonEBP/OREBP and PKAc coimmunoprecipitate. HEK293 cells were transfected with 77-1531V5-His (A and B, lanes 1–4) or empty pcDNA6-V5His (A, lanes 5–6). Twenty-four hours after transfection at 300 mosmol/kg, fresh medium was substituted that was either isotonic or hypertonic (500 mosmol/kg). (A) Cell extracts were immunoprecipitated by using mouse IgG (lanes 1 and 2) or mouse anti-His (lanes 3–6) and immunoblotted. The top of the membrane was probed with mouse anti-V5 to visualize TonEBP/OREBP. The bottom was probed with rabbit anti-PKAcα. Solid line indicates position of cut. (B) The reciprocal IP was performed on 77-1531V5-His transfected cells by using mouse IgG (lanes 1 and 2) or rabbit anti-PKAcα (lanes 3 and 4). The top of the membrane was probed with mouse anti-V5 and the bottom was probed with rabbit anti-PKAcα. Solid line indicates position of cut.
Because PKAc coimmunoprecipitates with TonEBP/OREBP, we would expect the reciprocal experiment to yield evidence of TonEBP/OREBP. The same extracts of cells transfected with 77-1531V5-His were used in conjunction with an antibody to PKAc. When PKAc antibody was used to immunoprecipitate PKAc from cells in isotonic (300 mosmol/kg) or hypertonic (500 mosmol/kg) medium, Western transfer showed that the fusion protein 77-1531V5-His (TonEBP/OREBP) coimmunoprecipitates (Fig. 5B). As for PKAc above, there is no consistent tonicity-dependent difference in the amount of TonEBP/OREBP coimmunoprecipitated. As a negative control, when the same extracts were immunoprecipitated with a nonspecific IgG antibody, no evidence of either PKAc or TonEBP/OREBP was found.
Discussion
In the present study, we demonstrate that PKA is necessary for increase in TonEBP/OREBP-mediated transcriptional activity in response to hypertonicity. This osmotic response is depressed by H89, an inhibitor of PKA. H89 reduces native TonEBP/OREBP-driven reporter activity as well as tonicity-dependent induction of AR and BGT1 mRNAs. H89 also reduces tonicity-dependent transactivating activity of the amino acids 548–1531 and 872–1271 regions of TonEBP. Overexpression of a catalytically inactive dominant-negative mutant of PKAc (11) also decreases the activity of an ORE/TonE reporter. Further, we demonstrate that PKA is sufficient for increased transactivation and transcription. Overexpression of wild-type PKAc increases both transactivation activity of amino acids 548–1531 and 872–1271 and activity of an ORE/TonE reporter. The increases are much greater under isotonic than under hypertonic conditions.
PKA is also known as cAMP-dependent kinase. Classically, an increase in intracellular cAMP levels induces activation of PKA. PKA exists as a tetramer comprising two regulatory subunits and two catalytic subunits. Two molecules of cAMP bind to each regulatory subunit, allowing the release of active catalytic subunits. A broad array of cellular responses are regulated by PKA with specificity determined by PKA isozymes formed of heterogenous regulatory and catalytic subunits, differential expression of PKA isozymes, and subcellular localization by A-kinase-anchoring-proteins (9, 18). However, PKA activation of TonEBP/OREBP does not appear to be cAMP dependent. As shown here, hypertonic stimulation of HepG2 cells does not cause an increase in intracellular cAMP levels. Additionally, treatment of HepG2 cells with agents that either increase intracellular cAMP (forskolin) or mimic cAMP (dibutyryl-cAMP) does not cause significant change in either TonEBP/OREBP-driven osmotic response or the activity of TADs contained in amino acids 548–1531 or amino acids 872–1271.
PKAc can be regulated by mechanisms that are cAMP independent. For example, the phosphorylation of the p65/RelA subunit of transcription factor NF-κB that is catalyzed by PKAc is independent of cAMP (11). NF-κB is maintained in an inactive state in the cytosol by association with the inhibitor protein IκB. The catalytic subunit of PKA is also inactivated by binding to IκB, forming an NF-κB–IκB–PKAc complex. Upon lipopolysaccharide stimulation, IκB kinase phosphorylates IκB, thereby targeting it for proteasomal degradation. PKAc is released in an active form and phosphorylates p65 on Ser-276, resulting in increased transactivating activity of NF-κB independent of nuclear translocation and increased DNA binding (11). Rather, increased transactivation is due to enhanced binding to the transcriptional coactivators, CBP/p300 (19).
PKAc stimulation of TonEBP/OREBP transactivating activity could be similar to that found for NF-κB. Like NF-κB, proteasome inhibitors such as MG-132 inhibit transcriptional activity of TonEBP/OREBP (7, 20), implying the existence of some protein inhibitor of TonEBP/OREBP. However, there is no direct evidence that IκB inhibits TonEBP/OREBP (20). Previous studies show that hypertonicity results in increased phosphorylation of serine and tyrosine residues in native TonEBP/OREBP (8), and phosphorylation of recombinant amino acids 548–1531 is coincident with increased tonicity-dependent transactivation (7). Also, there are several serines in two putative PKA phosphorylation sites (amino acids 404, 408, 618, and 619) in TonEBP/OREBP. However, the amino acid sequence 872–1271, which does not contain the recognized putative PKA phosphorylation sites, is responsive to PKAc activation. This indicates that any PKAc phosphorylation of the TonEBP/OREBP TAD would have to be at nonconsensus sequence PKA sites. Direct PKAc phosphorylation of TonEBP/OREBP remains to be demonstrated.
Alternative mechanisms of activation are possible. For example, PKAc might activate a protein that forms a complex with TonEBP/OREBP. TonEBP/OREBP binds DNA in solution as a homodimer (5) but residues sufficient for dimerization of TonEBP/OREBP are either identical or similar to counterparts in p52 and p65 (21), suggesting that mixed dimer formation is possible. Others have shown that TonEBP/OREBP exists not only as a dimer but also in a large complex with other unknown proteins (1, 2). Herein, we show by reciprocal coimmunoprecipitation that PKAc is part of a complex with TonEBP/OREBP.
Another possibility for PKAc stimulation of TonEBP/OREBP is activation of an upstream signaling molecule. The yeast high osmolarity glycerol (HOG) mitogen-activated protein (MAP) kinase pathway is functionally analogous to the mammalian TonEBP/OREBP pathway. Activation by hyperosmotic stress results in adaptive accumulation of organic osmolytes (glycerol). In yeast, two membrane proteins, Sho1p and Sln1p, the latter a histidine kinase, are thought to be osmosensors and are the most upstream elements identified in the HOG MAP kinase cascade. Also known are several upstream control proteins as well as a MAP kinase phosphorylation relay of serine/threonine and threonine/tyrosine kinases (22). However, histidine kinases are not found in mammalian cells, and the osmosensors leading to activation of TonEBP/OREBP have not been identified. General inhibitors of either serine/threonine or tyrosine kinases decrease tonicity-dependent TonEBP/OREBP transcription and transactivating activity (7), and two other kinases involved in activation of TonEBP/OREBP, in addition to PKA, have been identified, namely, p38 (23, 24) and Fyn (24). Hypertonicity increases activity of p38 (25) and Fyn (26). Inhibition of p38 or Fyn partially reduces hypertonicity-induced activation of both ORE/TonE reporter activity and increase of AR mRNA abundance; inhibition of both p38 and Fyn reduces them further (24).
A summary of our current knowledge of TonEBP/OREBP regulation follows. In the basal state, under isotonic conditions, TonEBP/OREBP is partially active and distributed in both the cytosol and the nucleus (7, 17). With an increase in tonicity, existing TonEBP/OREBP rapidly translocates to the nucleus (1). Nuclear translocation is abrogated by the proteasome inhibitor MG132, implying the existence of an inhibitor protein (20). More slowly, the abundance of TonEBP/OREBP protein increases through enhanced mRNA transcription and protein synthesis (1). The increase in nuclear TonEBP/OREBP results in increased binding to its cognate DNA element, ORE/TonE. TonEBP/OREBP is a constitutive homodimer that forms a circle around DNA (21). Dimerization, while not tonicity-dependent, is required for both DNA binding and transactivation (5). The C terminus contains an osmotically regulated TAD (7). Amino acids 872–1271, comprising the amino acid region bound at N and C termini by a series of glutamines, are sufficient for tonicity-dependent transactivation (this study). Hypertonicity results in increased serine and tyrosine phosphorylation (8). Increased phosphorylation of amino acids 548–1531 coincides with enhanced tonicity-dependent transactivation activity (7). PKA activity is necessary for TonEBP/OREBP-mediated osmotic response and is sufficient for increased transactivation and transcription (this study). Hypertonicity-induced activation of PKA is cAMP independent (this study). Activation of p38 and Fyn by hypertonicity is also necessary for the increased transcriptional activity of TonEBP/OREBP (24).
Abbreviations
TonEBP, tonicity-responsive enhancer-binding protein
OREBP, osmotic response element-binding protein
PKA, protein kinase A
PKAc, catalytic subunit of PKA
IBMX, 3-isobutyl-1-methylxanthine
AR, aldose reductase
BGT1, betaine/γ-aminobutyric acid transporter
TAD, transactivating domain
dbd, DNA-binding domain
RLU, relative light units
IP, immunoprecipitation
References
- 1.Miyakawa H., Woo, S. K., Dahl, S. C., Handler, J. S. & Kwon, H. M. (1999) Proc. Natl. Acad. Sci. USA 96, 2538-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ko B. C., Turck, C. W., Lee, K. W., Yang, Y. & Chung, S. S. (2000) Biochem. Biophys. Res. Commun. 270, 52-61. [DOI] [PubMed] [Google Scholar]
- 3.Burg M. B., Kwon, E. D. & Kultz, D. (1997) Annu. Rev. Physiol 59, 437-455. [DOI] [PubMed] [Google Scholar]
- 4.Trama J., Lu, Q., Hawley, R. G. & Ho, S. N. (2000) J. Immunol. 165, 4884-4894. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Rodriguez C., Aramburu, J., Jin, L., Rakeman, A. S., Michino, M. & Rao, A. (2001) Immunity 15, 47-58. [DOI] [PubMed] [Google Scholar]
- 6.Jauliac S., Lopez-Rodriguez, C., Shaw, L. M., Brown, L. F., Rao, A. & Toker, A. (2002) Nat. Cell Biol. 4, 540-544. [DOI] [PubMed] [Google Scholar]
- 7.Ferraris J. D., Williams, C. K., Persaud, P., Zhang, Z., Chen, Y. & Burg, M. B. (2002) Proc. Natl. Acad. Sci. USA 99, 739-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahl S. C., Handler, J. S. & Kwon, H. M. (2001) Am. J. Physiol. 280, C248-C253. [DOI] [PubMed] [Google Scholar]
- 9.Torgersen K. M., Vang, T., Abrahamsen, H., Yaqub, S. & Tasken, K. (2002) Cell. Signalling 14, 1-9. [DOI] [PubMed] [Google Scholar]
- 10.Ferraris J. D., Williams, C. K., Martin, B. M., Burg, M. B. & Garcia-Perez, A. (1994) Proc. Natl. Acad. Sci. USA 91, 10742-10746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong H., SuYang, H., Erdjument-Bromage, H., Tempst, P. & Ghosh, S. (1997) Cell 89, 413-424. [DOI] [PubMed] [Google Scholar]
- 12.Courey A. J. & Tjian, R. (1988) Cell 55, 887-898. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell P. J. & Tjian, R. (1989) Science 245, 371-378. [DOI] [PubMed] [Google Scholar]
- 14.Gill G., Pascal, E., Tseng, Z. H. & Tjian, R. (1994) Proc. Natl. Acad. Sci. USA 91, 192-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Cesare D., Fimia, G. M. & Sassone-Corsi, P. (1999) Trends Biochem. Sci. 24, 281-285. [DOI] [PubMed] [Google Scholar]
- 16.Cohen P. (2000) Trends Biochem. Sci. 25, 596-601. [DOI] [PubMed] [Google Scholar]
- 17.Woo S. K., Dahl, S. C., Handler, J. S. & Kwon, H. M. (2000) Am. J. Physiol. 278, F1006-F1012. [DOI] [PubMed] [Google Scholar]
- 18.Scott J. D., Dell'Acqua, M. L., Fraser, I. D., Tavalin, S. J. & Lester, L. B. (2000) Adv. Pharmacol. 47, 175-207. [DOI] [PubMed] [Google Scholar]
- 19.Zhong H., Voll, R. E. & Ghosh, S. (1998) Mol. Cell 1, 661-671. [DOI] [PubMed] [Google Scholar]
- 20.Woo S. K., Maouyo, D., Handler, J. S. & Kwon, H. M. (2000) Am. J. Physiol. 278, C323-C330. [DOI] [PubMed] [Google Scholar]
- 21.Stroud J. C., Lopez-Rodriguez, C., Rao, A. & Chen, L. (2002) Nat. Struct. Biol. 9, 90-94. [DOI] [PubMed] [Google Scholar]
- 22.Hohmann S. (2002) Microbiol. Mol. Biol. Rev. 66, 300-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nadkarni V., Gabbay, K. H., Bohren, K. M. & Sheikh-Hamad, D. (1999) J. Biol. Chem. 274, 20185-20190. [DOI] [PubMed] [Google Scholar]
- 24.Ko, B. C., Lam, A. K., Kapus, A., Fan, L., Chung, S. K. & Chung, S. S. (September 30, 2002) J. Biol. Chem., 10.1074/jbc.M208138200.
- 25.Han J., Lee, J. D., Bibbs, L. & Ulevitch, R. J. (1994) Science 265, 808-811. [DOI] [PubMed] [Google Scholar]
- 26.Ishino M., Aoto, H., Sasaski, H., Suzuki, R. & Sasaki, T. (2000) FEBS Lett. 474, 179-183. [DOI] [PubMed] [Google Scholar]