Abstract
Toxic recombination events are detected in vegetative Saccharomyces cerevisiae cells through negative growth interactions between certain combinations of mutations. For example, mutations affecting both the Srs2 and Sgs1 helicases result in extremely poor growth, a phenotype suppressed by mutations in genes that govern early stages of recombination. Here, we identify a similar interaction involving double mutations affecting Sgs1 or Top3 and Mus81 or Mms4. We also find that the primary DNA structures that initiate these toxic recombination events cannot be double-strand breaks and thus are likely to be single-stranded DNA. We interpret our results in the context of the idea that replication stalling leaves single-stranded DNA, which can then be processed by two competing mechanisms: recombination and nonrecombination gap-filling. Functions involved in preventing toxic recombination would either avoid replicative defects or act on recombination intermediates. Our results suggest that Srs2 channels recombination intermediates back into the gap-filling route, whereas Sgs1/Top3 and Mus81/Mms4 are involved in recombination and/or in replication to allow replication restart.
Keywords: replication fork arrest, helicases, endonucleases
During DNA replication, the elongation of the new strands can be impaired by the presence of lesions on the template strands, bound proteins, or secondary structures. The replication fork stalls, which may lead to the disassembly of the replicative protein complex. Different cellular processes ensure the rescue of the arrested replication forks, and it is now established that homologous recombination (HR) is one of the mechanisms involved (reviewed in ref. 1). In Escherichia coli, different models involving recombination reactions were proposed to account for the tolerance of UV lesions, notably the “strand switch” model (2) and the “fork regression” model (3) first proposed for mammalian cells (4). In the latter model, re-annealing of the nascent strands creates an X-shaped structure with one duplex arm containing a double-strand end, a structure sometimes referred to as a chicken foot. The formation of such structures has been evidenced in mutants of replication helicases deprived of the RecBC exonuclease activity that is required for double-strand break (DSB) repair (5, 6). In those mutant cells, the X structure is cleaved by the Holliday junction (HJ) resolvase RuvC, generating chromosomal DSBs and cell death. In recombination-proficient cells, it is believed that the double-strand tail resulting from fork regression is processed by RecBCD to form a recombination structure with the chromosome, allowing replication to resume (reviewed in ref. 7). In mammalian cells, sister chromatid exchanges occur spontaneously and likely reflect reciprocal exchanges occurring during replication (8, 9). At the molecular level, X-shaped recombination intermediates, similar to HJs, are formed during mitotic replication at different loci of Physarum polycephalum (10) and in the Saccharomyces cerevisiae ribosomal DNA locus (11).
In S. cerevisiae, HR is initiated by the formation of a Rad51 nucleoprotein filament on single-stranded DNA (ssDNA). Nucleation of the Rad51 filament is aided by the Rad52 and Rad55/Rad57 proteins (reviewed in ref. 12). During synapsis, the Rad51 filament is active in homology search and strand invasion to establish joint molecules in conjunction with the Rad54 protein (13–15). In vegetatively growing S. cerevisiae cells, none of these genes is essential. This finding implies that normal replication or any other part of the cell cycle can proceed in the absence of HR. However, there is evidence that in certain mutant situations recombination does occur during S-phase and is lethal. This situation is illustrated by our previous report (16) showing that simultaneous deletion of the Srs2 and Sgs1 helicases renders cells extremely sick, and that a vast majority of the cells in the colonies that succeed to develop are dead; however, this phenotype is suppressed by mutations in any of the RAD51, RAD52, RAD55, and RAD57 genes. Such events were also proposed to be responsible for the short life span of srs2 and sgs1 single mutants (17). That the defect of srs2 sgs1 mitotic cells relates to replication is strongly suggested by the findings that each protein plays a role during S-phase and possibly in S-phase-specific checkpoints (18, 19). Furthermore, their respective protein level increases during S-phase (20, 21) and the mutants are sensitive to hydroxyurea (HU), an inhibitor of ribonucleotide reductase that generates replicative defects (19, 22). The Sgs1 and Srs2 proteins share no homology with each other, but they do unwind DNA with the same 3′ to 5′ polarity (23, 24). Sgs1 belongs to the RecQ-type helicase subfamily and has several orthologues in mammalian cells. These include BLM, WRN, and RTS, responsible for the Bloom, Werner, and a subset of Rothmund-Thomson syndromes, respectively, which confer genetic instability when mutated (reviewed in ref. 25). The Srs2 helicase domains share homologies with the bacterial UvrD, Rep (26), or PcrA helicases and, to date, no Srs2 orthologue in mammalian cells has been described.
In the present study, we searched for mutants showing recombination-dependent growth defects in combination with a deletion of SGS1. Among the genes identified are MUS81 and MMS4. MUS81 was first discovered in S. cerevisiae through a search for proteins interacting with the Rad54 recombination protein (27). The Schizosaccharomyces pombe orthologue of Mus81 was discovered as interacting with the forkhead domain of Cds1, a checkpoint kinase involved in the tolerance of DNA damage during replication (28). The phenotype of the mutants in both organisms led the authors to propose that Mus81 is involved in the processing of stalled replication forks, as well as in meiotic functions. It was shown that the Mus81 proteins from S. cerevisiae (29), S. pombe (30, 31), and human cells (32) catalyze endonucleolytic activities. However, the different authors did not assign the same biological function to Mus81 (reviewed in ref. 33). The function of Mus81 from S. pombe and human cells was first proposed to be HJ cleavage (30, 32), whereas Mus81 from S. cerevisiae, which cleaves three branched structures with a much higher efficiency than HJ, was proposed to act at stalled replication forks (29). Recently, however, Mus81 from S. pombewas also shown to efficiently cleave three-branched structures (31), leaving open the possibility that the proteins from both yeasts have a similar role in replication or in recombination resulting from replication stalling.
We have also investigated the nature of the DNA interruptions leading to the toxic recombination seen in several mutants. Our findings indicate that the majority of such recombination events are not generated by DSBs, but likely by ssDNA resulting from replication arrest. We discuss two nonexclusive models in which Sgs1 works either in replication or in recombination processing.
Materials and Methods
Strains.
The strains used in this study are isogenic derivatives of either FF18-733 (MATa) and 18-734 (MATα leu2-3,112 trp1-289 ura3-52 his7-2 lys1-1) or of the W303 RAD5 derivatives D1841B (MATa) and D184-1C (MATα ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1). The mutants were constructed by the one-step gene disruption method using plasmids or PCR fragments. Details are available on request. The strains were verified by PCR or Southern blot analysis. The MATa haploids in the FF18 series are: 733, wild-type; 958, rad51:URA3; 1079-2, rad51:ura3, derived from 958; 742, rad52:URA3; 974, rad54:LEU2; 1461, rad55:URA3; WDHY1177, rad57:KAN; 1769, rad59:KAN; 1767, lig4:KAN; 1659, mus81:KAN; 1495, sgs1:URA3; 744, srs2:LEU2. The MATa strains in the W303 background are: wild-type, D184-1B; rad51:LEU2, D142-4B; mms4:URA3, D212-4C; mus81:URA3, D210-1A; top3:TRP1, D201-11B; srs2:LEU2, D195-4A; sgs1:TRP1, D190-4B. Multiple mutant strains were derived from meiotic segregants. In some crosses, the mutant segregants were identified by complementation analysis on plates containing methyl methanesulfonate (MMS).
Media, Growth Conditions, and Growth Rate Determination.
Standard yeast genetics and molecular techniques were performed according to Guthrie and Fink (34). MMS medium was prepared by adding 0.012% methyl methane sulfonate to the rich yeast extract/peptone/dextrose (YPD) medium. Doubling times were determined by measuring optical density at 600 nm during exponential growth in liquid YPD at 30°C. Aliquots were taken every 60 min for a period of at least 7 h.
Results
To investigate the relationships between replication and recombination, we first studied a number of mutations known to be either synthetically lethal or to show a strong negative interaction with sgs1. Next, we asked whether this interaction is suppressed by mutations in genes required for presynapsis and synapsis during HR (RAD51, RAD52, RAD54, RAD55, and RAD57). We then studied the interaction of the different mutations to define more precisely the pathways in which they act. Finally, we investigated whether DSBs could be the DNA lesions that lead to recombination detected in relevant mutant conditions, with the idea that if DSBs are not involved, the initiating DNA substrate must be single-stranded or, at least, must have “single-stranded character.”
Mus81/Mms4 and Sgs1/Top3 Act in Related Pathways to Prevent Toxic Recombination.
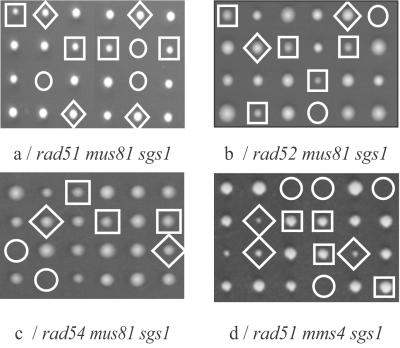
A functional relationship between Mus81 and Sgs1 was first demonstrated in S. pombe where the mus81 deletion was shown to be synthetically lethal with a deletion of rqh1+, the orthologue of SGS1 (28). In S. cerevisiae, a similar result was obtained by Mullen et al. (35) who reported that a mutation called slx3, an allele of MUS81, is synthetically lethal with sgs1, an interaction that we had also observed. To determine whether the sgs1 mus81 interaction depends on HR, we asked whether mutations in HR genes suppress the lethality of the double mutant. In a first experiment, diploid cells heterozygous for deletions of SGS1 and MUS81 were sporulated and tetrad analysis performed. All of the double-mutant spores failed to develop into a colony, as reported (35). A similar experiment was next performed with diploid cells heterozygous not only for SGS1 and MUS81 but also for RAD51 (Fig. 1a). Again, the sgs1 mus81 double-mutant spores were unable to form a visible colony. However, all of the rad51 sgs1 mus81 triple-mutant spores developed into colonies, as did the rad51 sgs1 or rad51 mus81 double-mutant spores. Similar results were obtained with rad52, rad54 (Fig. 1 b and c), rad55, and rad57 (data not shown), confirming that elimination of HR suppresses the lethality of mus81 sgs1 cells.
Fig 1.
Inactivation of recombination suppresses the synthetic lethality of mus81 sgs1 and mms4 sgs1 mutants. Tetrads from diploids heterozygous for the three mutations are indicated underneath the figures. The single “rad ” segregant clones are indicated by squares, the sgs1 mus81 and sgs1 mms4 double-mutant clones by circles, and the triple mutants by diamonds.
Mutations in six different genes were isolated by Mullen et al. (35) as synthetically lethal with sgs1. One of the genes was MUS81. We introduced deletions of the five other SLX genes in our strains to ask whether the synthetic lethality is suppressed when HR is eliminated. Confirming the published data, the tetrad analysis of diploid cells heterozygous for the slx and sgs1 mutations indicated that the double-mutant spores do not develop into colonies (data not shown). Diploids heterozygous for the three rad51, slx, and sgs1 mutations were then constructed and sporulated. Tetrad analysis showed that rad51 suppresses exclusively the slx2 sgs1 lethality (Fig. 1d). Thus, only slx2, an allele of MMS4 (35), behaves like mus81 with respect to recombination. We did not detect any growth interactions between mus81 and mms4 (data not shown), indicating that the two proteins act within the same pathway. This finding is in full agreement with the results of Mullen et al. (35) showing that the mms4 mutant shares the phenotype of mus81: elevated sensitivity to MMS, weak sensitivity to HU and to UV, and meiotic defects. MMS4 and MUS81 belong to the same epistasis group for repair after UV or MMS treatment. The two proteins coimmunoprecipitate and were proposed to act together during replication. Additionally, the requirement of Mms4 for the nuclease activity of Mus81 was recently demonstrated (29).
The mms4 and mus81 mutations were reported to be synthetically lethal not only with sgs1 but also with top3 (35). TOP3 encodes a type I-5′ DNA topoisomerase capable of decatenating ssDNA (36). The sgs1 mutant was originally isolated as a suppressor of the top3 slow growth phenotype, and both Top3 and Sgs1 proteins were shown to interact physically (37). This epistatic relationship was interpreted to mean that Top3 resolves topological constraints that result from the Sgs1 helicase activity. We now find that the mus81 top3 double-mutant lethality is suppressed by rad51. However, like top3 and top3 rad51 cells, the triple-mutant colonies are small and heterogeneous in size (data not shown).
Taken together, the several observations presented above imply that toxic recombination reactions occur when both Sgs1/Top3 and Mus81/Mms4 are absent.
Srs2 and Rad54 Are Also Important for Preventing the Occurrence of Toxic Recombination Reactions.
Two other combinations of mutations also confer synthetic lethality or a severe growth defect that is reversed by tertiary mutations in RAD51, RAD52, RAD55, or RAD57.
First, we reported previously that srs2 interacts negatively with sgs1 and that this interaction is alleviated by a rad51, rad52, rad55, or rad57 mutation (16). Similar results have also been reported for the srs2 top3 interaction (38). We have reproduced the latter results in our own strain background, with the minor difference that, in tetrads derived from srs2 and top3 heterozygous diploids, a few very slow growing srs2 top3 double mutants were recovered (data not shown), a phenomenon that we had previously observed for sgs1 srs2 cells. Therefore, in our strains, sgs1 and top3 behave in a similar way and show a strong negative interaction with srs2 that is reversed by the four indicated rad mutations.
Second, it has also been shown that srs2 interacts negatively with rad54 (39) and that this interaction is again suppressed by a rad51, rad52, rad55, or rad57 mutation (20, 39).
Thus, in total, three different partially overlapping combinations of functions lead to the occurrence of toxic recombination intermediates: Mms4/Mus81–Sgs1/Top3; Sgs1/Top3–Srs2, and Srs2–Rad54. These three combinations cannot represent three distinct but overlapping pathways: In that case, elimination of any two pathways would not confer lethality. Thus, more complex relationships among the interrelated functions must be involved. For example, because srs2 and mus81 are each synthetically lethal with sgs1, Srs2 might be a component of the Mms4/Mus81 pathway. Indeed, the mus81 srs2 double mutant exhibits only a slight decrease in growth rate as compared with each single mutant (Table 1). However, this simple model is precluded by the fact that rad54 alleviates the mus81 sgs1 lethality but confers synthetic lethality in combination with srs2. Thus, additional complexities would have to be included with respect to rad54 and/or srs2.
Table 1.
Doubling time of haploid mutants
| Relevant genotype | Strain | Doubling time, min |
|---|---|---|
| Wild type | FF18733 | 105 |
| rad51 | FF181079 | 105 |
| sgs1 | FF181495 | 107 |
| srs2 | FF18744 | 104 |
| mus81 | FF181659 | 105 |
| rad59 | FF181769 | 106 |
| lig4 | FF181767 | 105 |
| rad52 | FF18742 | 144 |
| srs2 mus81 | FF181661 | 119 |
| srs2 sgs1 | FF181611 | 372 |
| rad51 sgs1 mus81 | FF181667 | 132 |
| rad51 srs2 sgs1 | FF181787 | 107 |
| rad51 srs2 sgs1 rad59 | FF181806 | 105 |
| rad52 lig4 | FF181773 | 147 |
| rad52 srs2 sgs1 | FF181787 | 145 |
| rad52 srs2 sgs1 lig4 | FF181808 | 145 |
| rad52 sgs1 mus81 | FF181787 | 195 |
Strains are isogenic.
Mus81 and Sgs1 Also Play a Role Not Related to Recombination.
The fact that mutations in HR genes suppress the lethality of a mus81 sgs1 mutant indicates that HR is the main cause of death in that condition. However, we observed on the dissection plates that the rad51 sgs1 mus81 or rad52 mus81 sgs1 triple-mutant clones, although present, were smaller than the single rad51 or rad52 clones (Fig. 1 a and b). This difference suggests that the effect of mus81 sgs1 is not restricted to HR. To further explore this possibility, we determined the growth rates of these mutants as well as those of rad51 or rad52 strains that did or did not also contain srs2 sgs1 (Table 1).
Double mutants involving rad51 or rad52 and srs2, sgs1, or mus81 grow at the same rate as the corresponding single rad mutants (data not shown). Furthermore, rad51 srs2 sgs1 mutants grow at the same rate as rad51 cells (doubling time of 105 min) and the rad52 srs2 sgs1 mutants divide only slightly slower than rad52 cells (162 and 144 min, respectively; 12% of increase). That is, in a rad51 or rad52 background, srs2, sgs1, mus81, and srs2 sgs1 mutations do not confer any important growth defect. These results indicate that the negative interaction between srs2 and sgs1 is essentially dependent on HR.
A different situation holds for sgs1 and mus81. The generation time of the rad sgs1 mus81 triple mutants is increased by ≈30% with respect to the single rad mutant (132 and 105 min for rad51; 195 and 144 min for rad52), implying that the sgs1 mus81 mutant combination confers a significant defect beyond that which can be reversed by the rad51 mutation. However, rad51 or rad52 strains carrying in addition either the mus81 or sgs1 single mutation grew as well as the corresponding rad mutant (data not shown). Thus, the extra defect requires a negative interaction between mus81 and sgs1, and, conversely, the negative interaction between mus81 and sgs1 is only partially HR dependent. We infer that Mus81 and Sgs1 also play roles that are interdependent and are independent of recombination. Likely, the sgs1 slx interactions that are not suppressed by rad51 (see above) are related to these other roles (29).
Nature of the DNA Structures That Initiate Mitotic Recombination.
Recombination does not initiate on intact DNA but instead must be provoked by either a DSB or a single-stranded gap or, possibly, a duplex region that has single-stranded character, e.g., due to supercoiling. The following experiments were designed to determine whether DSBs could be responsible for the death or very slow growth observed in double mutants that undergo toxic recombination.
In S. cerevisiae, DSBs are essentially repaired by HR. All known and efficient HR mechanisms require RAD52. Thus, if DSBs initiate the toxic events observed in the double mutants, the suppression by rad52 should depend on nonhomologous end-joining (NHEJ), the only other known process that seals DSBs. We tested this idea by coupling rad52 to lig4, a mutation that inactivates the DNA ligase involved in NHEJ. The rad52 lig4 cells were found to grow like rad52 cells, implying that DSBs do not occur frequently in wild-type cells. In addition, lig4 also has no effect on the growth of rad52 srs2 sgs1 (Table 1). We interpret these patterns to mean that the spontaneous deleterious recombination events suppressed by rad52 are not be initiated by DSBs.
The above conclusion holds for toxic recombination events suppressed by rad52. However, there remains the possibility that a subset of the events may be initiated by DSBs and repaired in rad51 cells but not in rad52 cells. This possibility arises because rad52 cells exhibit a slow growth phenotype (Table 1) not shared by rad51 cells (in other strains rad51 confers a slight slow growth phenotype). In that case, the DSB repair processes acting in rad51 cells would be break-induced replication (BIR) and/or single strand annealing (SSA), both of which are known to be RAD52 dependent and largely RAD51 independent (reviewed in ref. 40). We tested this possibility by using rad59, a mutation shown to largely prevent BIR and SSA in rad51 cells (41, 42). rad59 has no effect on the growth rates of rad51 or of rad51 srs2 sgs1 cells (Table 1), suggesting that in rad51 cells, BIR and/or SSA are not involved in processing DSBs. Because it was shown that rad59 eliminates only ≈80% of BIR (41) and SSA (42) events induced in rad51 cells by the HO endonuclease, we cannot exclude the possibility that the residual repair is sufficient to ensure wild-type growth rates in rad51 cells. However, if DSBs were frequently formed and repaired by BIR or SSA, rad59 should have had at least some effect on the rad51 growth rate.
Taken together, these results strongly suggest that DSBs are rare events in wild-type cells and likely not the initiating lesions for the toxic recombination observed in the assayed mutant situations. We infer that recombination is most often initiated on ssDNA formed during replication.
Discussion
The occurrence of toxic recombination is defined by the observation of cell lethality that is alleviated by mutations in RAD51, RAD52, RAD55, or RAD57. We show here that such a condition arises in double mutants lacking either SGS1 or TOP3 and either MUS81 or MMS4. Previous studies have shown that this condition also occurs in double mutants lacking both SGS1 and SRS2 (16, 38) and in double mutants lacking both SRS2 and RAD54 (20, 39). These results implicate six different gene products in averting the formation of toxic recombination intermediates. We focus here on the nature of the DNA structure on which recombination is initiated and on the possible function of these different proteins
DSBs Are Not the Major Lesions Initiating Recombination Events.
Our evidence suggests that DSBs are likely not the initiating lesions that lead to toxic recombination in the various double mutants discussed in this report, because concomitant elimination by rad52 of all efficient HR mechanisms and by lig4 of NHEJ still results in suppression of the srs2 sgs1 phenotype (Table 1). Similarly, suppression by rad51 is not affected by rad59 (Table 1), which largely inhibits the Rad51-independent BIR and SSA DSB repair processes. Furthermore, because our rad51 and rad51 rad59 mutants grow as wild-type cells, it appears that DSBs do not occur frequently in wild-type cells. We believe that the slow growth of a rad52 mutant (Table 1) relates to events that are not initiated by DSBs and that depend on RAD52 but not on RAD51. In agreement with this idea, recombinant structures detected in the yeast rDNA during replication are dependent on RAD52 but not on RAD51 (11), and the spontaneous rate of unequal sister chromatid exchanges was also found to be unaffected by rad51 (43). In none of these cases were DSBs evidenced. This does not exclude the possibility that DSBs are occasionally formed, but they must be rare enough to be undetectable by growth rate studies.
Are Mus81/Mms4, Sgs1/Top3, and Srs2 Involved in Replication or Recombination?
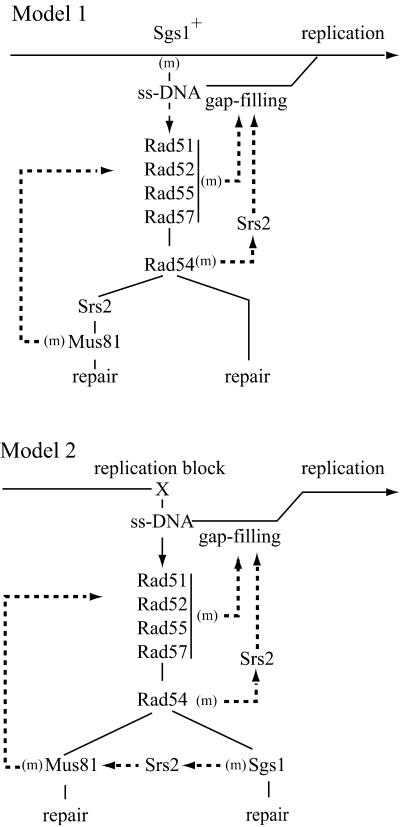
For simplification and because mus81 or mms4 on one hand, and sgs1 or top3 on the other, have similar effect in the interactions described above, we will now refer to Mus81/Mms4 as Mus81 and to Sgs1/Top3 as Sgs1. Our genetic data indicate that most of the spontaneous recombination events responsible for the death or sickness of srs2 sgs1, sgs1 mus81, and srs2 rad54 cells are initiated on single-stranded structures likely formed on replication arrest. Because rad51, rad52, rad55, or rad57 mutations result in suppression of the synthetic defect, we infer that the toxic recombination intermediates are initiated by Rad51-ssDNA filaments. Indeed, all these rad mutations affect the formation or stability of the Rad51 nucleofilament (ref. 44; reviewed in ref. 12). In the absence of recombination initiation, an alternative nonrecombinational gap-filling process must act to ensure replication restart. Two main hypotheses can explain the formation of toxic recombination structures. First, some functions governed by these genes play a role in replication to prevent formation of ssDNA on which recombination could be initiated and become toxic. Second, the gene products act on recombination intermediates formed on ssDNA generated in the course of normal DNA replication. They could then catalyze the formation of a structure that would allow replication restart by recombination or channel the structure into an alternative non recombinational gap-filling process. In the two models proposed here, Rad54, Srs2, and Mus81 act after recombination initiation, whereas Sgs1 functions either upstream or downstream of recombination.
These choices derive from the following considerations. First, all of the involved functions except SGS1 are placed downstream of DNA replication per se (1). Numerous data showed that Rad54 is involved in recombination reactions, and there is no indication that Rad54 affects replication independently of recombination (2). Srs2 was proposed to reverse intermediate recombination structures (45) on the basis that the SRS2 deletion suppresses the γ-ray or the MMS sensitivities of leaky alleles of RAD51 or RAD52 but has no suppressive effect on null alleles (39, 45–47). In the absence of Srs2, recombinational repair occurs because of the leakiness of the recombination system. It would indeed be difficult to interpret these results if the lack of Srs2 generates more recombination substrates. In addition, we have obtained preliminary evidence that purified Srs2 acts on intermediate recombination structures (unpublished data). We will therefore assign to Srs2 a role in recombination (3). Regarding Mus81, it interacts with Rad54 (27) and plays a role in meiotic recombination (29, 48) known to occur after replication, and we will assume that it acts in recombination. The two models arise because the situation is more complex for Sgs1. The sgs1 mutation decreases the rates of conversion induced by genotoxic agents as compared with the wild-type responses (16, 49) but increases that of untreated cells (37). Thus, Sgs1 plays both positive and negative roles in recombination, which may or may not be related to its role in the intra-S-phase checkpoint (19, 50). We will envisage separately the possibility that Sgs1 acts in replication to prevent recombination or acts in the maturation of recombination structures. However, we would like to emphasize that meiotic defects of sgs1 mutants result from a deficiency in processing DNA structures that are subsequent to recombination initiation (51) and that the biochemical properties of Sgs1 or its human orthologues suggest a role in HJ migration (52–54).
Specific Roles of Mus81/Mms4, Sgs1/Top3, and Srs2 During Vegetative Growth.
The two models shown in Fig. 2 account for the Rad51-dependent synthetic lethal interaction observed in sgs1 srs2, sgs1 mus81, and srs2 rad54 cells and for the suppression by rad54 of the sgs1 mus81 but not of sgs1 srs2 cells. In both models, ssDNA is formed on replication arrest and the gap can either be filled in by a nonrecombinogenic process or be bound by recombination proteins. Mutations in early recombination steps obviously prevent the occurrence of toxic recombination. When recombination is arrested at the later Rad54 step, Srs2 is required to channel the structure back into the alternative pathway.
Fig 2.
Pathways involved in replication recovery, in wild-type and mutant cells. On replication arrest, ssDNA is processed either by a nonrecombinogenic gap-filling mechanism or by HR. In model 1, Sgs1 acts in replication to prevent the formation of ssDNA. In model 2, Sgs1 acts in one of the recombination subpathways. “(m)” indicates the channeling of the intermediate in the corresponding single mutant (see text for explanations).
In the first model, Sgs1 helps replication progression and prevents formation of ssDNA on which recombination could be initiated. In the sgs1 mutant, replication arrest leads to recombination initiation. After the Rad54 step, two types of structures are formed, only one of which requiring Srs2 and Mus81 to be resolved. The sgs1 mus81 cells are saved by rad54 because in the triple mutant the upstream rad54 intermediate is a substrate for Srs2, which channels the structure into the alternate gap-filling process. Indeed, the sgs1 mus81 rad54 srs2 cells are dead and saved by a rad51 mutation (data not shown). This model accounts for the spontaneous sgs1 hyperrecombination phenotype. However, because srs2 and rad54 are synthetically lethal, we believe that recombination is also initiated in wild-type cells and is toxic in certain double mutants. Therefore, in the second model, naturally occurring ssDNA is eventually bound by recombination proteins and Sgs1 plays a role in recombination, downstream of Rad54. In the sgs1 mutant, Srs2 channels the intermediate to the Mus81 subpathway. These two models are not necessarily exclusive, in view of the different possible situations that trigger replication arrest, such as the nature of the initial DNA structure that might depend on the strand affected (leading or lagging) or the possible collapse of the replication fork. Furthermore, Sgs1 might be involved in both replication and recombination, so that in the mutant, recombination is more often initiated, as in model 1, and processing involves Srs2 and Mus81, as in model 2.
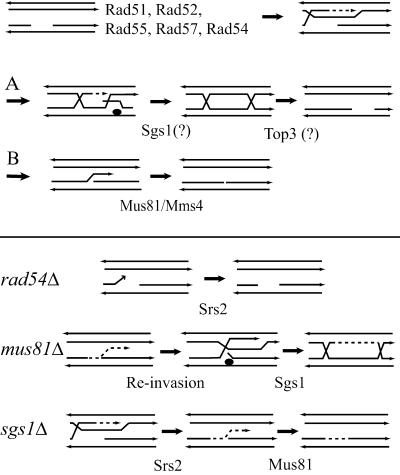
Both of our models require that two types of recombination intermediates be formed after the Rad54 step. Rad54 catalyzes the duplex invasion step (15) after which replication is primed. Two processes could then occur. Either this intermediate forms a recombination structure involving HJs (Fig. 3, A) that can be resolved with or without reciprocal exchange (55) or the newly synthesized strand is displaced from its template and re-anneals with the gapped molecule (Fig. 3, B). This synthesis-dependent strand-annealing (SDSA) process does not lead to a reciprocal exchange and was proposed to account for the large excess of spontaneous mitotic convertants not associated with crossovers (reviewed in ref. 40). Although SDSA was proposed to account for DSB repair events, there is no reason to believe that it could not be initiated from single-stranded gaps. Replication primed by the invading strand may extend beyond the region corresponding to the gap. This extension could be favored by the absence of complementary 3′ ssDNA believed to capture the D loop and consequently to limit replication. Re-annealing generates a 3′ single-stranded overhang protruding from the duplex, a structure reported to be an excellent substrate for the Mus81/Mms4 endonuclease (29). We see two possibilities for the fact that the single mus81 mutation does not affect growth rates. First, the uncleaved protruding DNA can re-invade the sister chromatid to form a structure processed through the alternate HJ pathway. Second, Sgs1 could unwind the 5′ end at the junction of the mus81 intermediate, allowing the re-annealing of the 3′ strand and transforming the 3′ flap into a 5′ flap structure cleaved by the Rad27 endonuclease. This possibility is supported by the synthetic lethality of both mus81 and srs2 with rad27 (38, 56). Unfortunately, the essential role of HR in rad27 cells (57) prevents a direct test of this hypothesis.
Fig 3.
Molecular model for the functions of Srs2, Mus81, and Sgs1 during recombinational repair of replicative defects. Recombination initiated from a single-stranded gap leads after strand invasion and DNA synthesis to intermediates that can be metabolized through two pathways leading to a double HJ structure (A) or, on strand displacement, to a 3′ tail protruding from the initially gapped molecule (B). This scheme applies to the second model shown in Fig. 2 (see text for explanations). (Lower) The fate of the intermediate structures in the rad54, mus81, or sgs1 mutants.
In our models, the activity of Srs2 in sgs1 mutants leads to a structure metabolized by Mus81. If the only role of Srs2 is to revert intermediate recombination structures, it is difficult to imagine that it creates HJ structures resolved by Mus81/Mms4, as proposed for S. pombe (30) and human cells (32). The finding that Mus81 cleaves 3′ flap structures (29) fits much better with our data. The absence of this activity was also proposed by de Los Santos et al. (48) to account for the meiotic defect of mms4 cells. In their model, the 3′ flap structures result also from re-annealing of the invading strand.
The role of Sgs1 in replication would be to help the polymerase to replicate through obstacles and thus prevent recombination initiation (model 1). On arrest, Sgs1 may regulate fork regression that could allow repair or bypass of the obstacle by a nonrecombinogenic process (1). Its role in the processing of recombination intermediates (model 2 of Fig. 3, A) could be to enlarge the single-stranded region by unwinding the DNA while the displaced DNA would be degraded, or to participate in D-loop elongation by opening the duplex DNA in front of the polymerase. Interestingly, the human Wrn helicase interacts with the replication proteins Polδ, PCNA, and RP-A (58, 59), and in the wrn mutants cells, toxic recombination events are formed (60). Because it has been shown that Sgs1 and Top3 can also work independently, we do not know whether Sgs1 always associates with Top3 to carry out these functions (51, 61). However, Top3 that decatenates single strands (36) could resolve the HJs. Such a role was proposed to be the essential function of Top3 during meiotic recombination (51) and has more recently been suggested to be a role of Top3 in mitotic cells (62).
Conclusion
Our results are consistent with the idea that in the absence of any genotoxic treatment, DNA replication leads to the formation ssDNA that is eliminated either by a nonrecombinogenic gap-filling process or by recombination. If both Mus81/Mms4 and Sgs1/Top3 functions are mutated, the recombination events are deleterious, indicating roles in prevention of recombination during replication and/or in maturation of intermediate structures. In S. cerevisiae, Srs2 would play an important role in eliminating potentially toxic recombination intermediates, thus allowing repair through other pathways. It can be predicted that such a function is conserved in higher eukaryotes, although we do not yet know whether this task is performed by an unidentified Srs2 orthologue or by another type of activity.
Acknowledgments
We thank Xavier Veaute, Laurent Maloisel, Laurence Leloup, Kirk Ehmsen, Mike Rolfsmeier, Jachen Solinger, and James Haber for comments and suggestions, and Delphine Dervins for excellent technical assistance. This work was supported by the Commissariat à l'Energie Atomique, the Centre National de la Recherche Scientifique and Electricité de France, and a grant to W.-D.H. from the National Institutes of Health (GM-58015).
Abbreviations
DSB, double-strand break
HJ, Holliday junction
HR, homologous recombination
ssDNA, single-stranded DNA
MMS, methyl methanesulfonate
References
- 1.Oakley T. J., Goodwin, A., Chakraverty, R. K. & Hickson, I. D. (2002) DNA Repair 1, 463-482. [DOI] [PubMed] [Google Scholar]
- 2.Rupp W. D., Wilde, C. E., III, Reno, D. L. & Howard-Flanders, P. (1971) J. Mol. Biol. 61, 25-44. [DOI] [PubMed] [Google Scholar]
- 3.Echols H. & Goodman, M. F. (1991) Annu. Rev. Biochem. 60, 477-511. [DOI] [PubMed] [Google Scholar]
- 4.Higgins N. P., Kato, K. & Strauss, B. (1976) J. Mol. Biol. 101, 417-425. [DOI] [PubMed] [Google Scholar]
- 5.Seigneur M., Bidnenko, V., Ehrlich, S. D. & Michel, B. (1998) Cell 95, 419-430. [DOI] [PubMed] [Google Scholar]
- 6.Seigneur M., Ehrlich, S. D. & Michel, B. (2000) Mol. Microbiol. 38, 565-574. [DOI] [PubMed] [Google Scholar]
- 7.Michel B., Flores, M. J., Viguera, E., Grompone, G., Seigneur, M. & Bidnenko, V. (2001) Proc. Natl. Acad. Sci. USA 98, 8181-8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonoda E., Sasaki, M. S., Morrison, C., Yamaguchi-Iwai, Y., Takata, M. & Takeda, S. (1999) Mol. Cell. Biol. 19, 5166-5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambert S. & Lopez, B. S. (2001) Oncogene 20, 6627-6631. [DOI] [PubMed] [Google Scholar]
- 10.Benard M., Maric, C. & Pierron, G. (2001) Mol. Cell 7, 971-980. [DOI] [PubMed] [Google Scholar]
- 11.Zou H. & Rothstein, R. (1997) Cell 90, 87-96. [DOI] [PubMed] [Google Scholar]
- 12.Sung P., Trujillo, K. M. & Van Komen, S. (2000) Mutat. Res. 451, 257-275. [DOI] [PubMed] [Google Scholar]
- 13.Mazin A. V., Bornarth, C. J., Solinger, J. A., Heyer, W. D. & Kowalczykowski, S. C. (2000) Mol. Cell 6, 583-592. [DOI] [PubMed] [Google Scholar]
- 14.Van Komen S., Petukhova, G., Sigurdsson, S., Stratton, S. & Sung, P. (2000) Mol. Cell 6, 563-572. [DOI] [PubMed] [Google Scholar]
- 15.Solinger J. A., Lutz, G., Sugiyama, T., Kowalczykowski, S. C. & Heyer, W. D. (2001) J. Mol. Biol. 307, 1207-1221. [DOI] [PubMed] [Google Scholar]
- 16.Gangloff S., Soustelle, C. & Fabre, F. (2000) Nat. Genet. 25, 192-194. [DOI] [PubMed] [Google Scholar]
- 17.McVey M., Kaeberlein, M., Tissenbaum, H. A. & Guarente, L. (2001) Genetics 157, 1531-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liberi G., Chiolo, I., Pellicioli, A., Lopes, M., Plevani, P., Muzi-Falconi, M. & Foiani, M. (2000) EMBO J. 19, 5027-5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frei C. & Gasser, S. M. (2000) Genes Dev. 14, 81-96. [PMC free article] [PubMed] [Google Scholar]
- 20.Heude M., Chanet, R. & Fabre, F. (1995) Mol. Gen. Genet. 248, 59-68. [DOI] [PubMed] [Google Scholar]
- 21.Cho R. J., Campbell, M. J., Winzeler, E. A., Steinmetz, L., Conway, A., Wodicka, L., Wolfsberg, T. G., Gabrielian, A. E., Landsman, D., Lockhart, D. J. & Davis, R. W. (1998) Mol. Cell 2, 65-73. [DOI] [PubMed] [Google Scholar]
- 22.Mankouri H. W., Craig, T. J. & Morgan, A. (2002) Nucleic Acids Res. 30, 1103-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rong L. & Klein, H. L. (1993) J. Biol. Chem. 268, 1252-1259. [PubMed] [Google Scholar]
- 24.Lu J., Mullen, J. R., Brill, S. J., Kleff, S., Romeo, A. M. & Sternglanz, R. (1996) Nature 383, 678-679. [DOI] [PubMed] [Google Scholar]
- 25.Mohaghegh P. & Hickson, I. D. (2001) Hum. Mol. Genet. 10, 741-746. [DOI] [PubMed] [Google Scholar]
- 26.Aboussekhra A., Chanet, R., Zgaga, Z., Cassier-Chauvat, C., Heude, M. & Fabre, F. (1989) Nucleic Acids Res. 17, 7211-7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Interthal H. & Heyer, W. D. (2000) Mol. Gen. Genet. 263, 812-827. [DOI] [PubMed] [Google Scholar]
- 28.Boddy M. N., Lopez-Girona, A., Shanahan, P., Interthal, H., Heyer, W. D. & Russell, P. (2000) Mol. Cell. Biol. 20, 8758-8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaliraman V., Mullen, J. R., Fricke, W. M., Bastin-Shanower, S. A. & Brill, S. J. (2001) Genes Dev. 15, 2730-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boddy M. N., Gaillard, P. H., McDonald, W. H., Shanahan, P., Yates, J. R., III & Russell, P. (2001) Cell 107, 537-548. [DOI] [PubMed] [Google Scholar]
- 31.Doe C. L., Ahn, J. S., Dixon, J. & Whitby, M. C. (2002) J. Biol. Chem. 277, 32753-32759. [DOI] [PubMed] [Google Scholar]
- 32.Chen X. B., Melchionna, R., Denis, C. M., Gaillard, P. H., Blasina, A., Van de Weyer, I., Boddy, M. N., Russell, P., Vialard, J. & McGowan, C. H. (2001) Mol. Cell 8, 1117-1127. [DOI] [PubMed] [Google Scholar]
- 33.Haber J. E. & Heyer, W. D. (2001) Cell 107, 551-554. [DOI] [PubMed] [Google Scholar]
- 34.Guthrie C. & Fink, G. R., (1991) Guide to Yeast Genetics and Molecular Biology (Academic, San Diego).
- 35.Mullen J. R., Kaliraman, V., Ibrahim, S. S. & Brill, S. J. (2001) Genetics 157, 103-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim R. A. & Wang, J. C. (1992) J. Biol. Chem. 267, 17178-17185. [PubMed] [Google Scholar]
- 37.Gangloff S., McDonald, J. P., Bendixen, C., Arthur, L. & Rothstein, R. (1994) Mol. Cell. Biol. 14, 8391-8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klein H. L. (2001) Genetics 157, 557-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schild D. (1995) Genetics 140, 115-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pâques F. & Haber, J. E. (1999) Microbiol. Mol. Biol. Rev. 63, 349-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Signon L., Malkova, A., Naylor, M. L., Klein, H. & Haber, J. E. (2001) Mol. Cell. Biol. 21, 2048-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugawara N., Ira, G. & Haber, J. E. (2000) Mol. Cell. Biol. 20, 5300-5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fasullo M., Giallanza, P., Dong, Z., Cera, C. & Bennett, T. (2001) Genetics 158, 959-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fortin G. S. & Symington, L. S. (2002) EMBO J. 21, 3160-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chanet R., Heude, M., Adjiri, A., Maloisel, L. & Fabre, F. (1996) Mol. Cell. Biol. 16, 4782-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Milne G. T., Ho, T. & Weaver, D. T. (1995) Genetics 139, 1189-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaytor M. D., Nguyen, M. & Livingston, D. M. (1995) Genetics 140, 1441-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de los Santos T., Loidl, J., Larkin, B. & Hollingsworth, N. M. (2001) Genetics 159, 1511-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Onoda F., Seki, M., Miyajima, A. & Enomoto, T. (2001) Mol. Gen. Genet. 264, 702-708. [DOI] [PubMed] [Google Scholar]
- 50.Myung K. & Kolodner, R. D. (2002) Proc. Natl. Acad. Sci. USA 99, 4500-4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gangloff S., de Massy, B., Arthur, L., Rothstein, R. & Fabre, F. (1999) EMBO J. 18, 1701-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bennett R. J., Keck, J. L. & Wang, J. C. (1999) J. Mol. Biol. 289, 235-248. [DOI] [PubMed] [Google Scholar]
- 53.Karow J. K., Constantinou, A., Li, J. L., West, S. C. & Hickson, I. D. (2000) Proc. Natl. Acad. Sci. USA 97, 6504-6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Constantinou A., Tarsounas, M., Karow, J. K., Brosh, R. M., Bohr, V. A., Hickson, I. D. & West, S. C. (2000) EMBO Rep. 1, 80-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holliday R. A. (1964) Genet. Res. 5, 282-304. [Google Scholar]
- 56.Tong A. H., Evangelista, M., Parsons, A. B., Xu, H., Bader, G. D., Page, N., Robinson, M., Raghibizadeh, S., Hogue, C. W., Bussey, H., et al. (2001) Science 294, 2364-2368. [DOI] [PubMed] [Google Scholar]
- 57.Symington L. S. (1998) Nucleic Acids Res. 26, 5589-5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brosh R. M., Orren, D. K., Nehlin, J. O., Ravn, P. H., Kenny, M. K., Machwe, A. & Bohr, V. A. (1999) J. Biol. Chem. 274, 18341-18350. [DOI] [PubMed] [Google Scholar]
- 59.Kamath-Loeb A. S., Johansson, E., Burgers, P. M. & Loeb, L. A. (2000) Proc. Natl. Acad. Sci. USA 97, 4603-4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saintigny Y., Makienko, K., Swanson, C., Emond, M. J. & Monnat, R. J., Jr. (2002) Mol. Cell. Biol. 22, 6971-6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duno M., Thomsen, B., Westergaard, O., Krejci, L. & Bendixen, C. (2000) Mol. Gen. Genet. 264, 89-97. [DOI] [PubMed] [Google Scholar]
- 62.Wang J. C. (2002) Nat. Rev. Mol. Cell. Biol. 3, 430-440. [DOI] [PubMed] [Google Scholar]