Abstract
The relationship between the risk of hantaviral pulmonary syndrome (HPS), as estimated from satellite imagery, and local rodent populations was examined. HPS risk, predicted before rodent sampling, was highly associated with the abundance of Peromyscus maniculatus, the reservoir of Sin Nombre virus (SNV). P. maniculatus were common in high-risk sites, and populations in high-risk areas were skewed toward adult males, the subclass most frequently infected with SNV. In the year after an El Niño Southern Oscillation (ENSO), captures of P. maniculatus increased only in high-risk areas. During 1998, few sites had infected mice, but by 1999, 18/20 of the high-risk sites contained infected mice and the crude prevalence was 30.8%. Only 1/18 of the low-risk sites contained infected rodents, and the prevalence of infection was lower (8.3%). Satellite imagery identified environmental features associated with SNV transmission within its reservoir population, but at least 2 years of high-risk conditions were needed for SNV to reach high prevalence. Areas with persistently high-risk environmental conditions may serve as refugia for the survival of SNV in local mouse populations.
In 1993 a disease outbreak marked by rapidly progressing pulmonary symptoms and a high likelihood of mortality reawakened interest in diseases caused by hantaviruses (1). These agents cause zoonotic diseases and are primarily associated with the rodent family Muridae. The viruses and rodent reservoir species are tightly linked so that a single rodent species tends to be infected by a single viral species (2). Thus, in general, at-risk areas for people are defined by the rodent reservoirs' geographic distributions (3). However, within these regions, risk varies based on the ecology of the rodent species and the availability of habitats (3, 4).
Recently, Glass et al. (5) used Landsat Thematic Mapper (TM) satellite data to identify environments associated with human risk of hantaviral disease caused by Sin Nombre virus (SNV). This retrospective study of human disease in the southwestern United States introduced an epidemiologic risk algorithm that combined several TM bands, taken nearly a year before the outbreak, and elevation, to estimate human risk in 1993, when disease was reported from nearly 30 locations. When the study was repeated using TM imagery from 1995 to predict risk in 1996 the extent of high-risk areas was substantially reduced and only a single human case was observed (5).
The hantaviral pulmonary syndrome (HPS) outbreak led to a series of studies of hantavirus–rodent reservoir systems examining SNV and its main reservoir, Peromyscus maniculatus, the deer mouse (3, 6–8). Three key observations emerged from this research. First, as with other hantaviruses, SNV was maintained within a single reservoir species. Although spillover to other mammalian species occurred, viral persistence in the ecosystem relied on the deer mouse (6). Second, SNV appeared to be transmitted horizontally, so that the prevalence of infection increased with age class (or its surrogate, body mass) (3, 6–8). Thus, the prevalence of infection in deer mouse populations tended to peak with an aging population rather than with the absolute size of the population (4, 8, 9). Consequently, HPS risk could be higher in areas where populations showed a preponderance of adult animals (4, 9). Third, there was a sex bias in infection; more adult male than adult female mice were infected (3, 6). Mills et al. (3) surveyed >50 locations in the southwestern United States and found a crude prevalence of infection in female deer mice of 6%, compared with 14% in males. Among adult deer mice the difference was more striking with <10% of adult females with antibody to SNV, whereas nearly 30% of adult males were similarly positive.
The underlying dynamics that generate these patterns in local populations remain a matter of investigation (4, 8–11). However, Parmenter and colleagues (12, 13) hypothesized that, for several zoonotic agents in the region, local environmental conditions were major drivers that affected rodent populations and led to the increased risk of human disease. These events were triggered by meteorological conditions that affected vegetation and invertebrate populations that, in turn, affected the rodent reservoir populations. The changes in the rodent populations altered SNV transmission within the rodent populations (12) and, subsequently, human risk at a time remote from the triggering events themselves. Parmenter and colleagues proposed the trophic cascade hypothesis and they hypothesized that El Niño events could alter precipitation patterns in the southwestern United States sufficiently to trigger the trophic cascade.
In the spring of 1997, sea surface temperature patterns in the equatorial Pacific indicated a new El Niño Southern Oscillation (ENSO) event. This ENSO continued through mid-1998 (NOAA Climate Prediction Center, www.cpc.noaa.gov/products/analysis_monitoring/ensostuff/ensoyears.html) and provided an opportunity to examine the trophic cascade hypothesis and to evaluate the relationship between the epidemiologic risk algorithm by using satellite data and the ecology of the hantavirus system. We developed a series of predictions relating human risk estimated from satellite imagery with key aspects of the SNV–deer mouse system. High-risk sites were expected to have a faunal structure that was predominated by members of the genus Peromyscus, especially P. maniculatus, and that these local populations would differ in their age and sex structure from lower-risk sites. A consequence would be that levels of SNV infection within local populations in low- and high-risk sites should differ so that human populations in high-risk areas would more likely acquire infection. We further predicted that if the ENSO was an environmental trigger driving the trophic cascade leading to HPS outbreaks, then the dynamics of the SNV–deer mouse system during the ENSO should differ between high- and low-risk areas.
We tested the hypotheses by classifying human risk by using the risk algorithm (5) with satellite imagery from mid-1997 and mid-1998. A series of locations was then selected and sampled for small mammals by using a standardized protocol in 1998 and 1999. We show that high-risk sites were ecologically distinct from low-risk sites and that, by the end of the study, SNV infection was especially prevalent among deer mice in high-risk areas.
Materials and Methods
Satellite Imagery and Risk Classification.
Landsat 5 TM imagery from mid-June in 1997 and 1998 was obtained for a 105,200-km2 study area in the southwestern United States where HPS was initially recognized in 1993 (1). Imagery was obtained from the Earth Resources Data Center [Earth Resources Observation Systems (EROS) Distributed Active Archive Center (DAAC); U.S. Geological Society (USGS), Sioux Falls, SD].
The images were processed and annual predicted HPS risk maps were generated by using described methods (5). Logistic regression was used to estimate risk for each year by using the digital numbers in each of three TM bands (bands 1, 5, and 7) and elevations at 28 HPS cases and 170 randomly selected control sites from the original 1993 outbreak. Risk at each pixel within the study region was assigned by weighting the digital numbers for each band, and elevation by the respective coefficients from the logistic models.
Pixels were categorized into three risk categories. Areas of low, moderate, or high human risk were aggregated by using a receiver operator characteristic (ROC) function (5). Low-risk areas were those with risk values accounting for ≤10% of cases, moderate-risk areas had values with >10% but <25% of cases, and high-risk areas had signatures associated with ≥25% of cases. These categories were used to sample rodent-trapping sites with similar levels of human risk.
Rodent Faunal Sampling.
In 1998, 40 sites encompassing a wide range of predicted HPS risk (0.05–0.86 from the 1997 images) within the study area were selected. Sites were chosen by an individual unfamiliar with the region to reduce selection bias. The criteria used in site selection were that the sites were within relatively homogeneous patches of risk categories and that they were within 300 m of a road to allow access. For the field surveys, sites were dichotomized into those above and below the high-risk threshold, and two sites above and two sites below predicted high risk were surveyed at a time. This methodology was devised to control for environmental conditions (e.g., meteorological and lunar conditions) that could affect trap success. The field crews were blinded to the predicted human risk level to further reduce sampling bias. These sites were resampled in the spring of 1999 by using the same protocols. The 1998 TM images used to generate the risk map for 1999 did not include two of the original survey sites in moderate-risk areas, and these were excluded from further analyses.
A standardized trapping protocol was used to sample the local small mammal fauna (3, 14). One hundred single-capture small mammal live traps were set for two nights as four parallel lines of 25 traps each. Traps were spaced at ≈10-m intervals. Longitude and latitude were recorded at the contralateral corners of the two outer trap lines by using global positioning system (GPS) receivers. The data were used to locate the sampling sites on the satellite images. The major vegetation in the overstory, understory, and herbaceous layers and the extent of cover, as well as the amount of cover by litter and bare soil or rock, were recorded by using a standardized protocol (14). These data were used to qualitatively compare sites for similarities in plant community structure.
Standard measures of faunal composition and abundance were generated from the trapping data. Trap success for selected species was measured as the number of animals captured divided by the number of trap nights (maximally 200) at each study site and used as an indication of abundance. Corrections were made for traps that were lost or sprung but empty (14). The age, sex, body mass, and species identification were recorded for each animal captured. These data were used to characterize the age and sex structure of populations for selected species (3). Collected animals were processed according to protocols for SNV detection (15). All animals were accessioned to the Museum of Southwestern Biology (MSB), University of New Mexico, and retained as voucher specimens [NK77969–78300 (1998) and NK84919–85500 (1999)]. Frozen tissues and blood samples from voucher specimens were deposited in the Division of Genomic Resources, MSB.
Serological Testing.
Sera from captured rodents were tested by ELISA for antibodies to hantaviruses by using described methods (16). Briefly, recombinant SNV nucleocapsid antigen cloned into a vector was expressed and coated onto ELISA plates. As control antigen, protein from the vector alone was expressed and coated onto plates. Sera were screened at a dilution of 1:100 in PBS–Tween-20 (0.1%) and 5% skim milk. Sera were incubated in duplicate wells and detected by using a combination of goat anti-Peromyscus IgG and goat anti-Rattus IgG conjugated with horseradish peroxidase followed with 2,2′-azinodi(3-ethyl)benzthiazoline-6-sulfonic acid (ABTS). Optical densities were read at 410 nm and the difference between the pair of SNV antigen wells and the pair of control wells for each sample was calculated.
Statistical Analysis.
The human risk for HPS at each trapping site was determined by averaging the risk for all of the pixels incorporated within the area enclosed by the trapping lines. Prevalence of SNV antibody at the small mammal sampling locations was estimated by dividing the number of individuals that were antibody positive by the numbers of individuals tested. Previously identified risk factors for hantavirus infection in small mammals (species, sex, and age composition) were used to compare risk sites (3).
We tested the hypothesis that the predicted HPS risk derived from the satellite imagery identified biologically relevant features associated with hantavirus transmission by predicting characteristics of local rodent populations. First, we examined the relationship between P. maniculatus abundance at the 38 trapping sites and the HPS risk by using multiple linear regression. The number of deer mice captured (logarithmically transformed captures + 1) was the dependent variable. The HPS risk obtained from the previous June's satellite image and the change in the risk from the prior year, for 1999, were used as the independent variables. Satellite images from 1996 were not available to allow us to calculate changes in risk from 1997 to 1998, so the analysis for 1998 was performed as a simple linear regression. We predicted that there should be a significant relationship between the abundance of deer mice captured at a site and the predicted risk of HPS because deer mice are the principal reservoir of SNV.
As a second test, the age and sex structures of deer mouse populations at high-risk sites were compared with low-risk sites. Adult male deer mice are disproportionately infected with SNV compared with females and juveniles of either sex. Thus, we hypothesized that high-risk sites would have a population sampled that was biased toward adult male deer mice.
Finally, we examined the prevalence of SNV infection, as measured by the presence of specific antibodies in individual animals, in high- and low-risk sites. We predicted that the prevalence of SNV infection should be higher in high-risk sites, especially in the year after the ENSO, as this was the time lag associated with the previous HPS outbreak in 1993.
Results
Sampling Locations.
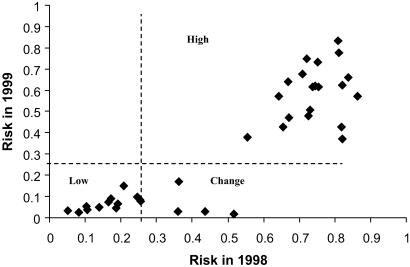
Three types of trapping sites were identified on the risk maps for 1998 and 1999. The categorization was based on the annual stability of local risk (Fig. 1). Low- and moderate-risk sites were combined because few low-risk sites were available in 1998 and because of the small contributions that both categories made to the number of human cases. Eleven sites were in low–moderate-risk categories during both years, 7 sites were classified as high-risk areas in 1998, but as low-risk areas in 1999, and 20 sites were in high-risk areas during both years (Fig. 1). No sites categorized as low–moderate risk in 1998 (during an ENSO) became high risk in 1999 (after an ENSO). Sites that changed from high risk in 1998 to low risk in 1999 were locations where the field crew could not access the originally provided coordinates. The crew selected nearby sites. These sites were adjacent to low–moderate-risk areas.
Fig 1.
Cross tabulation of predicted risk for 38 trapping sites in 1998 (x axis) and 1999 (y axis). Vertical and horizontal lines at 0.25 are thresholds between high- and low-risk categories (5). Low-risk sites were those that were predicted to be low risk during both years of the study (Lower Left). High-risk sites were those that remained at high risk during both years (Upper Right). Sites that changed from high risk in 1998 to low risk in 1999 (Change; Lower Right) were analyzed separately.
Trapping sites that remained high-risk areas both years were dominated by an overstory of woody plants, primarily associated with Pinus ponderosa (Ponderosa pine) or Pinus edulis (Piñon pine). Understories in these sites were dominated by Pinus spp. or Quercus spp. In contrast, low–moderate-risk sites either had no overstory or had a sparse overstory of Juniperus sp. (4/11 sites). Atriplex canescens (saltbush) was commonly observed in the understory at these locations. The remaining low-risk sites usually contained Gutierrezia sp. (snakeweed), Larrea tridentata (creosote bush), Artemisia sp. (sagebrush), Salsola kali (tumbleweed), or Prosopis sp. (mesquite) as the primary understory cover. Sites where risk decreased between 1998 and 1999 had a vegetation community that was similar to that found in persistently low–moderate-risk locations.
Trapping Results.
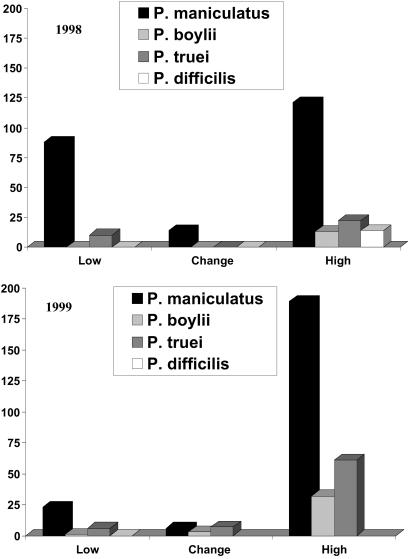
A total of 15,042 trap nights were completed during sampling at the 38 trapping sites (7,527 trap nights and 7,515 trap nights in 1998 and 1999, respectively). Thirteen species of small mammals were captured, with the preponderance (77.8%; n = 826) being members of the genus Peromyscus. Small mammal captures were more common in 1999 (494 animals = 6.4% trap success), the year after the ENSO, than during 1998 (332 animals = 4.41% trap success; P < 0.0001). P. maniculatus was the most common species captured and was found in both low- to moderate- and high-risk areas (Fig. 2). Peromyscus boylii and Peromyscus truei were more commonly observed in high-risk areas than in low- to moderate-risk areas.
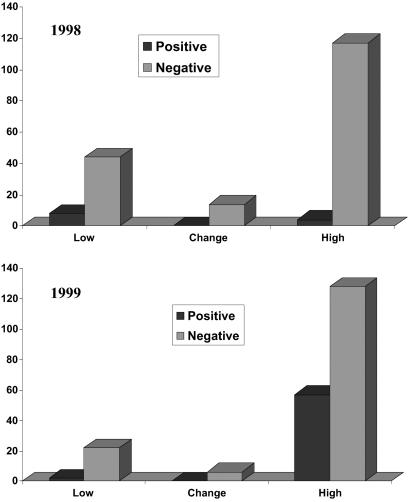
Fig 2.
Numbers of Peromyscus species captured in low–moderate (Low), high, and change sites during 1998 (Upper) and 1999 (Lower). P. maniculatus was the most common member of the genus captured. Its abundance decreased in low and change sites in 1999, but its abundance increased in high sites in 1999.
After the start of the ENSO, in 1998, deer mice were found at most trapping sites but generally were not abundant. Despite the low overall trap success, there was a statistically significant association between the numbers of P. maniculatus captured at a site in 1998 and the human risk predicted from the 1997 satellite images (r = 0.76; F = 53.38; df = 1,37; P < 0.0001). In 1999, the association between the local abundance of deer mice and a linear combination of the risk in 1998 plus the change in risk from 1997 was highly significant (r = 0.92; F = 105.32; df = 2,36; P < 0.0001) and took the form:
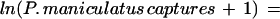 |
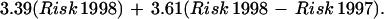 |
Overall, the abundance of deer mice varied between years and was related to the predicted human risk (Fig. 2). At least one deer mouse was captured at 10/11 low–moderate-risk sites in 1998 (x̄ = 8.0 mice per site; SD = 16.06; range, 0–56 mice). However, the average abundance was skewed by results from a single location. When that one moderate-risk site was excluded, capture statistics were substantially lower (x̄ = 3.2; SD = 2.20; range, 0–6 mice). Deer mice were captured at only 4/7 sites that changed from high risk in 1998 to low–moderate risk in the following year (x̄ = 2.0 mice per site; SD = 2.08; range, 0–5 mice). Deer mice were trapped at 17/20 sites in persistently high-risk areas in 1998 (x̄ = 5.4 mice per site; SD = 5.19; range, 0–17 mice). The differences in average local deer mouse abundance by risk category in 1998 were not statistically significant (Kruskal–Wallis; H = 1.37; df = 2,35; P = 0.27).
In 1999, the average abundance of deer mice in low–moderate risk sites was not significantly different from that observed in 1998 (x̄ = 2.1; SD = 4.50; range, 0–15). However, deer mice were captured at fewer low–moderate risk sites (4/11 sites vs. 10/11 sites). Sites that had changed from high risk to low risk in 1999 had marginally lower numbers of deer mice (x̄ = 0.9; SD = 0.90; range, 0–2) and deer mice were captured at only four of seven sites. In contrast, all high-risk sites had at least two deer mice and the numbers of deer mice increased in high-risk areas (x̄ = 9.4; SD = 0.5.79; range, 2–24). The average local abundance of deer mice at high-risk sites was significantly greater than at other sites (Kruskal–Wallis; H = 24.42; df = 2,35; P < 0.001).
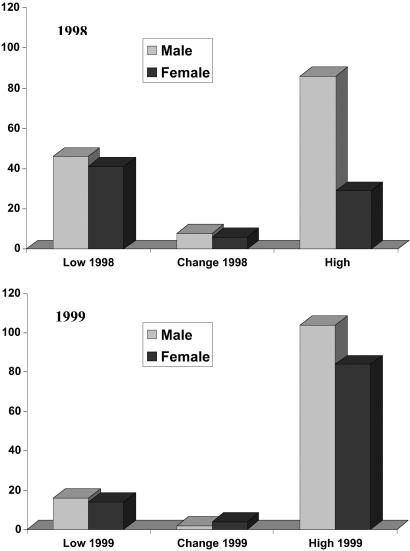
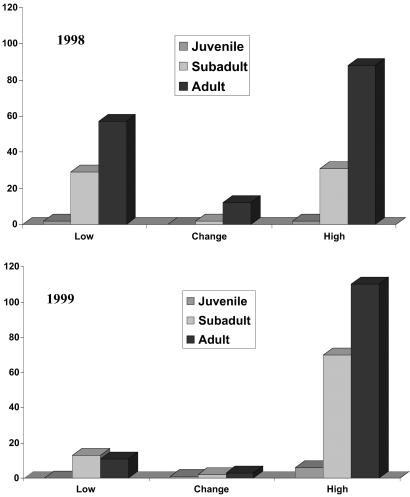
In addition to the increase in the abundance of reservoir populations in high-risk areas in 1999, there were differences in the deer mouse population structure depending on the level of risk. The sex ratios varied depending on the year and the risk categories of sites (Fig. 3). In both years the low–moderate-risk sites and sites where risk varied between years had sex ratios that did not differ from 1:1. Populations in high-risk sites, however, were biased toward males (2.97:1 in 1998 and 1.24:1 in 1999). In 1998, this difference was highly significant (χ2 = 15.11; df = 1; P = 0.0001), and, while the trend persisted in 1999, it was not statistically significant (χ2 = 1.07; df = 1; P = 0.30). Populations in high-risk areas also had a biased age structure (Fig. 4). Populations from high-risk areas during both 1998 and 1999 had a disproportionate fraction of adult animals compared with other risk areas (χ2 = 12.74; df = 5; P = 0.026). By 1999, subadults represented the largest class of individuals in populations from low–moderate-risk sites, whereas adults continued to represent the largest age class in high-risk areas (Fig. 4).
Fig 3.
Numbers of male and female P. maniculatus captured in low, high, and change sites during the 2 study years. Sex ratios were skewed toward males in high-risk sites.
Fig 4.
Age structure of P. maniculatus captured in low–moderate (Low), high, and change sites during 1998 (Upper) and 1999 (Lower). The y axis shows the number of individuals captured. High-risk sites were biased toward adults. Age classes were juvenile, ≤10 g; subadult, 10 to <16 g; adult, ≥16 g.
The prevalence of SNV infection in deer mouse populations varied among sites and between years. During the 1998 ENSO, the prevalence of infection was low and did not differ with the risk level. Deer mice with antibodies to SNV were captured at 5/38 sites (13.1%), three in low–moderate-risk areas and two high-risk areas (χ2 = 0.32; df = 1; P = 0.570). The crude prevalence of infection (6.6%; n = 181) did not differ significantly between high- and low-risk areas. Infection was predominantly in male (91.7%; n = 12), adult (75%; n = 12) mice. Three subadult P. maniculatus also were SNV-antibody-positive. Only one animal of another species (1/26; P. truei) had antibodies to SNV in 1998.
With the end of the El Niño in 1999 more high-risk sites had infected mice and the prevalence of infection in deer mice from the high-risk areas was markedly increased (Fig. 5). Overall, SNV-antibody-positive deer mice were captured at more sites than in 1998 (χ2 = 11.94; df = 1; P = 0.0006). In 1999, 19/38 (50.0%) of the sites had deer mice that were antibody positive. However, infected deer mice were found nearly exclusively in high-risk areas. Only 1/11 low–moderate-risk sites had antibody-positive animals, whereas infected deer mice were found at 18/20 high-risk sites (χ2 = 20.74; df = 1; P < 0.0001). The crude prevalence of infection among mice differed between low–moderate- and high-risk sites (Fig. 5). The prevalence (8.3%; n = 24) in low–moderate-risk sites did not differ from the prevalence in those sites in 1998, whereas the prevalence of SNV infection among deer mice in high-risk sites (30.8%; n = 185) increased from 1998 (χ2 = 32.97; df = 1; P < 0.0001). During 1999, infected deer mice were predominantly male (76.3%; n = 59), adult (67.8%; n = 59) mice, although 1 juvenile female and 18 subadults (3 females and 15 males) also were antibody positive. The prevalence of SNV in adult deer mice within high-risk sites was 36.4% (40/110). In addition to antibody-positive P. maniculatus, 5/36 (13.9%) P. boylii and 10/68 (14.7%) and P. truei also were positive to SNV during 1999. All positive P. boylii and P. truei were captured in high-risk areas.
Fig 5.
Numbers of P. maniculatus with antibody titers ≥100 (positive) or <100 (negative) for SNV captured in low–moderate (Low), high, and change sites during 1998 (Upper) and 1999 (Lower). The prevalence of infection did not differ among risk categories in 1998. Prevalence was significantly elevated in high sites in 1999.
Discussion
Previous work demonstrated that environmental conditions monitored by remote sensing, nearly a year before the 1993 HPS outbreak in the southwestern United States, were associated with the subsequent risk of human disease (5). That study also showed there was significant interannual variation in the level of predicted human risk between El Niño and La Niña events. However, the lack of field data prevented establishing a relationship between the remotely sensed data and known risk factors for disease. This study demonstrates that the algorithm used to predict human risk for HPS from the satellite imagery is also linked with key aspects of hantavirus ecology and SNV transmission within the primary reservoir species. The data provide strong evidence that the apparent year lag between the end of the ENSO and subsequent HPS outbreaks in humans is related to a biological process within the reservoir population that leads to the viral amplification in localized areas. The year-to-year variation in risk at some sites also suggests that ephemeral features of the landscape that affect the reservoir population, rather than vegetation per se, determine human disease risk.
Predicted high-risk areas for humans were characterized by a small mammal fauna dominated by the known reservoir species, P. maniculatus, and local rodent populations in high-risk areas were dominated by those subclasses of the population that were most likely to carry SNV. Deer mice occurred in persistently low-risk areas as well as sites that showed significant year-to-year fluctuations in risk. However, populations in these areas were subject to local extinction after the ENSO, as evidenced by the increasing number of these sites that failed to have any deer mice in 1999 (10/18 in 1999 vs. 4/18 in 1998) and decreases in the numbers of animals caught in the sites that did have mice (Fig. 2). The characteristics of local reservoir populations in high-risk areas differed from those of low-risk areas so much that, after the 1998 ENSO the chance of exposure to SNV was more likely in high-risk areas (Figs. 2 and 5). Populations of deer mice in high-risk areas continued to increase in abundance during 1999, and mice were captured in all high-risk sites. Most significantly, by 1999, the prevalence of infection with SNV exceeded 36% in adult deer mice from high-risk areas, while infection occurred only sporadically (1/18 sites) and at low prevalence (8.3%) in low-risk areas.
The precise ecological factors that link the increased levels of SNV in some local populations of deer mice, the rodent population dynamics, and the signature associated with the satellite imagery remain to be determined. We postulate that many local populations become established when animals colonize habitats as conditions ameliorate during an ENSO. The colonists are derived from animals that disperse from locations with persistently suitable environmental conditions, but colonizing populations are subject to periodic extinctions as local conditions become more stringent after an ENSO. The likelihood of extinction in the colonized areas depends on annual changes in environmental conditions that may be related to soil moisture, as indicated by rapid changes in predicted risk at some sites between 1998 and 1999 (Fig. 1). Low-risk sites represent environmental conditions where populations are able to survive only for short periods of time (<2 years), whereas environmental conditions in high-risk sites favor the establishment and persistence of deer mouse populations for longer periods (9, 17). Because SNV is horizontally transmitted, populations must persist for a sufficient time to allow for the transmission among local population members to reach a level where human risk is elevated. Empirically, this appears to require at least 2 years (Fig. 5). Rodent populations in sites where extinctions are common do not survive long enough (or reach sufficient population densities) for SNV to become widespread. The behavioral ecology of deer mice, with male-dominant dispersal and territoriality (18), may account for the observed demographic characteristics of high-risk areas (Figs. 3–5). Sites that favor population persistence and SNV transmission are predominantly colonized by male mice. These mice defend their territories from other males through aggressive interactions (18). Thus, when suitable sites for SNV persistence are initially colonized, they have sex- and age-biased populations, but later they evolve toward a more equitable sex ratio because of in situ reproduction (as seen in 1999). However, behavioral interactions would cause most juveniles and subadults to disperse from these more suitable habitats, resulting in persistent age-biased populations. In addition, aggressive interactions associated with SNV transmission would cause dispersing subadult and other adult male population members to spread SNV, leading to increased human HPS risk, and the dispersal of SNV to other areas. In contrast, low-risk areas, being less suitable over the long run, may have similar population characteristics to higher-risk sites during colonization but serve as population sinks, as environmental conditions became more severe in subsequent years. These sites have lower population densities and a higher likelihood of extinction, and would be primarily occupied by juvenile and subadult animals (Fig. 4) with associated lower levels of SNV infection.
This hypothesis is consistent with several surveys of deer mice that indicate either the presence of infected mice, or the occurrence of human disease, was associated with specific vegetational zones, and, presumably, environmental conditions (3, 7, 19, 20). Studies of the interplay between local habitat characteristics and the dynamics of reservoir populations show they affect SNV persistence and transmission in reservoir populations (9, 17) much as our analysis proposes. A primary difference is that we propose that vegetation community is a surrogate (albeit a highly correlated one) for the underlying environmental risk factors affecting mouse populations. Our qualitative characterization of habitat indicates that predicted high-risk areas are generally those associated with higher elevation and more mesic vegetation, whereas low-risk areas tended to be vegetation communities associated with lower elevation and xeric plant communities, as the earlier studies indicated. However, the subset of high-risk sites in 1998 that were reclassified as low-risk sites in 1999 had vegetation structure consistent with lower-elevation plant communities (Fig. 1). This finding indicates that the algorithm using the TM imagery recognized something in the environment, rather than vegetation as associated with human risk. Glass et al. (5) suggested that soil moisture may be a factor recognized by the classification algorithm which would account for the loss of some predicted high-risk areas after the end of El Niño in 1998.
Much of the high-risk area identified from the satellite imagery lay at elevations that are only rarely occupied by people. Areas that have persistently remained at high risk since HPS was recognized in 1993 correspond to a relatively small fraction of the environment (data not shown) associated with arroyos and valleys leading from higher-elevation areas. These areas may serve as refugia from which deer mice disperse to lower elevations as environmental conditions become more suitable.
The high prevalence of SNV infection in rodent populations in high-risk areas after an ENSO is consistent with observations from sites where human cases have occurred (6). Prevalences of 30% in those survey samples were not uncommon. Practically, this result suggests the reported prevalences in rodents during case investigations do not represent selection bias. This study also shows that a substantial elevation in prevalence occurs over a year's time in high-risk areas. The lag time between the end of the 1997–1998 ENSO and the increase in SNV prevalence in high-risk areas mirrored the 1993 outbreak of HPS, which occurred within a year of the 1991–1992 ENSO. This time lag also is consistent with the trophic cascade hypothesis (12, 13). These results also demonstrate that increased SNV prevalence occurs locally, while prevalence remains low or absent in nearby locations, emphasizing that local conditions significantly impact virus transmission. Equally important, areas of high SNV prevalence can be detected by satellite.
Future studies, using satellite imagery to identify areas that remain high risk, could characterize the ecological dynamics of local environmental conditions and monitor SNV transmission in deer mouse populations and compare these sites with lower-risk locations. We believe this approach will help identify the environmental determinants of SNV persistence in the environment. These sites may provide important insights into identifying the environmental conditions that lead to increased levels of SNV in reservoir populations and the subsequent increased risk of human disease.
Acknowledgments
G.E.G. was supported by an Intergovernmental Personnel Agreement from the Centers for Disease Control and Prevention (CDC), the United States Environmental Protection Agency (EPA), and National Aeronautics and Space Administration (NASA) Grant NCC5-305. J.A.P. and T.M.S. were also supported by EPA ORD Grant 96-NCERQA-1B. J.A.P. received additional support from a cooperative agreement from the EPA. Satellite imagery, and supplies were provided through Cooperative Agreement CR823143 and Grant 96-NCERQA-1B from the EPA and from NASA Grant NCC5-305. Field studies were supported by National Oceanic and Atmospheric Administration Grant NA96gp0419 and the Museum of Southwestern Biology. The CDC provided funding through a cooperative agreement (to T.L.Y.).
Abbreviations
ENSO, El Niño Southern Oscillation
TM, Thematic Mapper
HPS, hantaviral pulmonary syndrome
SNV, Sin Nombre virus
References
- 1.Nichol S. T., Spiropoulou, C. F., Morzunov, S., Rollin, P. E., Ksiazek, T. G., Feldmann, H., Sanchez, A., Childs, J., Zaki, S., Peters, C. J., et al. (1993) Science 262, 914-917. [DOI] [PubMed] [Google Scholar]
- 2.Schmaljohn C. S. & Hjelle, B. (1997) Emerg. Infect. Dis. 3, 95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mills J. N., Ksiazek, T. G., Ellis, B. A., Rollin, P. E., Nichol, S. T., Yates, T. L., Gannon, W. L., Levy, C. E., Engelthaler, D. M., Davis, T., et al. (1997) Am. J. Trop. Med. Hyg. 56, 273-284. [DOI] [PubMed] [Google Scholar]
- 4.Mills J. N., Ksiazek, T. G., Peters, C. J. & Childs, J. E. (1999) Emerg. Infect. Dis. 5, 135-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glass G. E., Cheek, J. E., Patz, J. A., Shields, T. M., Doyle, T. J., Thoroughman, D. A., Hunt, D. K., Ensore, R. E., Gage, K. L., Irland, C., et al. (2000) Emerg. Infect. Dis. 6, 238-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Childs J. E., Ksiazek, T. G., Spiropoulou, C. F., Krebs, J. W., Morzunov, S., Maupin, G. O., Gage, K. L., Rollin, P. E., Sarisky, J., Enscore, R. E., et al. (1994) J. Infect. Dis. 169, 1271-1280. [DOI] [PubMed] [Google Scholar]
- 7.Boone J. D., Otteson, E. W., Villard, P., McGwire, K. C., Rowe, J. E. & St. Jeor, S. C. (1998) Am. J. Trop. Med. Hyg. 59, 445-451. [DOI] [PubMed] [Google Scholar]
- 8.Calisher C. H., Sweeney, W., Mills, J. N. & Beaty, B. J. (1999) Emerg. Infect. Dis. 5, 126-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calisher C. H., Mills, J. N., Sweeney, W. P., Choate, J. R., Sharp, D. E., Canestorp, K. M. & Beaty, B. J. (2001) J. Wildl. Dis. 37, 280-288. [DOI] [PubMed] [Google Scholar]
- 10.Abbott K. D., Ksiazek, T. G. & Mills, J. N. (1999) Emerg. Infect. Dis. 5, 102-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuenzi A. J., Morrison, M. L., Swann, D. E., Hardy, P. C. & Downard, G. T. (1999) Emerg. Infect. Dis. 5, 113-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parmenter R. R., Brunt, J. W., Moore, D. I. & Ernest, S., (1993) The hantavirus epidemic in the Southwest: Rodent population dynamics and the implications for transmission of hantavirus-associated adult respiratory distress syndrome (HARDS) in the Four Corners region (Univ. of New Mexico, Albuquerque), Sevilleta Long-Term Ecological Research Site Pub. No 41.
- 13.Parmenter R. R., PratapYadav, E., Parmenter, C. A., Ettestad, P. & Gage, K. L. (1999) Am. J. Trop. Med. Hyg. 61, 814-821. [DOI] [PubMed] [Google Scholar]
- 14.Glass G. E., Johnson, J. S., Hodenbach, G. A., DiSalvo, C. L. J., Peters, C. J., Childs, J. E. & Mills, J. N. (1997) Am. J. Trop. Med. Hyg. 56, 359-364. [DOI] [PubMed] [Google Scholar]
- 15.Mills J. N., Yates, T. L., Childs, J. E., Parmenter, R. R., Ksiazek, T. G., Rollin, P. E. & Peters, C. J. (1995) J. Mammal. 76, 716-722. [Google Scholar]
- 16.Feldmann H., Sanchez, A., Morzunov, S., Spiropoulou, C. F., Rollin, P. E., Ksiazek, T. G., Peters, C. J. & Nichol, S. T. (1993) Virus Res. 30, 351-367. [DOI] [PubMed] [Google Scholar]
- 17.Root J. J., Calisher, C. H. & Beaty, B. J. (1999) J. Wildl. Dis. 35, 311-318. [DOI] [PubMed] [Google Scholar]
- 18.Waser P. M. (1987) in Mammalian Dispersal Patterns: The Effects of Social Structure on Population Genetics, eds. Chepko-Sade, B. D. & Halpin, Z. T. (Univ. of Chicago Press, Chicago), pp. 251–256.
- 19.Boone J. D., McGwire, K. C., Otteson, E. W., DeBaca, R. S., Kuhn, E. A., Villard, P., Brussard, P. F. & St. Jeor, S. C. (2000) Emerg. Infect. Dis. 6, 248-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engelthaler D. M., Mosley, D. G., Cheek, J. E., Levy, C. E., Komatsu, K. K., Ettestad, P., Davis, T., Tanda, D. T., Miller, L., Frampton, J. W., et al. (1999) Emerg. Infect. Dis. 5, 87-94. [DOI] [PMC free article] [PubMed] [Google Scholar]