Abstract
The correct spatial expression of two Drosophila bithorax complex (BX-C) genes, abdominal-A (abdA) and Abdominal-B (AbdB), is dependent on the 100-kb intergenic infraabdominal (iab) region. The iab region is known to contain a number of different domains (iab2 through iab8) that harbor cis-regulatory elements responsible for directing expression of abdA and AbdB in the second through eighth abdominal segments. Here, we use in situ hybridization to perform high-resolution mapping of the transcriptional activity in the iab control regions. We show that transcription of the control regions themselves is abundant and precedes activation of the abdA and AbdB genes. As with the homeotic genes of the BX-C, the transcription patterns of the RNAs from the iab control regions demonstrate colinearity with the sequence of the iab regions along the chromosome and the domains in the embryo under the control of the specific iab regions. These observations suggest that the intergenic RNAs may play a role in initiating cis regulation at the BX-C early in development.
In Drosophila, thoracic and abdominal segmental identities are specified by genes in the bithorax complex (BX-C) (1). The BX-C contains >300 kb of genomic DNA but codes for only three homeotic (Hox) transcription factors: Ultrabithorax (Ubx), abdominal-A (abdA), and Abdominal-B (AbdB) (2). Transcription of each of the three protein-coding genes is regulated by an extensive region of cis DNA (1, 3–5). In the cases of abdA and AbdB, the cis-regulatory DNA required for accurate spatial and temporal expression during development lies predominantly in the 100-kb intergenic region (6–8) (Fig. 1A). This region contains an organized array of genetically defined domains: infraabdominal (iab) regions iab2 through iab8 (Fig. 1A). Mutations in any given iab region disrupt the development of a corresponding abdominal segment; iab3 mutations affect abdominal segment 3 (more precisely, parasegment 8, which is composed of the posterior part of abdominal segment 2 and the anterior part of abdominal segment 3), iab4 mutations affect abdominal segment 4, and so on (9, 10).
Fig 1.
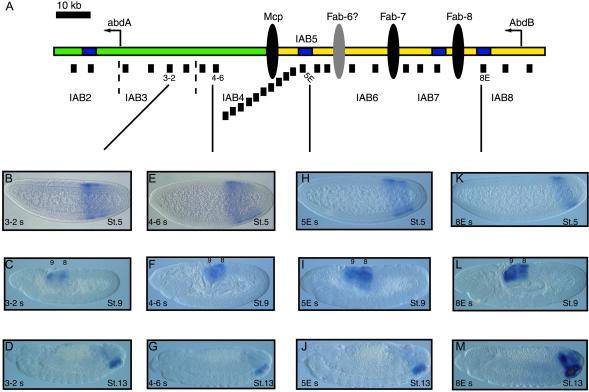
Intergenic transcription at the abdA-AbdB locus. (A) Summary of abdA-AbdB locus. The abdA and AbdB transcription start sites are indicated by leftward arrows. The intergenic region is ≈100 kb in length. The iab regions that control expression of the two Hox genes are indicated (IAB2 to IAB8). IAB2, IAB3, and IAB4 (shown in green) regulate expression of abdA. IAB5, IAB6, IAB7, and IAB8 (shown in yellow) direct AbdB expression. The insulator DNAs that separate the different iab regions are indicated (black ellipses). The presumptive Fab6 insulator (gray ellipse) has yet to be identified. Characterized enhancers within the iab regions are shown as blue rectangles. The locations of probes used for in situ hybridization analysis in this study are shown as black bars under the locus. (B–I) Transcription patterns detected with in situ hybridization probes. Embryos are orientated with anterior to the left and dorsal up. Probes against the abdA (B and C) and AbdB (F and G) coding regions detect the expected distribution of transcripts (see text). An APP probe detects transcription at blastoderm stage 5 (D) and later in development (E), unlike a BPP probe, which detects transcription in abdominal segments 8 and 9 only later in embryonic development (H and I).
Previous studies have shown that the abdA and AbdB transcripts are not the only RNAs produced from this region of the BX-C, because the iab regions also are transcribed in the early embryo (11–13). However, the resolution of the mapping was limited in these studies and, therefore, unable to characterize a specific function for the intergenic transcripts. In this study, we have performed high-resolution in situ hybridization mapping to more accurately analyze endogenous intergenic transcription in the iab regions. In blastoderm-stage embryos, these RNAs are abundant and their transcription patterns show spatial modulation along the anteroposterior axis of the embryo, exhibiting a colinear expression pattern correlating with the iab domain from which they originate. We discuss these findings with regard to regulation of abdA and AbdB expression.
Whole-Mount in Situ Hybridization
Probes from the Bithorax complex were PCR-amplified by using Drosophila yw67 adult genomic DNA as a template. The DNA probes were cloned into pGEMT-Easy (Promega). Sense and antisense riboprobes (relative to the direction of abdA and AbdB transcription; see Fig. 1) were prepared by using a digoxigenin (DIG) RNA-labeling kit (Roche, Gipf-Oberfrick, Switzerland). PCR primer sequences and positions in BX-C (14) were as follows: BPP s, 5′-TATTATTCGTCTCCAGTCGC-3′ (47980); BPP as, 5′-CTCAGATTGATGGTGGTGGTGG-3′ (49031); Bexon s, 5′-GAACAAGAAGAACTCACAGC-3′ (53954); Bexon as, 5′-TAGGCATAGGTGTAGGTGTAGG-3′ (55566); 8E s, 5′-CAAGTGTTGCCATCGTGG-3′ (59940); 8E as, 5′-CATTCCGTCCAGCAATAGAACC-3′ (61783); 7E s, 5′-AAGGCGACCATTATTAGAGTGC-3′ (66156); 7E as, 5′-TTGAAGTCACACAGATGAACGG-3′ (68096); 7-1 s, 5′-GCCACACTCATCGTTATTCTCC-3′ (71024); 7-1 as, 5′-TTGGAGTAGGAGAAGAAGAAGG-3′ (72858); 7-2 s, 5′-GACATCTAACTCTCCTTCAACC-3′ (76879); 7-2 as, 5′-TTATGAAGTCGTAGTTGTCGGC-3′ (78772); 6-1 s, 5′-ATTATGACGGACTGATTGGC-3′ (89455); 6-1 as, 5′-TTGCTGTTGTTGCTACACTACG-3′ (91210); 6-2 s, 5′-AGCAACCACTATGGCAGTCTGG-3′ (96681); 6-2 as, 5′-ATCCGCCTGATAAGGTTCCTCG-3′ (97937); 5-1 s, 5′-TTCCTCTGACCGTGCTCATTGG-3′ (99668); 5-1 as, 5′-AGTGTGTGGTCCGCAATACAGC-3′ (101631); 5-2 s, 5′-ATTGGAATGGAGACTCGCAGCC-3′ (101688); 5-2 as, 5′-ATTCCTTACTATTCGGTACACC-3′ (103688); 5E s, 5′-CAAGATGCTCGCTCGTAACG-3′ (103787); 5E as, 5′-GAAGGTGTGGATAGTTCAGTC C-3′ (105773); 5-3 s, 5′-CGCTGTCTGAATCTTGGC-3′ (106763); 5-3 as, 5′-AAGACACCTGCTTACTAACC-3′ (108463); MCP-1 s, 5′-GCCATTAGTCTGCTCTGAGG-3′ (110002); MCP-1 as, 5′-GACGATGACGATGACGAAGACC-3′ (112089); MCP-2 s, 5′-TTGAGTATTCCACTTACGCTCC-3′ (113068); MCP-2 as, 5′-CGGAGATAACGAATGGCG-3′ (114879); MCP-3 s, 5′-CACTCGCCATTCGTTATCTCCG-3′ (114858); MCP-3 as, 5′-ACCAGGAACGACAATGCC-3′ (116782); MCP-4 s, 5′-TCAATCTCCGTCCTCATTATCG-3′ (117013); MCP-4 as, 5′-TGCGCACTGAACGAATGC-3′ (118783); 4-1 s, 5′-GTATTAGGTGGTCCTGACAGCG-3′ (120611); 4-1 as, 5′-GGTAAGTGTGCCAGATGC-3′ (122366); 4-2 s, 5′-GGCAGCGAATGTTCAAGG-3′ (123505); 4-2 as, 5′-TCGGTATCGGTATCTCCAGTGC-3′ (125457); 4-3 s, 5′-TCACCACCTCCTTCTCATCG-3′ (125733); 4-3 as, 5′-GTCTTATGTGACAAGTGCTGGC-3′ (127486); 4-4 s, 5′-ATGATTGCGATAACCACAGACG-3′ (127544); 4-4 as, 5′-ACTGCTCCTTCTTGTGGTCC-3′ (129275); 4-5 s, 5′-ACCACAAGAAGGAGCAGTCG-3′ (129258); 4-5 as, 5′-GCACTCTCACCTACACGAATGC-3′ (131,319); 4-6 s, 5′-CGACAGCAACATCAGCAATCGC-3′ (135,904); 4-6 as, 5′-ATGCGGTCACCATTGCTCTTCG-3′ (137,616); 4-7 s, 5′-GTCTGCTGTTGAATGTTGACCG-3′ (138,200); 4-7 as, 5′-GAAGTTCTATTGTGTAGTGGCG-3′ (139,391); 3-1 s, 5′-CATAGATACGAACTCACAGACG-3′ (140,638); 3-1 as, 5′-TATTCCGCCATTCCGTTGGACC-3′ (142,398); 3-2 s, 5′-GTGACATTCTGTTGAGCCGACC-3′ (143,635); 3-2 as, 5′-TTATGCTGCGGATTATCTTGGC-3′ (144,635); 3-3 s, 5′-GGAATAGACGAAGATGCTCAGC-3′ (146,932); 3-3 as, 5′-CGCCATCTGTATTCCGTTCG-3′ (148628); APP s, 5′-GTGGTAGCAACAACATAAGG-3′ (150762); APP as, 5′-CTATTGCTCTCATCCTCCTTCG-3′ (152745); IAB2 s, 5′-TCTACCTATCTTCTTCTGCTCC-3′ (171019); IAB2 as, 5′-TAAGACGGTGTCAGACGG-3′ (172988); Aexon s, 5′-CACCAACAGCAGCAACAACAGC-3′ (173566); and Aexon as, 5′-CATTGTATTCAAGCGTTGGC-3′ 174756.
In situ hybridizations were carried out on 2- to 4-h and 0- to 10-h yw67 embryos as described previously (15). In situ hybridizations were repeated at least three times. Expression patterns in blastoderm embryos were measured by photographing at least 10 embryos and calculating the mean domain of expression as a percentage of the total embryo length (0 = anterior tip, 100 = posterior tip).
Results
Intergenic RNAs at the BX-C.
A comprehensive series of 1- to 2-kb probes that span the iab intergenic region between abdA and AbdB was generated (see Fig. 1A) and used for in situ hybridizations in Drosophila embryos. Almost all of these intergenic probes show distinct transcription patterns that are spatially modulated along the anteroposterior (A–P) axis of the blastoderm embryo.
In midstage 5 blastoderm embryos, probes mapping to the exons of the abdA (Fig. 1B) and AbdB genes (Fig. 1F) detect the expected patterns of expression (16–18). abdA is expressed from 53.5% to 81.4% along the A–P axis of the embryo, whereas AbdB is more posterior (66.6–91.1%). Additional transcription is detected with an abdA promoter-proximal probe (APP), which terminates ≈650 bp 5′ of the start site of the abdA transcription unit (14). This probe detects transcription in a sense orientation relative to abdA and AbdB expression and is restricted more toward the posterior of the embryo than the abdA transcript (54.9–86.6%) (Fig. 1D). However, a promoter-proximal probe upstream of the AbdB transcription unit (BPP) fails to detect any expression in blastoderm-stage embryos (Fig. 1H). Taken together, these results indicate that early transcription at this chromosomal location is tightly restricted to the intergenic region and the protein-coding Hox genes themselves. At later developmental stages, abdA is expressed strongly in abdominal segments 1–7 and more weakly in segment 8 (Fig. 1C), whereas AbdB is detected predominantly in segments 7–9 (Fig. 1G) and more weakly in segments 5 and 6 (data not shown). The APP (Fig. 1E) and BPP (Fig. 1I) probes detect similar patterns of late expression in the most posterior abdominal segments, 8 and 9. No transcription could be detected with any of these probes in the antisense orientation (data not shown).
The spatial pattern of transcription in the intergenic region is restricted in very specific anteroposterior patterns throughout early embryonic development. Distinct patterns are detected from probes mapping to each iab region (Fig. 2A). For example, probes from the iab3 region detect transcription extending from a position slightly more posterior than the anterior limit of the abdA expression domain toward the posterior of the embryo (56.6–90.6% embryo length) (Fig. 2B). Accordingly, probes from the other iab regions detect anterior limits of expression increasingly restricted more toward the posterior pole of the blastoderm embryo as they get closer in location to AbdB on the chromosome. Specifically, iab4 probes detect expression from 59.6% to 90.4% (Fig. 2E); iab5 probes, from 66.1% to 89.9% (Fig. 2H); iab6 probes, from 68.1% to 91.3%; iab7 probes, from 70.2% to 90.8%; and an iab8 probe, from 71.9% to 91.2% (Fig. 2K). Interestingly, in many of the embryos examined, the probes detect stronger expression at the anterior region of their expression domain when compared to the signal at the posterior region (Figs. 1 and 2). Furthermore, no significant differences are seen between probes from within the same iab region, even though some of the probes map to known cis-regulatory elements (for example, enhancers 5E, 7E, and 8E), whereas others are from intergenic sequences of currently unknown function. The IAB2 enhancer is located in an intron of the abdA transcription unit and, accordingly, gives the same pattern of expression as the probe against the abdA exon.
Fig 2.
Transcription in the iab regions. (A) The location of probes at the abdA-AbdB locus. (B–M) Patterns of sense transcription detected by in situ hybridization RNA probes. At stage 5, transcripts from the different iab regions show distinct distribution patterns along the A–P axis of the embryo. A probe from the iab3 region detects expression (B) extending farther toward the anterior of the embryo than iab4 (E). Expression patterns become increasingly restricted toward the posterior of the embryo in the iab5 region (H) and iab8 region (K). From stage 9 of development, transcription from all iab regions is restricted to the two most posterior abdominal segments, 8 and 9 (C, F, I, and L). This pattern persists through stage 13 of development (D, G, J, and M).
The temporal pattern of expression detected by almost the entire series of probes is consistent. In blastoderm embryos, transcription is detected from late stage 4/early stage 5 (19), before the completed cellularization of the blastoderm. The initiation of intergenic transcription therefore precedes activation of either abdA or AbdB, which, in agreement with the observations of earlier studies (12), is first detectable from midstage 5 onward. To confirm this observation, embryos were cohybridized with probes against the iab4s transcript and the segment polarity gene engrailed. Early in development, the distinct stripes of the engrailed pattern are seen initially as anterior stripes in stage 5 embryos (20, 21). In late stage 4 embryos, iab4s transcription is already detectable in posterior regions, before engrailed expression (Fig. 3A). By early stage 5, transcription is detected in the posterior iab4s pattern and in the emerging anterior engrailed stripes (Fig. 3B).
Fig 3.
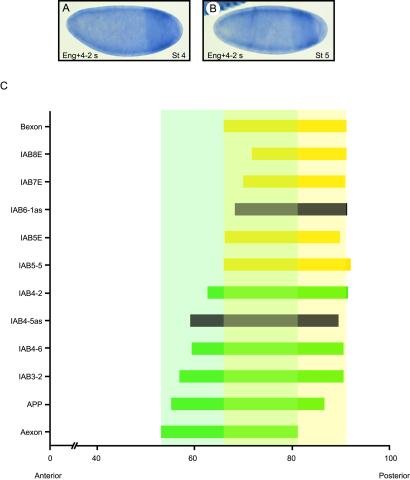
Temporal activation of sense iab transcripts. (A) At late stage 4 in embryos hybridized with probes against engrailed and iab4s transcripts, expression is detected only in the posterior iab4 domain, before the appearance of anterior engrailed expression. (B) In early blastoderm stage 5 embryos, sense transcripts can be detected in the iab4 domain and the anterior engrailed stripe 2. (C) Colinear distribution of transcripts in the iab regions. Measure of transcript distribution is shown as a percentage of embryo length. The anterior tip of the embryo corresponds to 0 and the posterior tip corresponds to 100. The probes are listed according to their order on the chromosome (see Fig. 1A), with the exception of probes against the two Hox genes, Aexon (green) and Bexon (yellow), which are shown at the bottom and the top of graph, respectively. Colinearity between the chromosomal order of probes and their transcription patterns is observed. The anterior limits of transcription for probes from the iab5, iab6, iab7, and iab8 regions are restricted within the AbdB domain of expression (pale yellow), whereas probes from iab3 and iab4 regions detect patterns extending into the abdA expression domain (pale green). The distribution of the antisense transcripts detected in iab4 and iab6 are also colinear with their chromosomal locations and are shown in black.
Colinear Transcription Program.
The anterior expression limit of the sense-orientation transcripts in the embryo correlates with their arrangement on the chromosome, demonstrating that the colinearity of expression previously characterized for the protein-coding Hox genes at the BX-C (22) is maintained throughout the intergenic iab region. This colinear relationship is demonstrated clearly when the domains of transcription are plotted on a graph (Fig. 3C). Probes from each iab region have distinct anterior limits that become restricted more toward the posterior of the embryo as the iab region is located closer to AbdB on the chromosome. Intriguingly, probes from iab5, iab6, iab7, and iab8, the regions known to regulate AbdB expression, detect transcription patterns that are restricted to the domain of AbdB expression, whereas probes from iab3 and iab4, from regions known to direct expression of abdA, detect transcription extending into the domain of abdA expression (Fig. 3C). The distinct transcription patterns observed indicate that the different transcripts may be firing from discrete promoters within each iab region.
Only four of our intergenic probes fail to detect early sense transcription by in situ hybridization: 4-5, 4-4, MCP-4, and 6-1. However, probes 4-5, 4-4, and 6-1 detect transcription in the antisense orientation. Their transcription patterns are presented in the following section. All other probes fail to detect transcription in the antisense orientation. Later in embryonic development, all probes detecting sense transcripts, except those against abdA and AbdB, detect uniform transcription only in the two most posterior abdominal segments, 8 and 9. During germ-band extension (stage 9), expression is in both the ectoderm and mesoderm (Fig. 2). By stage 13, the transcripts are expressed strongly in the ventral nerve cord and more weakly in other posterior tissues (Fig. 2).
Antisense Transcripts in iab Regions.
A further level of complexity in the cis-regulatory program of the iab regions was revealed with probes capable of detecting antisense transcription at the intergenic region. Almost no antisense transcription is detectable throughout the intergenic region. However, our panel of in situ probes does detect the previously characterized iab4as transcript (probes 4-5 and 4-4; see Fig. 1A) (23) as well as a previously unidentified antisense transcript in the iab6 region (probe 6-1; see Fig. 1A). Careful analysis of the in situ hybridization signal in embryos shows that the expression patterns for the antisense transcripts are distinct from those of the sense transcripts already described.
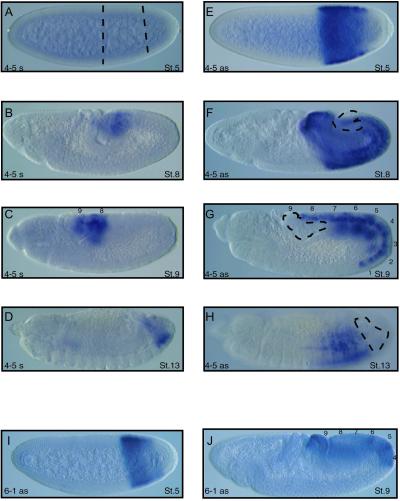
The iab4as transcript is expressed in blastoderm-stage embryos in the same domain as the sense transcript detected by the adjacent 4-6 probe (Figs. 4E and 2). However, the iab4as transcript appears to preclude sense transcription from the opposite strand on the chromosome, because no early sense transcription is detected with the 4-5 probe (Fig. 4 A and E). Sense transcripts can be detected around stage 8 of embryonic development in posterior abdominal segments 8 and 9 (Fig. 4B). Intriguingly, the broadly expressed iab4as transcript is absent from these segments (Fig. 4F). The mutually exclusive expression patterns of iab4as and iab4s become even more apparent slightly later in development; the iab4 sense transcription persists in abdominal segments 8 and 9, whereas the iab4as transcript is restricted predominantly to abdominal segments 2–7 (Fig. 4 C + G and D + H).
Fig 4.
Antisense transcripts in the iab regions. (A–D) Sense transcription pattern at iab4 region during embryonic development detected by probe 4-5. No transcript is detectable at stage 5 (A). Transcription is detectable at stage 8 and, by stage 9, is restricted to abdominal segments 8 and 9. Transcription persists in the two most posterior abdominal segments through stage 13 (D). (E–H) The iab4as transcript (23) also is detected by probe 4-5, although the pattern is distinct from that of sense transcription. At blastoderm stage 5, the antisense transcript is expressed strongly in the iab4 domain (E) and persists in abdominal segments 1–7 through developmental stages 8 (F), 9 (G), and 13 (H), but is excluded from the most posterior segments. The distributions of the iab4as and iab4 sense transcripts appear to be mutually exclusive. (I and J) A novel antisense transcript is detected by probe 6-1. At blastoderm stage 5, the transcript is restricted to the iab6 domain (I) and, by stage 9, is expressed strongly in abdominal segments 4, 5, and 6 and more weakly in 7, 8, and 9 (J). The sense transcription detected by probe 6-1 (data not shown) is mutually exclusive to the iab6as pattern.
The iab6as transcript demonstrates a similar mutual exclusivity of expression with sense transcription from its chromosomal location. The iab6as transcript is expressed early in the embryo in the iab6 domain (Figs. 4I and 2) and becomes predominantly restricted to abdominal segments 4–7 later in development (Fig. 4J). Further characterization of the iab6as transcript is needed. Exon prediction software fails to identify any significant ORFs in the iab6 region, suggesting the iab6as transcript is a noncoding RNA, as is the case for the iab4as transcript. The role of these enigmatic antisense transcripts will be discussed later.
Discussion
Colinear Transcription Program at BX-C iab Regions.
Previous studies have shown that the iab regions of the BX-C are transcribed, although the resolution provided by these studies was unsuitable to accurately assess the functional potential for these RNAs (11–13). Here, we show that transcription through these cis-regulatory regions is abundant and subject to a highly ordered developmental program. The early expression of sense transcripts (relative to the direction of abdA and AbdB expression) from the different iab regions is organized into sequential domains along the A–P axis of the developing embryo. This organization is reminiscent of the colinearity exhibited by the BX-C homeotic genes themselves, which are expressed in the same order along the A–P axis of the embryo as they are organized along the chromosome (1). The intergenic transcripts follow the same rule, because there is colinearity between the location of the iab regions on the chromosome and the anterior limit of transcription in the blastoderm-stage embryo. In this way, regions increasingly closer to AbdB are expressed in increasingly more posterior domains in the embryo, with transcripts from each individual iab region showing unique, spatially restricted patterns of expression (see Fig. 2). The pattern of transcription from each iab region corresponds to the segmental domain of the embryo that is affected by mutations in each particular iab region (10). Therefore, it is conceivable that the early sense transcripts could define the domains of activity for cis-regulatory elements within each iab region.
The timing of expression in the intergenic region is also significant. If the sense transcripts indeed are capable of defining the domains of activity for cis-regulatory elements in the iab regions, then it would be expected that they are transcribed before the time at which the cis-regulation is required. The iab transcripts in fact are detectable by late stage 4/early stage 5 of embryonic development, before the time at which expression is seen from the abdA or AbdB genes (16–18). Earlier studies also noted this temporally restricted order of transcription (12). The spatial and temporal distribution of the sense transcripts represents the earliest known response of the BX-C to the hierarchical positioning information inherited in the embryo from the gap and pair-rule genes (11, 24, 25). It appears that the early transcripts represent an initial primed state of the BX-C and, therefore, could act to define the domains of activity for the iab regions in the embryo. This activity has some parallels with the intergenic transcription that has been characterized at mammalian genes. For example, in the Ig genes, germ-line transcription through cis-regulatory elements is thought to activate interactions with regulatory proteins that are necessary to direct the switching of the class of Ig gene expressed in individual cells (26, 27). The iab transcripts may play a similar role in regulating abdA and AbdB gene expression.
Interplay of Transcription with Cis-Regulatory Elements.
Later in development, all of the intergenic sense transcripts are restricted to expression in the two most posterior abdominal segments. Why the transcription persists late in development is unclear. It is possible that once the domains of activity for the iab regions are established by the early transcripts, other factors may maintain the iab-regulated expression of the target homeotic genes. The transcriptional regulatory proteins of the Polycomb group and Trithorax group are good candidates for this role because they are known to be necessary to maintain expression states for the homeotic genes in the BX-C (28–30). They act through cis-regulatory elements in the iab regions, which have been identified as Polycomb response elements or cellular memory modules (31), to promote either a silenced or activated state throughout development by generating stable, higher-order chromatin structures (32). It is possible that the transcription we detect through the Polycomb response elements early in development primes their segment-specific activity, which is heritable through future cell divisions. Once the Polycomb response elements are activated, they are able to maintain the regulation of the homeotic gene-expression patterns, and, consequently, the iab transcripts are no longer required. One prediction of this model is that ectopic transcription through the iab Polycomb response elements later in development may interfere with Polycomb-mediated silencing. The continued expression of the iab transcripts in abdominal segments 8 and 9 late in development may indicate that homeotic gene expression in these segments is not regulated by the Polycomb or Trithorax group proteins and that the functional role for the transcripts consequently persists.
The potential role of the antisense transcripts remains enigmatic. The previously characterized iab4as transcript (23) is known to be processed, although it appears to have no protein-coding potential. Our identification of an additional antisense transcript in the iab6 domain may suggest a shared function. One possibility is that the antisense transcripts contribute to the inhibition of the spreading of the sense transcripts from one iab region to another, because they prevent transcription from the opposite DNA strand (Fig. 4 A–J). Their chromosomal locations, relatively close to the insulator elements Mcp and Fab-7, are consistent with this notion. It is possible that the antisense transcripts may be processed and function to inhibit the sense transcripts by an RNA interference mechanism, similar to the silencing characterized in Schizosaccharomyces pombe (33). However, despite extensive attempts, we have failed to identify antisense transcripts in the iab5 or iab7 regions. Further molecular characterization of the sense and antisense transcripts and analysis of genetic mutations at the BX-C will be necessary to further facilitate elucidation of their in vivo functional activities.
Acknowledgments
We thank Katharine Arney, Andrew Dowsett, and Joanne Topol for comments on the manuscript and Mark Stapleton for providing the engrailed probe. This work was supported by grants from the National Institutes of Health to M.L. (GM34431) and E.B.L. (HD06331). R.A.D. was funded by a Wellcome Trust Prize Traveling Fellowship.
Abbreviations
BX-C, bithorax complex
A–P, anteroposterior
APP, abdA promoter-proximal probe
BPP, AbdB promoter-proximal probe
References
- 1.Lewis E. B. (1978) Nature 276, 565-570. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez-Herrero E., Vernos, I., Marco, R. & Morata, G. (1985) Nature 313, 108-113. [DOI] [PubMed] [Google Scholar]
- 3.Duncan I. (1987) Annu. Rev. Genet. 21, 285-319. [DOI] [PubMed] [Google Scholar]
- 4.Lewis E. B., Knafels, J. D., Mathog, D. R. & Celniker, S. E. (1995) Proc. Natl. Acad. Sci. USA 92, 8403-8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morata G., Sanchez-Herrero, E. & Casanova, J. (1986) Cell Differ. 18, 67-78. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Herrero E. (1991) Development (Cambridge, U.K.) 111, 437-449. [DOI] [PubMed] [Google Scholar]
- 7.Boulet A. M., Lloyd, A. & Sakonju, S. (1991) Development (Cambridge, U.K.) 111, 393-405. [DOI] [PubMed] [Google Scholar]
- 8.Celniker S. E., Sharma, S., Keelan, D. J. & Lewis, E. B. (1990) EMBO J. 9, 4277-4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis E. B., (1996) Les Prix Nobel 1995 (Nobel Foundation, Stockholm), pp. 235–260.
- 10.Karch F., Weiffenbach, B., Peifer, M., Bender, W., Duncan, I., Celniker, S., Crosby, M. & Lewis, E. B. (1985) Cell 43, 81-96. [DOI] [PubMed] [Google Scholar]
- 11.Casares F. & Sanchez-Herrero, E. (1995) Development (Cambridge, U.K.) 121, 1855-1866. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Herrero E. & Akam, M. (1989) Development (Cambridge, U.K.) 107, 321-329. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J., Ashe, H., Burks, C. & Levine, M. (1999) Development (Cambridge, U.K.) 126, 3057-3065. [DOI] [PubMed] [Google Scholar]
- 14.Martin C. H., Mayeda, C. A., Davis, C. A., Ericsson, C. L., Knafels, J. D., Mathog, D. R., Celniker, S. E., Lewis, E. B. & Palazzolo, M. J. (1995) Proc. Natl. Acad. Sci. USA 92, 8398-8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohtsuki S., Levine, M. & Cai, H. N. (1998) Genes Dev. 12, 547-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karch F., Bender, W. & Weiffenbach, B. (1990) Genes Dev. 4, 1573-1587. [DOI] [PubMed] [Google Scholar]
- 17.Kuziora M. A. & McGinnis, W. (1988) EMBO J. 7, 3233-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeLorenzi M., Ali, N., Saari, G., Henry, C., Wilcox, M. & Bienz, M. (1988) EMBO J. 7, 3223-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bownes M. (1975) J. Embryol. Exp. Morphol. 33, 789-801. [PubMed] [Google Scholar]
- 20.Tabata T., Eaton, S. & Kornberg, T. B. (1992) Genes Dev. 6, 2635-2645. [DOI] [PubMed] [Google Scholar]
- 21.Drees B., Ali, Z., Soeller, W. C., Coleman, K. G., Poole, S. J. & Kornberg, T. (1987) EMBO J. 6, 2803-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGinnis W. & Krumlauf, R. (1992) Cell 68, 283-302. [DOI] [PubMed] [Google Scholar]
- 23.Cumberledge S., Zaratzian, A. & Sakonju, S. (1990) Proc. Natl. Acad. Sci. USA 87, 3259-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harding K. & Levine, M. (1988) EMBO J. 7, 205-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ingham P. W. & Martinez-Arias, A. (1986) Nature 324, 592-597. [DOI] [PubMed] [Google Scholar]
- 26.Laurencikiene J., Deveikaite, V. & Severinson, E. (2001) J. Immunol. 167, 3257-3265. [DOI] [PubMed] [Google Scholar]
- 27.Xu M. & Stavnezer, J. (1992) EMBO J. 11, 145-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karch F., Galloni, M., Sipos, L., Gausz, J., Gyurkovics, H. & Schedl, P. (1994) Nucleic Acids Res. 22, 3138-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busturia A. & Bienz, M. (1993) EMBO J. 12, 1415-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hagstrom K., Muller, M. & Schedl, P. (1997) Genetics 146, 1365-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavalli G. & Paro, R. (1999) Science 286, 955-958. [DOI] [PubMed] [Google Scholar]
- 32.Pirrotta V. (1998) Cell 93, 333-336. [DOI] [PubMed] [Google Scholar]
- 33.Volpe T. A., Kidner, C., Hall, I. M., Teng, G., Grewal, S. I. & Martienssen, R. A. (2002) Science 297, 1833-1837. [DOI] [PubMed] [Google Scholar]