Abstract
We report a case of bacteremia in puppies caused by Streptococcus dysgalactiae subsp. dysgalactiae. Identification was achieved by phenotypic and molecular genetic methods. This is the first report of the recovery of S. dysgalactiae subsp. dysgalactiae from dogs.
CASE REPORT
A 2-year-old Great Dane bitch that was housed at a breeding kennel gave birth at second parturition to eight puppies after normal gestation and parturition. However, one puppy died within 24 h of birth, five puppies died between 24 and 48 h, and the other two puppies died after 72 h of life. All puppies died suddenly without previous clinical signs of disease. The two puppies that died 72 h after birth were sent to the hospital of the Veterinary School at Complutense University in Madrid for necropsy. Postmortem examination showed irregular consolidation areas in the cranioventral region of the lungs and congestion in most of the internal organs. Samples from the lungs, livers, spleens, thymuses, and brains of both puppies were collected under aseptic conditions and submitted to the Microbiology and Pathology Laboratories for microbiological and histopathologic studies. Reports of previous reproductive disorders were absent, and the hygienic and sanitary conditions of the kennel were adequate. Animals in the kennel were annually revaccinated against canine distemper virus, canine parvovirus, canine adenovirus, canine parainfluenza virus, canine coronavirus, leptospirae, and Bordetella bronchiseptica. The dam had received this preventive vaccination program, and she was correctly dewormed.
Microbiology and identification.
Clinical samples were surface plated on Columbia blood agar (bioMérieux España, s.a.) and incubated for 48 h at 37°C under aerobic and anaerobic conditions. Biochemical characterization was achieved using the commercial Rapid ID32 Strep and API 20 Strep systems (bioMérieux España s.a.) according to the manufacturer's instructions. Lancefield serological group reaction was determined with the commercial Slidex Streptokit (bioMérieux España s.a) by following the manufacturer's instructions. CAMP reaction with Staphylococcus aureus CECT 4013 was also determined on sheep blood agar plates (9). Gram-positive, catalase-negative, facultative anaerobic coccus-shaped organisms were isolated in pure culture from all clinical samples taken from the two puppies with neonatal septicemia. Bacterial colonies grew on sheep blood agar as beta-hemolytic, small (0.5- to 1-mm), gray colonies after 24 h of incubation at 37°C. The 10 isolates (designated 69/02P to 78/02P) were catalase and CAMP test negative and gave a positive reaction for Lancefield group C but negative reactions for the other Lancefield groups tested. All isolates displayed identical biochemical profiles (numerical code, 15112061110) with the Rapid ID 32 Strep commercial system that corresponds to a doubtful identification of Streptococcus dysgalactiae subsp. equisimilis. The numerical profile with the API 20 Strep test was 0463417, corresponding to a doubtful discrimination between Streptococcus group L and S. dysgalactiae subsp. equisimilis.
Comparison of the 16S rRNA gene sequences of bacterial species is a very powerful approach to confirm the identities of clinical isolates by conventional phenotypic methods (4, 20, 21). Molecular phylogenetic characterization of the clinical isolates was performed by sequencing fragments of the 16S rRNA gene of each isolate as described previously (6). The determined sequences, which consisted of the almost complete 16S rRNA gene sequences (>1,400 nucleotides), revealed that the 10 isolates were genotypically identical (100% sequence similarity), with Streptococcus dysgalactiae subsp. dysgalactiae (99.5%) (GenBank accession number AB102730) and S. dysgalactiae subsp. equisimilis (99.4%) (GenBank accession number AB104839) as the closest phylogenetic relatives.
DNA-DNA hybridization experiments among two clinical isolates (71/02P and 74/02PT) and the type strains of S. dysgalactiae (NCTC 4335) and Streptococcus equi (ATCC 33398T) were carried out according to the method of De Ley et al. (8) with the modification described by Huss et al. (12). The two puppy isolates displayed 98.3% DNA relatedness to each other, demonstrating that they are members of the same species. Reassociation values for the clinical strain (71/02P) with respect to S. dysgalactiae (NCTC 4335) and S. equi (ATCC 33398T) were 93.2 and 36.9%, respectively, while the reassociation value between the strains NCTC 4335 and ATCC 33398T was 37.1%. DNA-DNA reassociation is considered the standard method for differentiating species, with DNA-DNA similarities of 70% or higher being considered the threshold value for the definition of bacterial species (23). The chromosomal DNA-DNA reassociation identified the puppy clinical isolates as S. dysgalactiae. These results were consistent with that of 16S rRNA sequencing. To further clarify the relationship of the puppy isolates to established (19) S. dysgalatiae subspecies (dysgalatiae and equisimilis), comparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis protein profiling was performed (14). The protein patterns of all 10 isolates from dogs formed a single tight protein electrophoretic cluster (correlation level, approximately 95%), which grouped with the protein profiles of S. dysgalactiae subsp. dysgalactiae (correlation level, approximately 85%). The protein patterns of the puppy isolates were significantly different from the profiles of S. dysgalactiae subsp. equisimilis, matching at less than 70% of residues (data not shown). The sodium dodecyl sulfate-polyacrylamide gel electrophoresis protein profiling results, together with the 16S rRNA gene sequencing and chromosomal DNA-DNA pairing data, clearly identified the puppy isolates as S. dysgalactiae subsp. dysgalactiae.
The clinical isolates were also genetically characterized by pulsed-field gel electrophoresis (PFGE) according to the specifications of Vela et al. (22). The restriction enzymes ApaI (Promega Co., Southampton, United Kingdom) and SmaI (MBI Fermentans, Vilnius, Lithuania) were used according to the manufacturer's recommendations. These enzymes have been successfully used for the molecular typing of S. dysgalactiae isolates (1). All puppy isolates displayed undistinguishable PFGE macrorestriction patterns with both ApaI and SmaI restriction enzymes (data not shown), which is indicative of the idea that all clinical isolates represent a single strain that was present in all tissues examined.
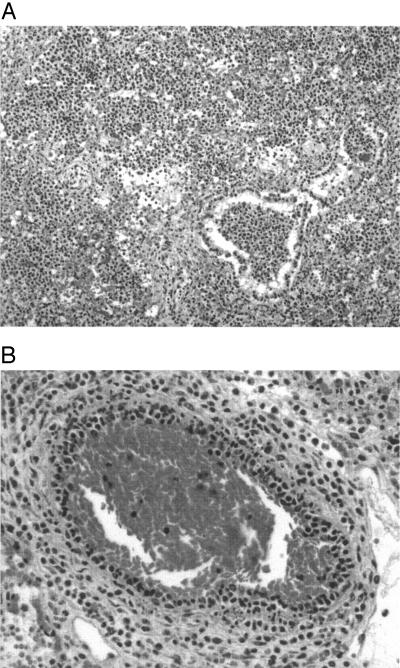
For histopathologic studies, tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, cut in 4-μm sections, and stained with hematoxylin and eosin and a tissue Gram stain. These sections were examined by light microscopy for evidence of inflammatory and degenerative lesions and for the presence of a bacterium with morphology consistent with that of members of the genus Streptococcus. Histological examination of the lungs of both puppies showed a hemorrhagic lobar pneumonia (Fig. 1A) with leukocytoclastic vasculitis (Fig. 1B) composed mainly of neutrophils and macrophages, which is indicative of the existence of a pneumonic process. Gram-positive cocci were observed in the pulmonary bronchioles and alveoli. Congestion and microscopic hemorrhages were the more prominent findings in other organs studied.
FIG. 1.
Lesions in the lungs of puppy 1. Hematoxylin and eosin staining. (A) Acute lobar pneumonia with edema, congestion, hemorrhage, neutrophilic cell exudation, and bacterial cells in the pulmonary bronchioles and alveoli (×10); (B) leukocyte infiltration within the vascular wall (×20).
Discussion.
Episodes of neonatal mortality occur in many dog breeding kennels and are related to different factors, such as management conditions, malnutrition, congenital abnormalities, parasitism, and infectious diseases (7). Bacterial infections are one of the major causes of neonatal deaths in many dog-breeding kennels (11). Factors that predispose a puppy to bacterial infections include endometritis in the bitch, a prolonged delivery or dystocia, vaginal discharges, and environmental exposure. Such infections may lead to diarrhea, pneumonia, peritonitis, septic arthritis, septicemia, and other clinical conditions which are usually debilitating and are often fatal. Staphylococci and gram-negative bacteria, especially Staphylococcus aureus and Escherichia coli, are the most common bacteria associated with systemic infections in newborn puppies (16). In addition to these microorganisms, different species of beta-hemolytic streptococci have been associated with neonatal septicemias in puppies (11, 15, 17). Streptococcus canis is the species most frequently identified from cases of septicemia in newborn puppies, but other species of beta-hemolytic streptococci, such as Streptococcus agalactiae or Streptococcus equi subsp. zoopeidemicus, have also been implicated (11). These species of streptococci have also been associated with other different sporadic and opportunistic infections in dogs, such as otitis, abscesses, necrotizing fasciitis, toxic shock syndrome endocarditis, wound infections, acute necrotizing hemorrhagic pneumonia, and canine infectious respiratory disease (2, 5, 10, 15, 24). However, apart from these three well-recognized species, many streptococcal isolates are identified only to the genus level (13, 17), which limits the knowledge of the diversity of species associated with septicemia in newborn puppies.
We report the first isolation of S. dysgalactiae subsp. dysgalactiae from dogs. In the present case, S. dysgalactiae subsp. dysgalactiae was isolated in pure culture from all the organs examined in both puppies, which, together with the histopathologic data, strongly suggests that S. dysgalactiae subsp. dysgalactiae was responsible for the neonatal mortality episode. Although the disease was bacteriologically confirmed only in two out of the eight puppies which died and no bacteriological analysis was performed on the other animals of the same litter, it is reasonable to assume that S. dysgalactiae subsp. dysgalactiae was also responsible for the disease in the other affected puppies. Canine herpesvirus infections are also a common cause of neonatal mortality in puppies (18). Nevertheless, the histological studies did not reveal the existence of multifocal necrosis with intranuclear inclusion bodies in liver, spleen, or kidney, which is characteristic of neonatal canine herpesvirus infections (18). These results reinforce the clinical significance of the isolations. Despite this, it must be pointed out that all affected puppies came from a single disease episode, and there is no clear evidence for considering S. dysgalactiae subsp. dysgalactiae as a consistent pathogen for dogs. Factors that predispose puppies to neonatal bacteremia and septicemia include malnutrition, incomplete developed thermoregulatory capacity, stress, or low birth weight (7). None of these specific factors were observed in the present study. The fact that the same streptococcus was isolated in both puppies also suggests a common source of infection. Beta-hemolytic streptococci can be isolated as normal flora of the urogenital tracts of dogs (3). Therefore, contamination of the puppies during the delivery may be the most likely origin of the infection.
Acknowledgments
A. I. Vela has a fellowship from the Ramon y Cajal Program (Spanish Ministry of Science and Technology/U.C.M.).
We thank A. Casamayor for her help technical assistance in PFGE typing.
REFERENCES
- 1.Bert, F., C. Branger, B. Poutrel, and N. Lambert-Zechovsky. 1997. Differentiation of human and animal strains of Streptococcus dysgalactiae by pulsed-field gel electrophoresis. FEMS Microbiol. Lett. 150:107-112. [DOI] [PubMed] [Google Scholar]
- 2.Biberstein, E. L., C. Brown, and T. Smith. 1980. Serogroups and biotypes among beta-hemolytic streptococci of canine origin. J. Clin. Microbiol. 11:558-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjurstrom, L., and C. Linde-Forsberg. 1992. Long-term study of aerobic bacteria of the genital tract in breeding bitches. Am. J. Vet. Res. 53:665-669. [PubMed] [Google Scholar]
- 4.Cai, H., M. Archambault, and J. F. Prescott. 2003. 16S ribosomal RNA sequence-based identification of veterinary clinical bacteria. Vet. Diagn. Investig. 15:465-469. [DOI] [PubMed] [Google Scholar]
- 5.Chalker, V. J., H. W. Brooks, and J. Brownlie. 2003. The association of Streptococcus equi subsp. zooepidemicus with canine infectious respiratory disease. Vet. Microbiol. 29:49-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins, M. D., E. Falsen, G. Foster, R. Monasterio, L. Domínguez, and J. F. Fernández-Garayzábal. 1999. Characterization of some novel Helcococcus-like organisms from sheep: description of Helcococcus ovis sp. nov. Int. J. Syst. Bacteriol. 49:1429-1432. [DOI] [PubMed] [Google Scholar]
- 7.Davidson, A. C. 2003. Approaches to reducing neonatal mortality in dogs. In P. W. Concannon, G. England, J. Verstegen, and C. Linde-Forsberg (ed.), Recent advances in small animal reproduction. Document A1226.0303. International Veterinary Information Service, Ithaca, N.Y. [Online.] http://www.ivis.org/advances/Concannon/davidson/chapter_frm.asp?LA=1.
- 8.De Ley, J., H. Cattoir, and A. Reynaerts. 1970. The quantitative measurement of DNA hybridization from renaturation rates. Eur. J. Biochem. 12:133-142. [DOI] [PubMed] [Google Scholar]
- 9.Facklam, R. R., and J. A. Washington. 1991. Streptococcus and related catalase-negative gram-positive cocci, p. 238-257. In A. Balows, W. J. Hausler, Jr., K. L. Herrmann, H. D. Isenberg, and H. J. Shadomy (ed.), Manual of clinical microbiology, 5th ed. American Society for Microbiology, Washington, D.C.
- 10.Garnett, N. L., R. S. Eydelloth, M. M. Swindle, S. L. Vonderfecht, J. D. Strandberg, and M. B. Luzarraga. 1982. Hemorrhagic streptococcal pneumonia in newly procured research dogs. J. Am. Vet. Med. Assoc. 181:1371-1374. [PubMed] [Google Scholar]
- 11.Greene, C. E., and J. F. Prescott. 1998. Streptococcal and other Gram-positive bacterial infections, p. 205-214. In C. E. Greene (ed.), Infectious diseases of the dog and cat, 2nd ed. W. B. Saunders Company, Philadelphia, Pa.
- 12.Huss, V. A. R., H. Festl, and K. H. Schleifer. 1983. Studies on the spectrophotometric determination of DNA hybridization from renaturation rates. Syst. Appl. Microbiol. 4:184-192. [DOI] [PubMed] [Google Scholar]
- 13.Kornblatt, A. N., R. L. Adams, S. W. Barthold, and G. A. Cameron. 1983. Canine neonatal deaths associated with group B streptococcal infections. J. Am. Vet. Med. Assoc. 183:700-701. [PubMed] [Google Scholar]
- 14.Pot, B., P. Vandamme, and K. Kersters. 1994. Analysis of electrophoretic whole-organism protein fingerprints, p. 493-521. In M. S. Goodfellow and A. G. O'Donnell (ed.), Chemical methods in prokaryotic systematics. Wiley, Chichester, United Kingdom.
- 15.Quinn, P. J., M. E. Carter, B. Markey, and G. R. Carter. 1999. The streptococci and related cocci, p. 127-136. In P. J. Quinn, M. E. Carter, B. Markey, and G. R. Carter (ed.), Clinical veterinary microbiology. Mosby, Edinburgh, Scotland.
- 16.Sager, M., and C. Remmers. 1990. Perinatal mortality in dogs. Clinical, bacteriological and pathological studies. Tierarztl. Prax. 18:415-419. [PubMed] [Google Scholar]
- 17.Schafer-Somi, S., J. Spergser, J. Breitenfellner, and J. E. Aurich. 2003. Bacteriological status of canine milk and septicaemia in neonatal puppies—a retrospective study. J. Vet. Med. B 50:343-346. [DOI] [PubMed] [Google Scholar]
- 18.Smith, K. C. 1997. Herpesviral abortion in domestic animals. Vet. J. 153:253-268. [DOI] [PubMed] [Google Scholar]
- 19.Vandamme, P., B. Pot, E. Falsen, K. Kersters, and L. A. Devriese. 1996. Taxonomic study of Lancefield streptococcal groups C, G, and L (Streptococcus dysgalactiae) and proposal of S. dysgalactiae subsp. equisimilis subsp. nov. Int. J. Syst. Bacteriol. 46:774-781. [DOI] [PubMed] [Google Scholar]
- 20.Vela, A. I., E. Fernandez, A. las Heras, P. A. Lawson, L. Dominguez, M. D. Collins, and J. F. Fernández-Garayzábal. 2000. Meningoencephalitis associated with Globicatella sanguinis infection in lambs. J. Clin. Microbiol. 38:4254-4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vela, A. I., E. Fernandez, P. A. Lawson, M. V. Latre, E. Falsen, L. Dominguez, M. D. Collins, and J. F. Fernández-Garayzábal. 2002. Streptococcus entericus sp. nov., isolated from cattle intestine. Int. J. Syst. Evol. Microbiol. 52:665-669. [DOI] [PubMed] [Google Scholar]
- 22.Vela, A. I., J. Goyache, C. Tarradas, I. Luque, A. Mateos, M. A. Moreno, C. Borge, J. A. Perea, L. Domínguez, and J. F. Fernández-Garayzábal. 2003. Pulsed-field gel electrophoresis genetic diversity of Streptococcus suis clinical isolates from pigs in Spain. J. Clin. Microbiol. 41:2498-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wayne, L. G., D. J. Brenner, R. R. Colwell, P. A. D. Grimont, O. Kandler, M. I. Krichevsky, L. H. Moore, W. E. C. Moore, R. G. E. Murray, E. Stackebrandt, M. P. Srarr, and H. G. Trüper. 1987. Report of the Ad Hoc Committee on Reconciliation of Approaches to Bacterial Systematics. Int. J. Syst. Bacteriol. 37:463-466. [Google Scholar]
- 24.Yildirim, A. O., C. Lämmler, R. Weib, and P. Kopp. 2002. Pheno- and genotypic properties of streptococci of serological group B of canine and feline origin FEMS Microbiol. Lett. 212:187-192. [DOI] [PubMed] [Google Scholar]