Abstract
Cellular RNA extracted from quiescent human foreskin fibroblasts harvested at 1, 3, 7, or 12 h after infection was profiled on Affymetrix HG-U95Av2 arrays designed to detect 12,626 unique human transcripts. We also profiled RNA extracted from cells harvested at 1 and 7 h after infection with a mutant lacking the gene (ΔUL41) encoding a protein (vhs) brought into cells by the virus and responsible for nonselective degradation of RNA early in infection. We report the following: (i) of the 12 tested genes, up-regulated at least 3-fold relative to the values of mock infected cells, 9 were confirmed by real-time PCR. The microchip assays analyses indicate that there were 475 genes up-regulated ≥3-fold. The up-regulated genes were clustered into 15 groups with respect to temporal pattern of transcript accumulation, and classified into 20 groups on the basis of their function. The preponderance of cellular genes up-regulated early in infection play a predominant role in transcription, whereas those up-regulated at later times respond to intracellular stress or concern themselves with the cell cycle and apoptosis. (ii) The number of genes up-regulated early in infection was higher in cells infected with the ΔUL41 mutant. Conversely, more genes were down-regulated late in infection with wild-type virus than with mutant viruses. Both observations are compatible with the known function of the UL41 gene product early in infection and with degradation of cellular RNAs in the absence of replenishment by de novo transcription of cellular genes.
Herpes simplex viruses 1 and 2 (HSV-1 and -2) have evolved an elaborate strategy to block host responses to infection. Of the numerous functions expressed by viral gene products designed to shut off the host, two are particularly important. A protein designated as virion host shutoff (vhs) (a product of the UL41 ORF) that is brought into the cell as a component of the viral tegument causes the degradation of preexisting and newly transcribed mRNA during the first few hours after infection (1). At later times after infection, newly made vhs is sequestered and rendered inactive by another viral protein, αTIF (VP16) (2). Another protein made immediately after infection, the infected cell protein 27 (ICP27), the product of the α27 ORF, contributes to the reduction in cellular mRNA levels by blocking pre-mRNA splicing (3). Late in infection, ICP27 shuttles between nucleus and cytoplasm, promotes the export of viral mRNAs (4), and no longer appears to block splicing (reviewed in ref. 5). The functions of ICP27 are not harmful to the virus. Of the four viral genes containing introns, three (α0, α22, and α47) are expressed immediately after infection, concurrently with the α27 gene. The fourth gene, UL15, is expressed late in infection at the time when ICP27 promotes mRNA export. The known functions of the UL41 and α27 gene products suggest that accumulation of cellular mRNA early in infection would be significantly reduced, and that even less cellular mRNA would be processed into mRNA and translated. Nevertheless, the known functions of these proteins would also predict that cellular transcripts could accumulate in larger amounts late in infection.
The predictions based solely on the basis of the known functions of viral gene products raise several important questions. On one hand, the interdiction of cellular gene expression early in infection raises the question whether the intent of the elaborate strategy is to block specific cellular responses that could block viral replication. Another scenario, equally plausible, given that viral mRNA is also subject to the same nucleolytic attack early in infection, is that cellular transcripts made at a very high rate could be expressed notwithstanding the efficacy of the blockade. On the other hand, the equally elaborate window of opportunity for cellular gene expression at mid or late times after infection raises the question whether the cellular takeover is complete, and hence the cell response inconsequential, or whether the virus induces the expression of specific cellular genes whose products it requires for its own use.
In the studies described here, we profiled cellular RNAs after infection to characterize transcriptional response to herpes simplex virus infection as a function of time after infection. DNA microarray technology provides a powerful tool to profile cellular responses to many environmental stimuli, including infections with a variety of viruses (6–9). For these studies, we have chosen GeneChip microarrays HG-U95Av2 (Affymetrix, Santa Clara, CA), containing probes representative of 12,626 human transcripts, to profile the RNA accumulating at different times after infection with wild-type virus or a mutant ΔUL41 lacking the gene encoding the vhs protein. Because of the nonselective degradation of RNA caused by vhs, we focused on genes up-regulated after HSV-1 infection. The studies were done on quiescent human foreskin fibroblasts (HFF) because they most closely resemble the human cells likely to be infected in vivo. Moreover, growth arrest induced by confluency and serum starvation could be expected to reduce transcriptional activity to the bare minimum necessary to keep the cells alive. Lastly, by selecting this system, we could compare our results with those obtained following human cytomegalovirus virus (HCMV) infection (9).
Materials and Methods
Cells and Viruses.
Primary HFF cells were cultured in DMEM supplemented with 10% FCS. The stock of HSV-1(F), the prototype wild-type HSV-1 strain used in this laboratory, was prepared as follows: at 24 h after infection HEp-2 cells were harvested, rinsed, and suspended in ice-cold 0.4 M NaCl for 30 min. The cells were then centrifuged and the pellet discarded. The supernatant fluid was diluted 10-fold in serum-free medium (mixture 199) and stored at −80°C. The stock ΔUL41 (mutant R2621; ref. 10) was prepared by the same procedure. All virus stocks were titered in Vero cells.
Cell Infection.
Confluent HF cells (1 × 107 cells in a 150-cm2 flask) were stored for 7 days in the same (spent) medium and were then mock infected or exposed to 20 plaque-forming units (pfu) of HSV-1(F) or ΔUL41 per cell for 90 min at 6°C in the spent medium. The inoculum was then replaced with 25 ml of the spent medium and the cultures shifted to 37°C (0 h) to synchronize the infection. Three independent infections with HSV-1(F) were carried out. The medium was removed from infected cells at 1, 3, 7, or 12 h after the shift to 37°C, and the cells were lysed in situ by the addition of 8 ml of TRIzol reagent (Life Technologies, Rockville, MD) and frozen at −80°C. The ΔUL41 mutant-infected cells were harvested at 1 and 7 h after infection. Viral infection was confirmed by Northern analyses for the presence of the α22 ORF transcripts (data not shown).
RNA Purification and Hybridization to GeneChip.
Total RNA was purified from TRIzol suspension according to the manufacturer's instructions and then digested with DNase (Life Technologies), extracted with phenol-chloroform and precipitated with ethanol (Fisher) to remove contaminating DNA. The RNA samples were hybridized to Affymetrix HG-U95A array by the Functional Genomics Facility at the University of Chicago. Three different sets of arrays were used for the three independent experiments of HSV-1(F) infection.
Data Analysis.
Chip data sets were analyzed with the Affymetrix GeneChip analysis software (Microarray Suite Version 5) and transformed to a Microsoft EXCEL file. Data were first sorted based on the P value that defines the presence or absence of the signal for each probe set (>0.06 = absent; <0.06 and >0.04 = marginal; <0.04 = present). In the present study, the P value has been changed to 0.08 to limit false-negatives. The intensity values of each gene present in at least one sample, including mock-infected cells, were extracted and recompiled. Fold increase for each gene was calculated with the aid of genespring software (Version 4.2.1, Silicon Genetics, Mountain View, CA), using mock-infected cell data as baseline. Genes were assigned to specific functional groups with the aid of the Netaffx analysis center (www.affymetrix.com/analysis/index.affx).
Real-Time PCR.
Total RNA (1 μg) from mock-infected and HSV-1-infected cell samples was reverse transcribed with random hexamers and Multiscribe Reverse transcriptase (Applied Biosystems). cDNA quantities were normalized to 18S rRNA quantities (primers from Ambion, Austin, TX) obtained from the same plate. Real-time PCR primers were selected for each gene by using primer express software (Version 2.0, Applied Biosystems). Reactions were performed in a 50-μl volume that included diluted cDNA sample, primers (1 μg/ml), and SYBR Green PCR Master mix (Applied Biosystems) that contained nucleotides, AmpliTaq Gold DNA polymerase, and optimized buffer components. Real-time PCR reactions were performed on an Applied Biosystems Prism 7000 sequence detection system. Predicted cycle threshold (Ct) values were exported directly into excel worksheets for analysis. After cycling, a melting curve was produced by slow denaturation of the PCR end products to validate the specificity of amplification. To compare mock and infected cell samples, relative changes in gene expression were determined using the 2−ΔΔCt method as described (11, 12).
Results
Experimental Design.
Differential expression of HFF genes during a 12-h interval after HSV-1 infection was investigated as described in Materials and Methods. Because the vhs protein, a component of the viral tegument, causes the degradation of preexisting and newly transcribed mRNA during the first few hours after infection (1), we focused on RNAs whose expression was up-regulated compared with that of mock-infected cells. Three independent experiments were done, and the RNAs were analyzed on three different sets of GeneChips. To capture a truly representative snapshot of the RNAs accumulating in response to HSV-1 infection, cellular RNAs whose transcripts showed up-regulation at least in two of the three experiments were included in the final tally.
Time Course of Accumulation of Cellular Transcripts.
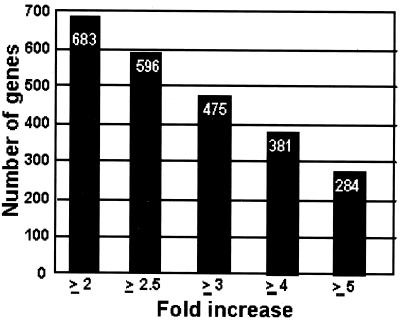
Based on the RNA accumulation profile during the time course, the genes could be divided into 15 groups, from A to O (Table 1). The total number of genes up-regulated in HSV-1-infected cells from ≥2- to ≥5-fold relative to the corresponding value of mock-infected cells is also shown (Fig. 1). Several aspects of this table are of interest. Only a relatively small number of genes were up-regulated throughout the 12 h of study (group A). As expected, a larger number of genes was up-regulated at 7 or 12 h after infection compared with earlier time points. Nevertheless, even in the presence of vhs activity, a certain number of genes were up-regulated early in the infection (groups A–D), suggesting either that these RNAs were resistant to vhs or that the genes were transcribed at a very high level. Finally, the largest number of up-regulated genes accumulated at a single time point after infection (groups L–O). Indeed, they represented 80% of the total number of up-regulated genes at the ≥5-fold level compared with those of mock-infected cells.
Table 1.
Distribution of genes up-regulated in HSV-1-infected cells according to temporal pattern of RNA expression relative to those of mock-infected cells
| Group
|
Hours postinfection | HSV-1-infected/mock-infected | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 7 | 12 | >2.0 | >2.5 | >3.0 | >4.0 | >5.0 | |
| A | + | + | + | + | 12 | 9 | 6 | 3 | 2 |
| B | + | + | + | − | 12 | 5 | 4 | 1 | 0 |
| C | + | + | − | − | 30 | 18 | 14 | 8 | 4 |
| D | + | + | − | + | 2 | 1 | 0 | 1 | 0 |
| E | − | + | + | − | 45 | 32 | 22 | 9 | 4 |
| F | − | + | + | + | 43 | 30 | 16 | 7 | 4 |
| G | − | − | + | + | 64 | 61 | 62 | 42 | 30 |
| H | + | − | + | + | 10 | 5 | 2 | 0 | 0 |
| I | + | − | + | − | 13 | 12 | 8 | 6 | 5 |
| J | − | + | − | + | 16 | 10 | 8 | 3 | 1 |
| K | + | − | − | + | 5 | 4 | 3 | 1 | 1 |
| L | + | − | − | − | 120 | 93 | 75 | 50 | 38 |
| M | − | + | − | − | 123 | 111 | 85 | 56 | 38 |
| N | − | − | + | − | 111 | 115 | 75 | 88 | 70 |
| O | − | − | − | + | 77 | 90 | 95 | 106 | 87 |
+, Increased by the indicated ratio compared to mock-infected cells; −, absent or below the indicated ratio compared to mock-infected cells.
Fig 1.
Total number of up-regulated genes in HSV-1(F)-infected cells according to fold increase relative to mock-infected cells. The total number of genes for each ratio value was calculated based on the distribution of genes reported in Table 1.
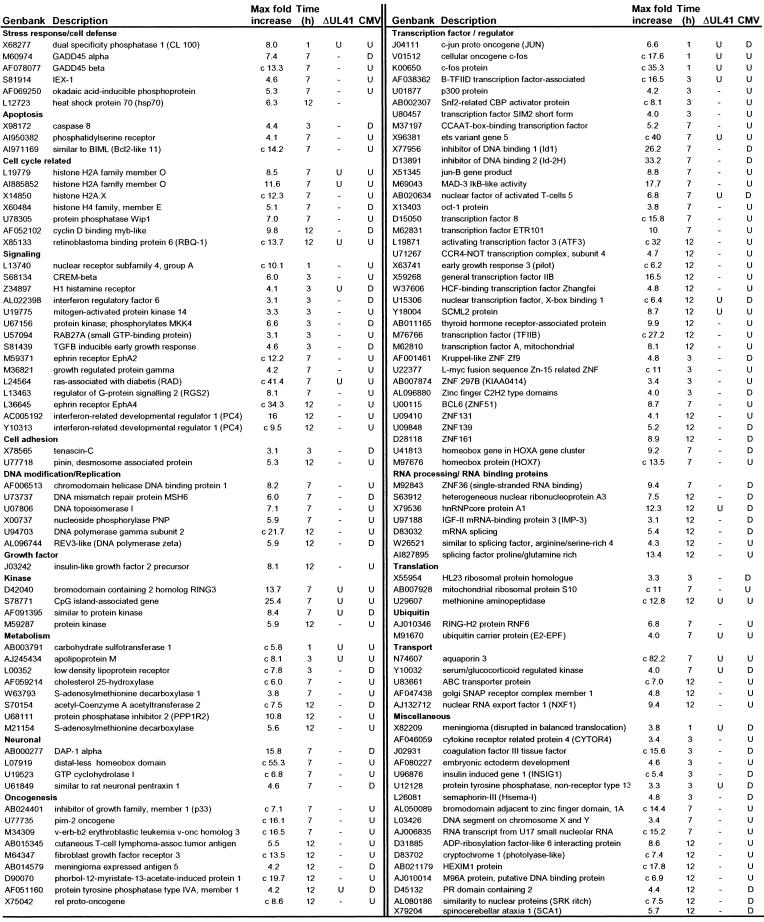
Validation of the results obtained with the array analysis by alternative techniques (see below for confirmation by real-time PCR) led us to consider all of the genes identified from groups A to O that were up-regulated ≥3-fold relative to those of mock-infected cells. These genes were clustered in 20 groups on the basis of known function by using the GeneOntology database (Table 2). Some of the up-regulated genes for each functional group are listed in Table 3. The time after HSV-1 infection at which the highest level of the RNA was detected (Max fold increase) is indicated, as well as the modulation of these genes in cells infected with the ΔUL41 mutant virus of HCMV. The complete list of genes is reported in Table 5, which is published as supporting information on the PNAS web site, www.pnas.org.
Table 2.
Number of up-regulated genes for each functional group
| Functional group | No. |
|---|---|
| Transcriptional factors and regulators | 69 |
| Signaling | 36 |
| Cell cycle | 24 |
| Oncogenesis | 24 |
| RNA processing and binding | 24 |
| Transport | 20 |
| Metabolism | 19 |
| Translation | 15 |
| Kinases | 14 |
| Enzymes general | 13 |
| DNA modification/replication | 11 |
| Structural proteins | 10 |
| Stress response/cell defense | 9 |
| Apoptosis | 8 |
| Ubiquitin | 8 |
| Neuronal | 7 |
| Cell adhesion | 4 |
| Growth factor | 4 |
| Miscellaneous | 69 |
| Unknown sequence/new proteins | 87 |
Table 3.
Genes modulated by HSV-1(F): Comparison with ΔUL41 mutant and HCMV
The “c” in front of the fold change indicates that the transcript was scored absent in mock-infected cells. In column ΔUL41, genes modulated by ΔUL41 mutant infection: —, no change or absent compared to mock-infected cells; U, up-regulated compared to mock-infected cells. In column CMV, genes modulated by CMV infection: U, up-regulated; D, down-regulated compared to mock-infected cells.
Validation of Chip Data by Real-Time PCR.
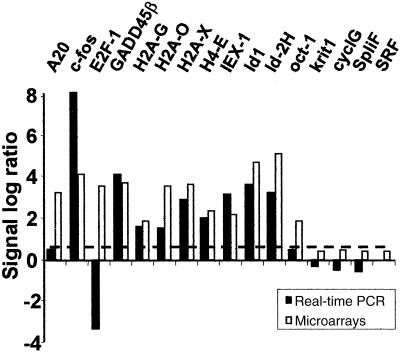
To validate the results obtained by microchip analyses, we selected 16 genes for confirmation by real-time PCR. These genes were chosen to be representative of a large range of ratios and different RNA accumulation profiles. The results are shown in Table 4 and Fig. 2. Although the sampling was limited, it helped establish the dimensions of the gene pool subject to further studies. The inclusion in Table 5 of all of the 475 genes up-regulated ≥3-fold relative to corresponding genes in the control set was based on two main observations. (i) The genes showing a peak of induction <3-fold in HSV-1-infected cells compared with those of mock-infected cells showed no increase in real-time PCR assays [Serum response factor, Krit-1, Cyclin G- associated kinase, and Splicing factor SF3a60 (Table 4)]. Of the 12 remaining genes with a peak of induction >3-fold relative to controls, 75% were confirmed by real-time PCR. The remaining three genes (A20, Oct-1, and E2F1) yielded discordant results. (ii) GADD45β, included among up-regulated genes, was up-regulated 3-fold relative to the values of mock-infected cells at a single time point (7 h) after infection. Real-time PCR showed an up-regulation at all of the time points tested with a peak at 7 h. The increase of GADD45β mRNA, along with that of IEX-1 mRNA in wild-type virus-infected HFF, HeLa, or HEp-2 cells was further validated by Northern analyses (data not shown).
Table 4.
Correlation of selected genes by real-time PCR
| No.
|
GenBank accession no.
|
Gene
|
Real-time PCR | Microarray | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 7 | 12 | 1 | 3 | 7 | 12 | |||
| 1 | V01512 | c-fos | 256 | 16 | 36 | 4.2 | 18 | 3.9 | 12 | 9 |
| 2 | AF078077 | GADD45b | 4.7 | 3.1 | 17 | 3.6 | — | — | 13 | — |
| 3 | Z80776 | Histone H2A-G | 2.1 | 1.3 | 3 | 0.7 | 1.2 | — | 3.5 | — |
| 4 | L19779 | Histone H2A-O | 1.1 | 1.4 | 2.9 | 0.7 | 2.3 | 1 | 12 | 6 |
| 5 | X14850 | Histone H2A.X | 0.6 | 0.9 | 7.2 | 1.4 | — | — | 12 | — |
| 6 | X60484 | Histone H4-E | 1.4 | 1.5 | 3.9 | 1.2 | 1.1 | — | 3.1 | — |
| 7 | S81914 | IEX-1 | 12 | 14 | 8.9 | 1.9 | 2.8 | 3.1 | 4.6 | — |
| 8 | X77956 | Id-1 | 2.1 | 3.2 | 12 | 2.1 | — | 3.2 | 11 | — |
| 9 | D13891 | Id-2H | 3.1 | 2.7 | 9.1 | 2.7 | 1.6 | 1.5 | 6.5 | 5 |
| 10 | M59465 | A20 | 2.3 | 1.7 | 1.4 | 1.6 | — | — | — | 9 |
| 11 | X13403 | Oct-1 | 1.4 | 1.1 | 1.7 | 1.2 | — | — | 3.6 | 4 |
| 12 | M96577 | E2F-1 | 0.5 | 0.1 | 0 | 0 | 11 | 11 | — | — |
| 13 | J03161 | Serum response factor | 1 | 1 | 0.3 | 0.1 | 1.3 | 2.5 | 1.6 | 2 |
| 14 | U90268 | Krit-1 | 0.6 | 0.4 | 0.4 | 0.2 | — | 2.5 | — | — |
| 15 | D88435 | Cyclin G-associated kinase | 0.4 | 0.3 | 0.1 | — | — | 2.7 | 1.8 | 2 |
| 16 | X81789 | Splicing factor SF3a60 | 0.3 | 0.4 | 0.2 | 0.1 | 1.9 | 1.6 | 2.8 | — |
—, No change or absent as compared to mock-infected cells.
Hours postinfection.
Fig 2.
Real-time PCR analysis of selected genes whose expression was modulated in microarray profiling by HSV-1. Fold change (expressed as log ratio) observed by microarray analysis at the peak of induction and by real-time PCR at the same time for 16 selected genes. The dashed line corresponds to a 3-fold increase in the accumulated RNAs of infected cells compared with those of mock-infected cells.
The Role of the HSV-1 UL41 Gene.
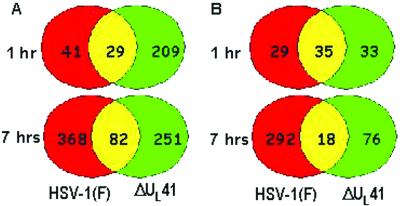
UL41 gene product, the vhs protein, is responsible for the shutoff of cellular protein synthesis induced by HSV-1 early in infection. To analyze the host RNA transcriptional profile after HSV-1 infection in the absence of the vhs protein, HFF were infected with the ΔUL41 mutant and total RNA was extracted at 1 and 7 h after infection and processed as described above. Genes whose RNA was up- or down-regulated in ΔUL41-infected cells relative to those of mock-infected cells were compared with those up- or down-regulated after infection with HSV-1(F). The observed patterns of cellular responses were consistent with the predicted effects of the degradation of RNA mediated by the vhs protein. Thus, a larger number of genes was specifically up-regulated very early after infection (1 h) in ΔUL41 mutant-infected cells than in wild-type virus-infected cells (128 vs. 17; Fig. 3). The unexpected finding was that the large number of genes predicted to be down-regulated early in infection with wild-type virus actually occurred late in infection. As shown in Fig. 3, the number of down-regulated genes at 1 h after infection with wild-type or ΔUL41 viruses was similar (29 vs. 33 and 35 genes in common). The difference becomes more pronounced at 7 h, where 292 genes were down-regulated in HSV-1(F)-infected cells as compared with 76 genes in ΔUL41 mutant-infected cells (Fig. 3). Moreover, there were numerous genes up-regulated in ΔUL41- but not in HSV-1(F)-infected cells; these are listed in Table 6, which is published as supporting information on the PNAS web site. The genes that were up-regulated after HSV-1(F) infection and also induced in ΔUL41 mutant-infected cells are listed in Tables 3 and 5. It is noteworthy that several RNAs up-regulated in HSV-1(F)-infected cells were not modified in ΔUL41 mutant-infected cells.
Fig 3.
Venn diagrams showing the distribution of differentially regulated genes during infection with HSV-1(F) or ΔUL41 mutant relative to those of mock-infected cells. (A) Up-regulated genes. (B) Down-regulated genes.
Discussion
Several aspects of these studies are of particular interest.
(i) Of the 69 mRNAs encoding transcription factors and/or proteins involved in transcription regulation up-regulated in infected cells (Tables 3 and 5), many attained peak levels very early in infection (between 1 and 3 h). Furthermore, most of the small number of genes up-regulated throughout the 12-h time course (Table 1 A group) belong to this functional group.
(ii) Several of the genes shown to be up-regulated in this study have already been reported to be up-regulated after HSV-1 infection by measurement of RNA and/or protein levels. These include c-fos (13), HSP70 (14), or TGF-β (15). A previous report showed up-regulation of transcriptional factor and stress response genes after HSV-1 infection, using a different DNA array system (16). Most of these genes are induced in our system.
(iii) Several mRNAs encoding proteins with roles in cell cycle regulation and oncogenesis were up-regulated after HSV-1 infection (Tables 3 and 5). Because HSV-1 inhibits cell cycle progression (17), the significance of up-regulation of these genes requires further investigation. Similarly, HSV-1 has been reported to inhibit apoptosis induced by a wide variety of exogenous and endogenous inducers (18). At least eight up-regulated genes were classified as pro- or antiapoptotic. The specific role of these genes in HSV-1 replication needs to be elucidated.
(iv) HSV-1 induces a persistent NF-κB nuclear translocation (19) as a result of the activation of the IκB kinase (IKK) and degradation of IκBα early in infection (20). After translocation to the nucleus, NF-κB activates a variety of genes encoding adhesion molecules, inflammatory and chemotactic cytokines, proteins involved in stress response and apoptosis, growth factors, and transcription factors (21). As result of the NF-κB activation mediated by HSV-1, several genes reported to be NF-κB targets were found to be up-regulated by microarrays (Tables 3 and 5). These genes are dispersed among the 19 functional groups and include Bcl-xL, HIAP-1, IEX-1, and GADD45β among the apoptotic and stress related genes; c-myc, c-jun, IκBα, and NF-κB p105 as transcriptional factors/oncogenes; or TNF-α and TNF-related proteins as part of signal transduction pathways. The significance of up-regulation of some of these genes is dealt with elsewhere (B.T. and B.R., unpublished work; T.-R. Luo, B.T., and B.R., unpublished work).
(v) The apparent decrease in the accumulation of transcripts late in infection with wild-type virus suggests that vhs is still active or that the results of its nucleolytic attack are still detectable later than expected. The synergistic activities of vhs, which degrades RNA, and ICP27, which inhibits the splicing and the export of cellular RNA, are responsible for the lack of cellular RNA pool reconstruction, resulting in a limitation of the cell defense response and a protection of the virus. However, there is also the possibility that in the absence of de novo transcription, the existing RNA decays and the rate of decay depends on the properties of the mRNAs. Furthermore, the observation that several cellular genes whose mRNAs were up-regulated in HSV-1(F)-infected cells were found not to be up-regulated or even completely absent in ΔUL41-infected cells could be at least in part explained by the biological properties of the mutant virus, such as the slower replication profile compare with wild-type HSV-1 (10).
(vi) Modulation of cellular gene expression in HFF infected with HCMV was reported recently (9). Table 3 identifies the genes up-regulated in HSV-1(F)-infected cells that are up- or down-regulated in HCMV-infected cells (www.molbio.princeton.edu/labs/shenk/browneetal2001/). Although both studies were done with the same Affymetrix microarrays, the analyses of the data were done with different software (9). Of the total of 135 cellular genes that overlap between HCMV and HSV-1, 43 were down-regulated and 92 were up-regulated. The genes are scattered among the most functional groups but are particularly prevalent in the groups comprising transcriptional factors and signaling. It is noteworthy that five of the seven overlapping genes in the group comprising RNA processing factors are down-regulated.
The next steps in the analysis of cellular responses to herpes simplex virus infection are: (i) confirmation that the changes observed in accumulation of transcripts results in a consequent accumulation of the relative protein products; and (ii) evaluation of the impact of the up-regulated genes on the outcome of viral infection.
Supplementary Material
Acknowledgments
We thank the University of Chicago Functional Genomics Facility and Dr. Xinmin Li for support with data analyses, Dr. Chris Dyanov for initial support with the microarray hybridizations, Sunil Advani for helpful discussion, and Weiran Zhang for technical support. We also thank Dr. Beatrice Fineschi and the Biological Sciences Collegiate Divisions for making the real-time PCR machine available. These studies were aided by National Cancer Institute Grants CA78766, CA71933, CA83939, CA87661, and CA88860 and grants from the U.S. Public Health Service. A.E. is a recipient of a fellowship from the Association pour la Recherche sur le Cancer (ARC France).
Abbreviations
HCMV, human cytomegalovirus
HFF, human foreskin fibroblasts
HSV-1, herpes simplex virus 1
References
- 1.Karr B. M. & Read, G. S. (1999) Virology 264, 195-204. [DOI] [PubMed] [Google Scholar]
- 2.Lam Q., Smibert, C. A., Koop, K. E., Lavery, C., Capone, J. P., Weinheimer, S. P. & Smiley, J. R. (1996) EMBO J. 15, 2575-2581. [PMC free article] [PubMed] [Google Scholar]
- 3.Hardwicke M. A. & Sandri-Goldin, R. M. (1994) J. Virol. 68, 4797-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandri-Goldin R. M. (1998) Genes Dev. 12, 868-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roizman B. & Knipe, D. M. (2001) in Virology, eds. Knipe, D. M. & Howley, P. M. (Lippincott, Philadelphia), pp. 2399–2459.
- 6.Geiss G. K., Bumgarner, R. E., An, M. C., Agy, M. B., van 't Wout, A. B., Hammersmark, E., Carter, V. S., Upchurch, D., Mullins, J. I. & Katze, M. G. (2000) Virology 266, 8-16. [DOI] [PubMed] [Google Scholar]
- 7.Cuadras M. A., Feigelstock, D. A., An, S. & Greenberg, H. B. (2002) J. Virol. 76, 4467-4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geiss G. K., Salvatore, M., Tumpey, T. M., Carter, V. S., Wang, X., Basler, C. F., Taubenberger, J. K., Bumgarner, R. E., Palese, P., Katze, M. G. & Garcia-Sastre, A. (2002) Proc. Natl. Acad. Sci. USA 99, 10736-10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Browne E. P., Wing, B., Coleman, D. & Shenk, T. (2001) J. Virol. 75, 12319-12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poon A. P. & Roizman, B. (1997) Virology 229, 98-105. [DOI] [PubMed] [Google Scholar]
- 11.Livak K. J. & Schmittgen, T. D. (2001) Methods 25, 402-408. [DOI] [PubMed] [Google Scholar]
- 12.Pfaffl M. W. (2001) Nucleic Acids Res. 29, E45-E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gieroba Z. J., Zhu, B.-S., Blessing, W. W. & Wesselingh, S. L. (1995) Brain Res. 675, 329-332. [DOI] [PubMed] [Google Scholar]
- 14.Phillips B., Abravaya, K. & Morimoto, R. I. (1991) J. Virol. 65, 5680-5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendez-Samperio P., Hernandez, M. & Ayala, H. E. (2000) J. Interferon Cytokine Res. 20, 273-280. [DOI] [PubMed] [Google Scholar]
- 16.Khodarev N. N., Advani, S. J., Gupta, N., Roizman, B. & Weichselbaum, R. R. (1999) Proc. Natl. Acad. Sci. USA 96, 12062-12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehmann G. L., McLean, T. I. & Bachenhiemer, S. L. (2000) Virology 267, 335-349. [DOI] [PubMed] [Google Scholar]
- 18.Galvan V. & Roizman, B. (1998) Proc. Natl. Acad. Sci. USA 95, 3931-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel A., Hanson, J., McLean, T. I., Olgiate, J., Hilton, M., Miller, W. E. & Bachenheimer, S. L. (1998) Virology 247, 212-222. [DOI] [PubMed] [Google Scholar]
- 20.Amici C., Belardo, G., Rossi, A. & Santoro, M. G. (2001) J. Biol. Chem. 276, 28759-28766. [DOI] [PubMed] [Google Scholar]
- 21.Pahl H. L. (1999) Oncogene 18, 6853-6866. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.