Abstract
Ecosystem services are critical to human survival; in selected cases, maintaining these services provides a powerful argument for conserving biodiversity. Yet, the ecological and economic underpinnings of most services are poorly understood, impeding their conservation and management. For centuries, farmers have imported colonies of European honey bees (Apis mellifera) to fields and orchards for pollination services. These colonies are becoming increasingly scarce, however, because of diseases, pesticides, and other impacts. Native bee communities also provide pollination services, but the amount they provide and how this varies with land management practices are unknown. Here, we document the individual species and aggregate community contributions of native bees to crop pollination, on farms that varied both in their proximity to natural habitat and management type (organic versus conventional). On organic farms near natural habitat, we found that native bee communities could provide full pollination services even for a crop with heavy pollination requirements (e.g., watermelon, Citrullus lanatus), without the intervention of managed honey bees. All other farms, however, experienced greatly reduced diversity and abundance of native bees, resulting in insufficient pollination services from native bees alone. We found that diversity was essential for sustaining the service, because of year-to-year variation in community composition. Continued degradation of the agro-natural landscape will destroy this “free” service, but conservation and restoration of bee habitat are potentially viable economic alternatives for reducing dependence on managed honey bees.
Keywords: ecosystem service, native bee, Apis mellifera, agriculture, biodiversity
Ecosystem services are the set of diverse ecological functions that are essential to human welfare (1); these services can provide significant, measurable benefits to humanity, potentially providing an economic argument for ecosystem conservation (2, 3). Yet, ecosystem services are poorly understood both ecologically and economically (1, 2, 4). A key question is whether natural ecosystems provide comparable or superior benefits to highly managed systems; only in such cases are ecosystem service arguments aligned with those of biodiversity conservation (4, 5). We examine this issue through an in-depth study of the crop pollination services provided by native bee communities under different land management regimes.
Animals, particularly bees, pollinate one or more cultivars of >66% of the world's 1,500 crop species (6) and are directly or indirectly essential for an estimated 15–30% of food production (7). Currently, farmers that manage pollination on farms or in glasshouses rely on <11 of the 20,000–30,000 bee species worldwide (8). The value of crop pollination by the most important managed pollinator, the honey bee Apis mellifera, is estimated to be 5–14 billion dollars per year in the United States alone (9, 10). This critical service is now compromised by the decline of beekeeping (≈50% since 1950) due to diseases, loss of subsidies, and insecticide poisoning (11, 12); coupled with increasing demand (10), this decline is leading to price increases (M. Burgett, unpublished data). In the United States, beekeeping is likely to decline further as the Africanized race of A. mellifera continues to spread northwards from its introduction site in Brazil. In the southwestern United States, Africanized A. mellifera already hybridize with managed colonies of European honey bees, conferring an aggressive trait and creating liability concerns for beekeepers (11).
Native bee communities might provide an insurance policy in the event of honey bee shortages and may already contribute substantially to crop pollination (8, 11, 13). Little is known, however, about such “unsolicited” pollination services by wild bees, or about their susceptibility to environmental changes such as habitat loss and degradation (12–15). In a crop system in California, we investigated the contribution of native bee communities to crop pollination and how this varied with increasing agricultural intensification. We examined the influence of temporal turnover in the bee community on the relationship between species diversity and function, an aspect of diversity–function relationships not yet well studied (16, 17), by comparing community composition and pollination function across years.
Methods
Crop Type and Study Sites.
In the Central Valley and the eastern edge of the Coast Range of Yolo County, California, we studied the pollination of watermelon on farms that varied in the level of agricultural intensification along two axes: farm management type (organic versus conventional) and isolation from large areas of oak woodland and chaparral habitat (near versus far). Watermelon is obligately dependent on multiple bee visits for pollination (18), requires deposition of 500–1,000 pollen grains on the stigma for production of marketable fruit (7, 19), and is visited by up to 39 native bee species and by honey bees in our area (20).
Near sites (N) contained ≥30% natural habitat within a 1-km radius of the farm, whereas far sites (F) had <1% natural habitat within a 1-km radius. This N–F classification represented the extremes of the landscape gradient for natural habitat, based on previous work showing that both the aggregate rate of visitation of native bees to watermelon and diversity (species richness) were significantly positively related to the proportional area of surrounding natural habitat in circles ≥1 km (log-transformed visitation rate, F1,19 = 12.05, P = 0.003, r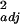 = 0.36; diversity, F1,19 = 11.37, P = 0.003, r
= 0.36; diversity, F1,19 = 11.37, P = 0.003, r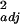 = 0.35; C.K., R.W.T., R. L. Bugg, and J. M. Fay, unpublished work). Organic sites (O) were certified according to the California Organic Foods Act (1990), usually grew multiple crops within fields of smaller area, left weedy field borders, and used drip or spray irrigation. Conventional farms (C) used three insecticides with moderate to high toxicity to bees (Admire 2F, Ambush Insecticide, and Lannate SP), as well as herbicides, inorganic fertilizer, and three or more other insecticides; grew only muskmelon and watermelon; ploughed borders; and used flood irrigation.
= 0.35; C.K., R.W.T., R. L. Bugg, and J. M. Fay, unpublished work). Organic sites (O) were certified according to the California Organic Foods Act (1990), usually grew multiple crops within fields of smaller area, left weedy field borders, and used drip or spray irrigation. Conventional farms (C) used three insecticides with moderate to high toxicity to bees (Admire 2F, Ambush Insecticide, and Lannate SP), as well as herbicides, inorganic fertilizer, and three or more other insecticides; grew only muskmelon and watermelon; ploughed borders; and used flood irrigation.
From least to most intensive management, farms were therefore classified as organic near (ON), organic far (OF), and conventional far (CF); no conventional near farms occurred in the study area. Over both years, all CF, one OF, and no ON farms brought managed honeybee colonies on watermelon fields. In 2001, we studied five ON, four OF, and five CF farms; in 2000 we studied six ON, four OF, and six CF farms. Attributes of individual farms, including elevation, sample date, average temperature, and wind speed on the date of sample, are presented in Table 3, which is published as supporting information on the PNAS web site, www.pnas.org. There were no significant differences in climate conditions between farm types during either year.
Pollen Deposition.
We estimated the contribution of each bee species to watermelon pollination at each farm site by counting the number of visits by each sex of that species over the flower's lifetime (full-day samples), then multiplying this quantity by the median per-visit pollen deposition for that species and sex (see below). Visits were counted within 1-m2 quadrats continuously along 50-m transects. Transects were initiated within 5 m of the field edge to minimize potential effects of field size. Transects were walked for twelve 10-min periods during continuous half-hour intervals beginning no earlier than 07:30 and ending no later than 14:30 (the hours during which flowers were open; both opening and bee activity depended on sunrise and occurred later in late summer than early spring). The total visits per flower day were calculated for each species and sex as 3∑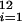 (visits per 10 min)/(flower density), where i is half-hour time period, and flower density was measured in five 1-m2 quadrats per transect. Watermelon flowers remain open for a single day; therefore, visits per flower day estimate all of the visits contributing to pollination.
(visits per 10 min)/(flower density), where i is half-hour time period, and flower density was measured in five 1-m2 quadrats per transect. Watermelon flowers remain open for a single day; therefore, visits per flower day estimate all of the visits contributing to pollination.
For certain genera, individuals could only be identified to genus or subgenus on the wing [Melissodes, four species; Lasioglossum (Evylaeus), four morphospecies; Lasioglossum (Dialictus), four morphospecies; Lasioglossum (Lasioglossum), two species; Hylaeus, three species; voucher specimens for all species and morphospecies of watermelon visitors are stored at the Harry H. Laidlaw, Jr., Honey Bee Research Facility at the University of California, Davis]. We use “species” henceforth to refer to the lowest taxonomic level discernable in the field. Transect data provided both diversity (species richness) and relative abundance, as measured by visitation rate.
In 2000, we used the same transect method but conducted four 10-min samples during 1 h only between 9 and 12:30 a.m. (hourly samples), except for four ON farms that also had full-day samples. We also grouped certain species as “tiny black” [Lasioglossum (Dialictus) spp., Hylaeus spp., and C. nanula] or “small striped” [Halictus tripartitus, Halictus ligatus, Lasioglossum (Lasioglossum) spp.] bees. We kept track of honey bee–native bee interactions on watermelon flowers to assess competition for resources over a total of 156 h of sample time, recording whether honey bees displaced native bees from flowers or vice versa, and whether honey bees or native bees investigated flowers but did not visit if the flower was already occupied by a bee.
Pollen deposition was measured by presenting newly opened female flowers (previously bagged with bridal veil to prevent visitation) to individual bees foraging in watermelon fields (21). Following a single visit, flowers were protected from further visitation. Stigmas were excised the following day, treated with 10% KOH for 6 h, briefly stained in 3% basic fuchsin, squashed onto microscope slides, and scored at 40× for watermelon pollen grains. Between 1 and 58 female bees (mean number of bees = 24.9) and 1 and 21 male bees (mean = 7.3) were studied per species, except for Hylaeus (only female visits). Control flowers (continuously bagged flowers) had an average of 0.18 pollen grains (n = 10, range = 0–1).
We measured pollen deposition rather than fruit production to separate the contributions of individual bee species to pollination. Median rather than mean pollen deposition per species provided more conservative estimates of pollen deposition per visit, given right-skewed distributions of pollen deposition per visit.
Total pollen deposition per flower equaled the sum of the contributions of each species. As a check, we measured pollen deposition at open-pollinated flowers (nine flowers on ON farms) and found no significant differences from our estimates of total pollen deposition for native bees plus A. mellifera for the ON farms (Xobs = 2,237 grains, SDobs = 1,056; Xest = 2,330, SDest = 776, F1,12 = 0.03, P = 0.87).
Statistical Analysis.
We tested differences in total pollen deposition by native bees among farm types by using ANOVA followed by paired tests. Nonparametric comparisons were used when data did not meet normality or variance assumptions. To explore effects of honeybees and farm type on native bee diversity and abundance, we used analysis of covariance (ANCOVA).
Simulation of Changes in Diversity.
We explored whether agricultural intensification disproportionately affected functionally important pollinator species by using Monte Carlo simulations in which y species, equivalent to the number lost from a given farm type, were deleted from the initial number on ON farms. Total pollen deposition was then calculated for the remaining set over 100 iterations [<10% of the total possible species combinations that equal n!/y!(n − y)!]. Alternative null models are being explored elsewhere.
Results
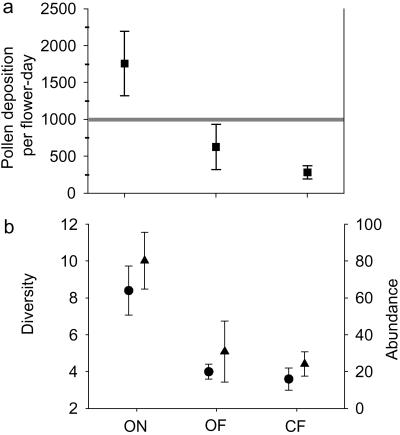
In 2001, we found that the native bee community alone could provide sufficient pollination services for watermelon at ON farms, but that agricultural intensification diminished these pollination services by roughly 3- to 6-fold (Fig. 1a; F2,11 = 6.34, r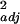 = 0.54, P = 0.015). A similar effect was found in 2000, based on hourly rather than full-day samples (F2,13 = 4.97, r
= 0.54, P = 0.015). A similar effect was found in 2000, based on hourly rather than full-day samples (F2,13 = 4.97, r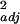 = 0.35, P = 0.025). The effect of isolation from natural habitat appeared potentially to be more important than that of management (2001: ON versus OF, P = 0.03; OF versus CF, P = 0.47; 2000: ON versus OF, P = 0.03; OF versus CF, P = 0.90, Bonferroni-adjusted P = 0.025), although both factors may contribute. In 2001 (full-day samples), the native bee community alone provided sufficient pollination (≥1,000 grains deposited per flower) at 80% of the ON farms and 50% of the OF farms, but none of the CF farms (Gcorrected = 5.9, df = 2, P = 0.05).
= 0.35, P = 0.025). The effect of isolation from natural habitat appeared potentially to be more important than that of management (2001: ON versus OF, P = 0.03; OF versus CF, P = 0.47; 2000: ON versus OF, P = 0.03; OF versus CF, P = 0.90, Bonferroni-adjusted P = 0.025), although both factors may contribute. In 2001 (full-day samples), the native bee community alone provided sufficient pollination (≥1,000 grains deposited per flower) at 80% of the ON farms and 50% of the OF farms, but none of the CF farms (Gcorrected = 5.9, df = 2, P = 0.05).
Fig 1.
(a) Total estimated pollen deposition by native bees ± SE in 2001 on ON, OF, and CF farms. The gray line indicates pollen deposition for production of marketable fruit. (b) Native bee diversity (circles) and abundance (triangles) ± SE in 2001.
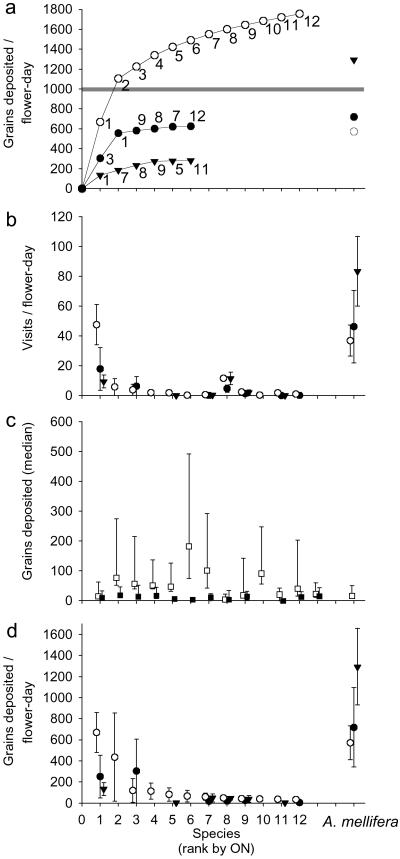
Because of the honey bee contribution, all farms received ample pollination for watermelon fruit set (Fig. 2a). Honey bees were not sufficiently abundant on organic farms, however, to provide the full pollination service; thus, organic farms, both near and far from wildland areas, relied in part on native bee pollinators for watermelon pollination. In contrast, on CF farms, where native bees could not provide sufficient pollination services (Fig. 1a), farmers routinely rented honey bee colonies to obtain adequate pollination.
Fig 2.
(a) Cumulative estimated mean pollen deposition per flower for ON (open circles), OF (filled circles), and CF (filled inverted triangles) in 2001 based on individual means in d. The gray line indicates pollen deposition threshold. 1, H. tripartitus; 2, Bombus californicus; 3, Peponapis pruinosa; 4, Bombus vosnesenskii; 5, Melissodes lupina, robustior, stearnsi, or tepida timberlakei; 6, H. farinosus; 7, Lasioglossum (Evylaeus) spp. (n = 4); 8, L. (Dialictus) spp. (n = 4); 9, H. ligatus; 10, L. mellipes or titusi; 11, Hylaeus rudebeckiae, stevensi, or conspicuus; 12, Agapostemon texanus. (b) Mean visits per flower day ± SE for each bee species (order and symbols as above) at each farm type. (c) Median pollen deposition per visit with quartiles for females (open squares) and males (filled squares) of each bee species. (d) Estimated mean pollen deposition per flower day ± SE by each bee species on each farm type.
The decline of native bee pollination services with agricultural intensification resulted from significant reductions in both diversity and total abundance of native bees (Fig. 1b). Agricultural intensification affected functionally important pollinator species disproportionately (Fig. 2d), i.e., those species that contributed the most to pollen deposition either because of high deposition per visit (e.g., B. californicus) or high visitation frequency (e.g., H. tripartitus; Fig. 2 b and c). The second, fourth, and fifth-ranked contributors for ON 2001 (B. californicus, B. vosnesenskii, and Melissodes spp.) occurred in neither the OF nor CF treatments (Fig. 2 a and d); the third-ranked contributor (P. pruinosa) occurred on only one OF of the eight far farms. Relative to a null model, in which species were chosen at random for deletion, bee communities at CF scored significantly lower for aggregate pollination function than random communities. This finding suggests that these communities contained fewer of the functionally important species than expected (Table 1). OF farms did not differ from the null model, however, because of high variability in contributions from H. tripartitus and P. pruinosa. Each of these species was exceptionally abundant on one of the four farms, possibly because of site-specific conditions such as presence of nesting aggregations or of squash flowers, an obligate pollen resource for Peponapis (22).
Table 1.
Comparison of total estimated pollen deposition on CF and OF farms against a null model of species deletion
| Farm type | No. of deleted species | Observed value | Significance, P value |
|---|---|---|---|
| Mean CF + SE | 6 | 371.56 | 0.09 |
| Mean CF | 6 | 282.38 | 0.01 |
| Mean CF − SE | 6 | 193.21 | <0.01 |
| Mean OF + SE | 6 | 934.19 | 0.55 |
| Mean OF | 6 | 625.72 | 0.22 |
| Mean OF − SE | 6 | 317.24 | 0.02 |
The P value equals the proportion of random communities out of 100 trials whose total pollen deposition values were less than the observed values. For example, only one of 100 trials produced a lower pollen deposition estimate than the observed mean deposition on CF. Significant results suggest loss of functional dominants. The null model assumed no changes in abundance of these species relative to ON farms, a conservative assumption because it reduces the expected pollen deposition relative to a model with density compensation. Means ± SE were included to encompass the among-farm variation within farm types.
We found no evidence that native bee abundance and diversity declined in response to increased honey bee abundance. In both 2000 and 2001, neither total native bee abundance nor diversity was significantly related to honey bee abundance across farm sites (Table 2). Honey bee abundances were also uncorrelated with abundances of individual native bee species across farm sites (see Table 4, which is published as supporting information on the PNAS web site). Interactions between honey bees and native bees were infrequent (<1 interaction per h); when they occurred, honey bees did not displace native bees from watermelon flowers more frequently than the converse (n = 106, Gcorrected = 1.58, df = 2, P = 0.45).
Table 2.
General linear models of the effects of farm type and honey bee (HB) abundance on native bee abundance and diversity, showing an effect of farm type but not HB abundance
| Source of variation
|
Native bee abundance | Native bee diversity | ||||||
|---|---|---|---|---|---|---|---|---|
| Est. | df | F | P | Est. | df | F | P | |
| 2000 | ||||||||
| HB abundance | −0.24 | 1 | 0.44 | 0.52 | −0.22 | 1 | 0.18 | 0.68 |
| Farm type | 2 | 2.92 | 0.09 | 2 | 9.09 | <0.01 | ||
| 2001 | ||||||||
| HB abundance | −0.47 | 1 | 3.08 | 0.11 | −0.03 | 1 | 0.03 | 0.88 |
| Farm type | 2 | 4.49 | 0.04 | 2 | 6.51 | 0.02 | ||
Analyses were run initially with an interaction effect; nonsignificant interactions (all models) were then removed for the final analysis. HB abundances were log-transformed. In 2001, diversity was square root-transformed; in 2000, native bee abundance was log-transformed.
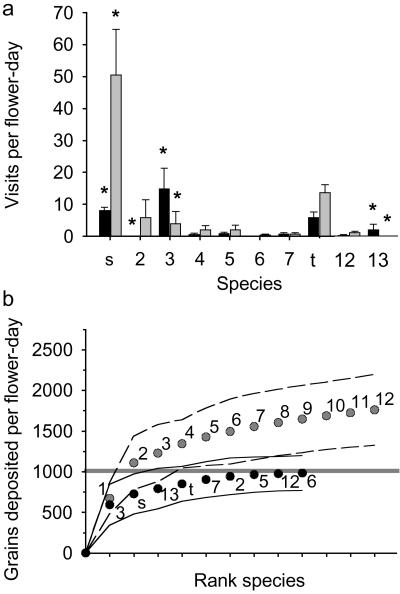
The composition of the native bee community visiting watermelon changed significantly between years (Gcorrected = 32.1, df = 9, P < 0.001; Fig. 3a). The change affected both the rank order of functionally important species and the number of species required to provide sufficient pollination on ON farms in different years. In 2001, on average, the two most important bee species, H. tripartitus and B. californicus, provided ample pollination, whereas on some farms in 2001 and most farms in 2000, meeting the pollination threshold by using unmanaged native bees required the contributions of the entire native bee community (see standard error bars in Fig. 3b).
Fig 3.
(a) Abundance of each bee species at watermelon during 2000 (black bars) and 2001 (gray bars). Stars indicate significant differences from expectation (Freeman–Tukey deviates). Species are in rank order of contribution to pollination service in 2001 (see Fig. 2 legend); because selected groups were lumped in 2000, s (small striped) includes 1, 9, and 10, and t (tiny black) includes 8, 11, and C. nanula (not observed in 2001). Anthophora urbana (13) was also observed only in 2000. (b) Means ± SE of cumulative pollen deposition for ON farms in 2000 (black with solid line) and 2001 (gray with dashed line). Numbers and letters refer to species identity as above.
Discussion
A central question in conservation biology and environmental economics is whether natural ecosystems provide comparable or superior benefits to highly managed systems. Our results show that under favorable circumstances, the native bee community can provide an equivalent service to that of managed honey bee pollinators for watermelon, a crop that has heavy pollination requirements. About 50% of the bee species that visit watermelon visit other crops (20) with equivalent or lower pollination requirements (23); thus, it is likely that this bee community is sufficient to provide services for multiple crops, including some that honey bees do not service (e.g., cherry tomatoes; S. Smith and C.K., unpublished work).
Another critical issue is whether biodiversity is important in maintaining ecosystem services (4, 17). We found that the diversity of native bee communities is important in providing crop pollination services because of temporal fluctuations in bee populations, which are known to be highly variable across space and time (24, 25). Diversity buffered pollination against asynchronous fluctuations of bee abundances between years (Fig. 3b). A diverse set of species (≈20 species) was necessary for sufficient pollination function in one year, and relatively unimportant species in one year became crucial functional dominants in the next year. Different bee species are also differentially effective as pollinators both within (Fig. 2c) and among crops (8, 26), and honey bees are known to be ineffective or less effective pollinators of selected crops (15, 27). Managing for bee diversity could therefore meet the pollination requirements of a greater number of crops, provide insurance in the event of shortages of any specific pollinator (managed or unmanaged), and provide options for new or alternative crops, either as a supplement or alternative to current protocols for single-species management. These findings suggest that for crop pollination, ecosystem services arguments can be aligned with arguments to conserve biodiversity (5).
Agricultural intensification reduced the diversity and abundance of native bees such that pollination services they provided were below the necessary threshold to produce marketable products (Fig. 1). There was no evidence that competition with honey bees explained this pattern (Table 2). The absence of certain species from OF and CF sites was not due to historical differences in bee community composition between the Central Valley (OF, CF, and ON sites) and the eastern edge of the Coast Range (ON sites), caused, for example, by soil or elevational differences between these areas, because all species missing at OF and CF sites have been collected since 1999 in isolated fragments of natural to seminatural vegetation within the Central Valley (C.K., unpublished observations) and are also known historically from Central Valley localities (R.W.T., unpublished observations).
Instead, isolation from critical floral and nesting resources present in wildlands is likely to be the key factor explaining the decline in abundance and diversity of native bees, and attendant loss of pollination services. In other studies in the same region (C.K., R.W.T., R. L. Bugg, and J. M. Fay, unpublished work; S. Smith and C.K., unpublished work), we show that both native bee diversity and abundance are significantly related to the proportional area of wild habitat surrounding the farm (28). The use of insecticides and herbicides in conventional agriculture is also likely to reduce bee populations (8, 13). Because most farms in the developed world operate in areas isolated from natural habitat and use pesticides and herbicides, many farmers must be relying on only one or several managed species, plus a depauperate native bee community. Globally, current agricultural trends are leading to increased degradation of agro-ecosystems, natural habitats, and the services these lands provide (29, 30). In the case of pollination, these trends will enforce reliance by farmers on the available managed species, which are vulnerable to disease outbreaks (31–33), unless wild bee communities can be restored.
Restoring pollination services in areas of greatest agricultural intensity would require both reducing insecticide use and restoring native or surrogate vegetation to provide nesting habitat and floral resources for bees when they are not using crops (34). Although this would incur opportunity costs from potentially reduced crop productivity due to losses from pest insects, weeds, and diminished crop area, it is likely that restoration strategies could be devised for net economic benefit. Bees can seek out patchy resources (35) and persist within small fragments of habitat (36); thus, restored patches could be largely located in less productive, larger “source” areas off-farm and as small patches of “stepping-stone” habitat on nonproductive farm areas [e.g., around tail water ponds and ditches, as hedgerows (37)]. Opportunity costs might be partially offset by restoration of native habitat because this may also increase abundances of other beneficial insects (37, 38).
In the United States, restoration costs might be partially defrayed through the Conservation Reserve Program. In addition, a simple estimate suggests that collectively farmers could redirect up to 30.1 million dollars per year to conserving and restoring bee habitat simply by reducing honey bee rentals 15–50% (see Table 5, which is published as supporting information on the PNAS web site). In this manner, they could hedge their bets in the event of honey bee scarcity through partial replacement of honey bee by native bee services. The actual amount required to offset opportunity costs and restore the service partially or fully is unknown, including how financial resources and restoration would need to be distributed spatially across the agro-natural landscape to provide adequate services. These issues are important areas of future economic and ecological research within ecosystem services (5, 39). Because crop-pollinating species are often generalists that pollinate many native plants (20), restoring pollination services for agriculture could also benefit wild plants and thereby promote conservation of biodiversity across the agro-natural landscape.
Supplementary Material
Acknowledgments
C. Fineman provided the perl program for the null model. N. Nicola, R. Hatfield, and L. Kropuenske assisted with collection of field data. This paper benefited from the comments of P. Balavanera, J. Cane, A. Dobson, J. Gould, H. Horn, S. Pacala, T. Ricketts, S. Smith, V. Tepedino, N. Waser, R. Winfree, and several anonymous reviewers. This research was funded by the Mead Foundation, the Organic Farming Research Foundation, the National Fish and Wildlife Foundation, the McDonnell Foundation, the Center for Conservation Biology, the Wildlife Conservation Society, Princeton University, and Stanford University. N.M.W. was supported by a Nature Conservancy Smith Postdoctoral Fellowship. The University of California, Davis, provided laboratory facilities.
Abbreviations
ON, organic near
OF, organic far
CF, conventional far
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Daily G. C., (1997) Nature's Services: Societal Dependence on Natural Ecosystems (Island, Washington, DC).
- 2.Heal G., (2000) Nature and the Marketplace: Capturing the Value of Ecosystem Services (Island, Covelo, CA).
- 3.Kremen C., Niles, J. O., Dalton, M. G., Daily, G. C., Ehrlich, P. R., Fay, J. P., Grewal, D. & Guillery, R. P. (2000) Science 288, 1828-1832. [DOI] [PubMed] [Google Scholar]
- 4.Myers N. (1996) Proc. Natl. Acad. Sci. USA 93, 2764-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balvanera P., Daily, G. C., Ehrlich, P. R., Ricketts, T. H., Bailey, S. A., Kark, S., Kremen, C. & Pereira, H. (2001) Science 291, 2047-2047. [DOI] [PubMed] [Google Scholar]
- 6.Roubik D. W., (1995) Pollination of Cultivated Plants in the Tropics (Food Agric. Org. U.N., Rome).
- 7.McGregor S. E., (1976) Insect Pollination of Cultivated Crop Plants (U.S. Department of Agriculture–Agricultural Research Service, Washington, DC).
- 8.Parker F. D., Batra, S. W. T. & Tepedino, V. J. (1987) Agric. Zool. Rev. 2, 279-304. [Google Scholar]
- 9.Southwick E. E. & Southwick, L., Jr. (1992) J. Econ. Entomol. 85, 621-633. [Google Scholar]
- 10.Morse R. A. & Calderone, N. W., (2000) Beeculture, http://bee.airoot.com/beeculture/pollination2000/pg1.html.
- 11.Nabhan G. P. & Buchmann, S. (1997) in Nature's Services: Societal Dependence on Natural Ecosystems, ed. Daily, G. C. (Island, Washington, DC), pp. 133–150.
- 12.AllenWardell G., Bernhardt, P., Bitner, R., Burquez, A., Buchmann, S., Cane, J., Cox, P. A., Dalton, V., Feinsinger, P., Ingram, M., et al. (1998) Conserv. Biol. 12, 8-17. [Google Scholar]
- 13.Kearns C., Inouye, D. & Waser, N. (1998) Annu. Rev. Ecol. Syst. 29, 83-112. [Google Scholar]
- 14.Kremen C. & Ricketts, T. (2000) Conserv. Biol. 14, 1226-1228. [Google Scholar]
- 15.Kevan P. G., Clark, E. A. & Thomas, V. G. (1990) Am. J. Altern. Agric. 5, 12-22. [Google Scholar]
- 16.Schwartz M. W., Brigham, C. A., Hoeksema, J. D., Lyons, K. G., Mills, M. H. & van Mantgem, P. J. (2000) Oecologia 122, 297-305. [DOI] [PubMed] [Google Scholar]
- 17.Loreau M., Naeem, S., Inchausti, P., Bengtsson, J., Grime, J., Hector, A., Hooper, D., Huston, M., Raffaelli, D., Schmid, B., Tilman, D. & Wardle, D. (2001) Science 294, 804-808. [DOI] [PubMed] [Google Scholar]
- 18.Stanghellini M. S., Ambrose, J. T. & Schultheis, J. R. (1997) Am. Bee J. 137, 386-391. [Google Scholar]
- 19.Adlerz W. C. (1966) J. Econ. Entomol. 59, 28-30. [DOI] [PubMed] [Google Scholar]
- 20.Kremen, C., Bugg, R. L., Nicola, N., Smith, S. A., Thorp, R. W. & Williams, N. M. (2003) Fremontia 31,in press.
- 21.Thomson J. D. (1981) Am. Midl. Nat. 105, 377-380. [Google Scholar]
- 22.Hurd P. D., Linsley, E. G. & Whitaker, T. W. (1971) Evolution (Lawrence, Kans.) 25, 218-234. [DOI] [PubMed] [Google Scholar]
- 23.Free J. B., (1993) Insect Pollination of Crops (Academic, San Diego).
- 24.Williams, N. M., Minckley, R. L. & Silveira, F. A. (2001) Conserv. Ecol. 5, www.consecol.org/vol5/iss1/art7.
- 25.Roubik, D. (2001) Conserv. Ecol. 5, www.consecol.org/vol5/iss1/art2.
- 26.Freitas B. M. & Paxton, R. J. (1998) J. Appl. Ecol. 35, 109-121. [Google Scholar]
- 27.Parker F. D. (1981) J. Kans. Entomol. Soc. 54, 61-67. [Google Scholar]
- 28.SteffanDewenter I. & Tscharntke, T. (1999) Oecologia 121, 432-440. [DOI] [PubMed] [Google Scholar]
- 29.Vitousek P. M., Mooney, H. A., Lubchenco, J. & Melillo, J. M. (1997) Science 277, 494-499. [Google Scholar]
- 30.Tilman D., Fargione, J., Wolff, B., D'Antonio, C., Dobson, A., Howarth, R., Schindler, D., Schlesinger, W. H., Simberloff, D. & Swackhamer, D. (2001) Science 292, 281-284. [DOI] [PubMed] [Google Scholar]
- 31.Macfarlane R. P., Lipa, J. J. & Liu, H. J. (1995) Bee World 76, 130-148. [Google Scholar]
- 32.Morse R. A., (1978) Honey Bee Pests, Predators and Diseases (Cornell Univ. Press, Ithaca, NY), pp. 430.
- 33.Sammataro D., Gerson, U. & Needham, G. (2000) Annu. Rev. Entomol. 45, 519-548. [DOI] [PubMed] [Google Scholar]
- 34.Fussell M. & Corbet, S. A. (1992) J. Appl. Ecol. 29, 451-465. [Google Scholar]
- 35.Rust R. W. (1990) Environ. Entomol. 19, 332-338. [Google Scholar]
- 36.Cane J. H. (2001) Conserv. Ecol. 5, 3. [Google Scholar]
- 37.Bugg R. L., Anderson, J. H., Thomsen, C. D. & Chandler, J. (1998) in Enhancing Biological Control: Habitat Management to Promote Natural Enemies of Agricultural Pests, ed. Bugg, R. L. (Univ. of California Press, Berkeley), pp. 339–374.
- 38.LeTourneau D. & Goldstein, B. (2001) J. Appl. Ecol. 38, 557-570. [Google Scholar]
- 39.Heal G., Daily, G. C., Ehrlich, P. R., Salzman, J., Boggs, C., Hellmann, J., Hughes, J., Kremen, C. & Ricketts, T. (2001) Stanford Environ. Law Q. 20, 333-364. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.