Abstract
There is currently no “gold standard” test for the diagnosis of late-stage Chagas' disease. As a result, protection of the blood supply in areas where Chagas' disease is endemic remains problematic. A panel of 709 serum samples from subjects with confirmed Chagas' disease (n = 195), healthy controls (n = 400), and patients with other parasitic diseases (n = 114) was used to assess enzyme-linked immunosorbent assays (ELISAs) based on a concentrated extract of excretory-secretory antigens from either Brazil or Tulahuen strain Trypanosoma cruzi trypomastigotes (total trypomastigote excretory-secretory antigens [TESAs]). The total TESA-based assays had excellent overall sensitivity (100%) and specificity (>94%), except for cross-reactivity with Leishmania-infected sera. In an attempt to increase the specificity of the assay, immunoaffinity chromatography was used to purify the TESA proteins (TESAIA proteins). By Western blotting, a series of polypeptide bands with molecular masses ranging from 60 to 220 kDa were recognized by pooled sera positive for Chagas' disease. An ELISA based on TESAIA proteins had a slightly lower sensitivity (98.6%) but an improved specificity (100%) compared to the sensitivity and specificity of the total TESA protein-based ELISAs. A 60-kDa polypeptide was identified as a major contributor to the cross-reactivity with Leishmania. These data suggest the need for field validation studies of TESA- and TESAIA-based assays in regions where Chagas' disease is endemic.
Trypanosoma cruzi is the causative agent of Chagas' disease, a major health problem in Central and South America (31). A large number of antigen preparations have been used for the serological diagnosis of this infection (1-5, 7, 8, 11, 12, 15, 18-20, 22, 27, 28). Of these preparations, trypomastigote excretory-secretory antigens (TESAs) appear to provide good sensitivity and specificity as reagents for the diagnosis of Chagas' disease. Major components of TESA proteins are transialidases secreted into the culture supernatant (27, 29). Parasite transialidases are implicated in the penetration of host cells as a result of the transfer of exogenous sialic acid to acceptor molecules located on the trypomastigote surface. Transialidases are highly immunogenic, and both the C-terminal and the N-terminal regions stimulate strong B-cell responses (9, 24). Transialidase-like proteins are predominant antigens on the surfaces of bloodstream trypomastigotes, metacyclic trypomastigotes, and intracellular amastigotes (13).
Both enzyme-linked immunosorbent assay (ELISA) and immunoblot assays with TESA proteins have been reported for the diagnosis of Chagas' disease. Immunoblots have typically been used in parasite diagnostics to rule out false-positive ELISA results (27). However, immunoblots are expensive, difficult to standardize, and time-consuming. In contrast, the ELISA format is inexpensive, simple to automate, and rapid (20). To date, the TESA proteins evaluated for use in the diagnosis of Chagas' disease have been complex mixtures that cross-react with sera from subjects with other parasitic diseases, particularly leishmaniasis (28). We evaluated the performance of ELISAs based on concentrated TESA proteins or immunoaffinity chromatography-purified TESA (TESAIA) proteins for the diagnosis of latent Chagas' disease. Both assays performed well in comparison with the performances of the serologic tests currently used in Central and South American blood banks. Of particular note, we identified a 60-kDa TESA protein that contributes significantly to the known cross-reactivity with Leishmania sera.
MATERIALS AND METHODS
Serum samples.
A panel of 709 serum samples was used in this study. One hundred ninety-five samples were obtained from Venezuelan subjects with Chagas' disease, as confirmed by a battery of three different serological tests, including immunofluorescence, indirect hemagglutination, and ELISA, at the National Chagas Immunodiagnosis Laboratory (NCIL; Maracay, Venezuela). Samples were considered positive if the results of two of the three assays were positive. Sera from 114 subjects with the following parasitic diseases were obtained from the Canadian National Reference Centre for Parasitology serum bank: leishmaniasis (n = 20), ascariasis (n = 6) fascioliasis (n = 8), malaria (n = 23), toxoplasmosis (n = 17), trichinosis (n = 11), filariasis (n = 8), cysticercosis (n = 8), and schistosomiasis (n = 13). These sera were not screened by the NCIL reference assays. Sera from 234 consecutive healthy Venezuelan blood donors negative by all three serological tests performed by NCIL and 166 healthy, nontraveling Canadians were also tested as controls.
TESA proteins.
TESA proteins from two T. cruzi strains (strains Tulahuen and Brazil) were obtained from infected Vero cell supernatants, as described by Umezawa et al. (27), with slight modifications. Briefly, Vero cell monolayers at 65% confluence were infected with T. cruzi trypomastigotes (1 × 109 parasites/ml/175 cm2) and incubated at 37°C with 5% CO2 for 4 days in Eagle's minimum essential medium (Wisent, St. Bruno, Quebec, Canada) supplemented with 1 M HEPES (1%) and gentamicin reagent solution (0.1%) without fetal bovine serum and phenol red. After 4 days of incubation, the infected monolayers were washed twice with the medium and reincubated for 18 to 20 h at 37°C in 5% CO2 in complete medium. The supernatants were then harvested, centrifuged at 2,800 × g for 15 min at 4°C, and filtered through a membrane (pore size, 0.22 μm; Millipore, Bradford, MA). The supernatant proteins were concentrated ∼32-fold (Ultra device; 30,000-molecular-weight cutoff; Amicon, Bradford, MA) and either used immediately or stored at −80°C. Total concentrated TESAs retained the high-molecular-mass (150- to 170-kDa) polypeptide bands, which correspond to the most immunogenic antigens (20). The protein content of the fetal bovine serum-free TESA was quantified by using a Micro-BCA protein assay reagent kit (Pierce Co., Rockford, IL).
Purification of chagasic antibodies.
Antibodies with specificity for T. cruzi were purified by following the procedure described by Curtis and Chase (10), with some modifications. A 10-ml pool of sera from subjects with serologically confirmed Chagas' disease (NCIL assays) and no clinical or serological evidence of leishmaniasis was precipitated with 50% ammonium sulfate for 24 h and then centrifuged at 3,000 × g for 5 min. The resulting pellet was resuspended in 10 ml distilled water, and then 37% ammonium sulfate (vol/vol) was added and the mixture was incubated for 24 h. After centrifugation at 3,000 × g for 5 min, the final pellet was resuspended in 10 ml of 0.02 M phosphate buffer (pH 8.0), and the residual ammonium sulfate was removed by dialysis (with 0.02 M phosphate buffer for 24 h). The final antibody concentration was determined by spectrophotometry by using the extinction coefficient (1.4) described previously (14). Antibodies were purified by chromatography on a DEAE-cellulose column equilibrated with 0.02 M phosphate buffer (pH 8.0). Column elution fractions were monitored spectrophotometrically at 280 nm, and protein purity was determined by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). The fractions containing antibodies were pooled, and the final protein concentration was determined as reported by Bradford (6). Purified antibodies were dialyzed against 0.1 M NaHCO3-0.5 M NaCl (pH 8.5) for 24 h and coupled to CNBr-activated Sepharose 4 Fast Flow, following the manufacturer's instructions (Amersham Biosciences).
Immunoaffinity purification of TESA proteins.
Aliquots of the total concentrated TESA (850 μg/ml) were mixed with the chagasic antibodies-Sepharose resin and incubated overnight in a nutator at 4°C. After the unbound proteins were washed with 0.1 M NaHCO3-0.5 M NaCl (pH 8.5), the adsorbed antigens were eluted with 0.1 M glycine (pH 2.5). Fractions of 1 ml were collected in tubes containing 0.1 ml of 1 M Tris-HCl (pH 8.0) to neutralize the eluting buffer. Fractions were read at 280 nm, and the purity of the eluted proteins was assayed by SDS-PAGE, followed by silver staining. Fractions containing the immunoaffinity chromatography-purified proteins (TESAIA proteins) were dialyzed against phosphate-buffered saline (PBS; pH 7.4) for 24 h.
TESA-based ELISAs.
Coating of 96-well polystyrene plates (Immulon 2; Thermo Labsystems, Franklin, MA) was accomplished by incubating either total TESA protein (1 μg/ml) or TESAIA (3 μg/ml) at 4°C overnight (100 μl/well) in 1 M sodium carbonate buffer (pH 9.6). The plates were washed four times with PBS (pH 7.4; 0.01 M phosphate buffer, 0.14 M NaCl) containing 0.05% Tween 20 (A&C, American Chemicals, Ltd., St-Laurent, Quebec, Canada) (PBST), blocked for 1 h at 37°C either with PBS containing 5% bovine serum albumin (Sigma, St. Louis, MO) and 0.1% Tween 20, in the case of the total TESA proteins, or with PBS containing 5% skim milk (Parmalat, Montreal, Quebec, Canada) and 0.1% Tween 20, in the case of TESAIA. The sera were diluted 1:800 (total TESA) or 1:200 (TESAIA) in the corresponding blocking solution (100 μl/well) and incubated for 1 h at 37°C. These dilutions were selected to permit optimal differentiation between positive and negative control sera, as tested by checkerboard titration (data not shown). Assays were completed with an optimal dilution of horseradish peroxidase-conjugated goat anti-human immunoglobulin G (IgG; 100 μl/well; Perkin-Elmer Life Science, Boston, MA) for 30 min at 37°C, four washes with PBST, and a final incubation with 3,3′,5,5′-tetramethylbenzidine (100 μl/well; Serologicals Corporation, Massachusetts) for 10 min at room temperature. The reaction was stopped with 1 N H2SO4 (50 μl/well). The optical density (OD) was measured at 450 nm by using an automated ELISA reader (a Titertek Multiskan MCC/340 reader [Labsystem and Row Laboratories, Finland] or a TECAN ELISA reader). All experiments were performed in duplicate on different days, and pooled positive and negative controls for which the results were confirmed by NCIL were included on each plate. The results were accepted only when the coefficient of variation within and among plates was ≤15%; otherwise, the samples were tested again. The results for healthy control sera were used to establish the cutoff values that yielded the optimal sensitivity and specificity results for each assay.
SDS-PAGE.
Protein purity was evaluated by SDS-PAGE carried out on 1.5-mm-thick slabs containing 7% polyacrylamide (for TESAIA) or 11% polyacrylamide (for purified antibodies), as described by Laemmli (16). Electrophoresis was performed for 2 h in Tris-glycine electrode buffer (25 mM Tris, 192 mM glycine, 0.1% SDS, pH 8.3) at a constant voltage of 100 V. Coomassie blue R-250 (for purified antibodies) or silver staining (for TESAIA) was used for protein visualization. Broad-range molecular weight markers (Promega, Madison, WI) and prestained protein standards (Invitrogen, Carlsbad, CA) were included in each run.
Western blot analyses.
Proteins separated by SDS-PAGE were transferred to nitrocellulose sheets (MFS, Pleasanton, CA), as described by Towbin et al. (26), by using a minitank electroblotter (Owl Scientific, Wsoburn, MA). Transfer was performed for 1 h at 4°C in 25 mM Tris, 192 mM glycine, and 20% (vol/vol) methanol (pH 8.3) at a constant current of 0.25 A. Membranes were blocked overnight at 4°C with PBS containing 5% skim milk (Parmalat) and 0.1% Tween 20 and were incubated for 2 h with sera diluted 1:400 in the blocking solution. Following four 5-min washes with PBST, the antigenically reacting proteins were incubated for 2 h with the appropriate dilution of horseradish peroxidase-conjugated goat anti-human IgG diluted in PBS containing 5% skim milk and 0.1% Tween 20. The nitrocellulose filters were then washed four times with PBST, and the immune complexes were revealed by chemiluminescence (Super signal West Pico; Pierce).
Statistical analyses.
Statistical analyses were performed by using SAS software (version 8.2; SAS, Cary, NC). Sensitivity and specificity were determined by examining all possible cutoff values. Analyses of variance (ANOVAs; linear model) were used to examine the effect of parasite type on the optical density in each study group. Tukey's post hoc test was used to evaluate differences between parasite antigens. Comparison among tests was determined by using the kappa index. P values of <0.05 were considered significant. The chi-square test was used to determine significant differences in specificity. The sensitivities and specificities of the tests were evaluated over a range of arbitrary OD cutoff values (OD value range, 0.2 to 0.4).
RESULTS
Concentrated TESA fraction.
The total TESA was concentrated with an Amicon Ultra device that maintains the high-molecular-mass (150- to 170-kDa) immunogenic proteins (20). The final protein concentrations determined by using Micro BCA for TESA both before concentration (550 μg/ml) and after concentration (850 μg/ml) were similar for both strains. However, the OD values obtained from the strain Brazil- and the strain Tulahuen-based ELISAs before and after concentration were significantly different for a pool of positive sera by ANOVA (P values, <0.0001). Following concentration, the background reactivity for the negative control pool increased from ODs of 0.078 ± 0.021 and 0.059 ± 0.015 to ODs of 0.150 ± 0.018 and 0.130 ± 0.003 in the Tulahuen- and Brazil-based TESA ELISAs, respectively. In contrast, concentration of the TESAs increased the reactivity for the pooled positive sera 4.3-fold for the Tulahuen strain (OD range, 0.596 ± 0.033 to 2.162 ± 0.143) and 15-fold for the Brazil strain (OD range, 0.152 ± 0.013 to 2.237 ± 0.102).
ELISA based on total TESA proteins.
The sensitivities of the ELISAs based on total TESA proteins were excellent for antigens from both strains (100%) at several cutoff values (OD cutoff values, 0.200 to 0.400) (Table 1). The assay based on the total TESA proteins derived from the Tulahuen strain showed a slightly higher specificity at the various cutoff values. However, when the results for all the negative control samples were combined for evaluation, these differences did not reach statistical significance. There were minor differences in the specificity estimates observed between the Tulahuen- and the Brazil-based assays for the individual control populations at various cutoff values (Table 2). However, the agreement between tests (kappa index) was excellent and increased progressively at higher cutoff values (Table 1). No differences between the assays based on the two strains were observed in terms of the ODs for the sera from patients with Chagas' disease, the sera from healthy patients from an area of endemicity, or the sera from patients with other parasitic diseases. The mean OD values for sera from patients with leishmaniasis were generally higher than those observed for the sera from the other groups of healthy and parasitic disease controls (Table 2).
TABLE 1.
Sensitivity, specificity, and agreement of the enzyme-linked immunoabsorbent assays with TESAs of T. cruzi at different arbitrary OD cutoff valuesa
| OD cutoff value and parameter | Tulahuen TESA-based assay | Brazil TESA-based assay |
|---|---|---|
| 0.200 | ||
| Specificity (%) | 96.30 | 95.33 |
| Sensitivity (%) | 100 | 100 |
| Agreement (kappa) | 0.93 | |
| 0.300 | ||
| Specificity | 99.03 | 98.83 |
| Sensitivity | 100 | 100 |
| Agreement (kappa) | 0.99 | |
| 0.400 | ||
| Specificity | 99.61 | 99.42 |
| Sensitivity | 100 | 100 |
| Agreement (kappa) | 0.99 |
Sera from 195 patients with Chagas' disease and 514 individuals without Chagas' disease were tested. Presumed false-positive test results occurred with the cutoff of an OD of 0.400 with samples from the following subjects: by the Tulahuen TESA-based assay, one subject with cutaneous leishmaniasis and one healthy control; by the Brazil TESA-based assay, one subject with cutaneous leishmaniasis, two subjects with toxoplasmosis, and one healthy control.
TABLE 2.
Mean, standard deviation, and range of OD values as well as specificity estimates for serum groups by ELISA based on TESAs from trypomastigote forms of T. cruzia
| Patient group (n) | TESA sourceb | Mean OD ± SD | OD range | Specificity (%) at an OD of:
|
||
|---|---|---|---|---|---|---|
| 0.200 | 0.300 | 0.400 | ||||
| Canadian healthy patients (166) | B | 0.069 ± 0.040 | 0.019-0.275 | 98.29 | 100 | 100 |
| T | 0.060 ± 0.032 | 0.018-0.181 | 100 | 100 | 100 | |
| Venezuelan healthy patients (234) | B | 0.088 ± 0.060 | 0.031-0.820 | 97.44 | 99.57 | 99.57 |
| T | 0.087 ± 0.052 | 0.028-0.476 | 95.30 | 99.14 | 99.57 | |
| Patients with other parasitic diseases (114) | B | 0.134 ± 0.200 | 0.027-2.077 | 94.02 | 97.86 | 98.72 |
| T | 0.107 ± 0.194 | 0.018-2.038 | 96.58 | 98.72 | 99.57 | |
| Chagas' disease patients (195) | B | 1.479 ± 0.506 | 0.450-2.628 | |||
| T | 1.558 ± 0.465 | 0.437-2.60 | ||||
The mean ODs for patients with other parasitic diseases were as follows: for patients with ascariasis (n = 6), Brazil TESA assay, 0.145; Tulahuen TESA assay, 0.127; for patients with cysticercosis (n = 8), Brazil TESA assay, 0.088; Tulahuen TESA assay, 0.064; for patients with faciolasis (n = 8), Brazil TESA assay, 0.085; Tulahuen TESA assay, 0.070; for patients with filariasis (n = 8), Brazil TESA assay, 0.092; Tulahuen TESA assay, 0.070; for patients with leishmaniasis (n = 20), Brazil TESA assay, 0.255; Tulahuen TESA assay, 0.216; for patients with malaria (n = 23), Brazil TESA assay, 0.128; Tulahuen TESA assay, 0.099; for patients with toxoplasmosis (n = 17), Brazil TESA assay, 0.133; Tulahuen TESA assay, 0.091; for patients with trichinosis (n = 11), Brazil TESA assay, 0.084; Tulahuen TESA assay, 0.065; for patients with schistosomiasis (n = 13), Brazil TESA assay, 0.082; Tulahuen TESA assay, 0.073.
B, strain Brazil; T, strain Tulahuen.
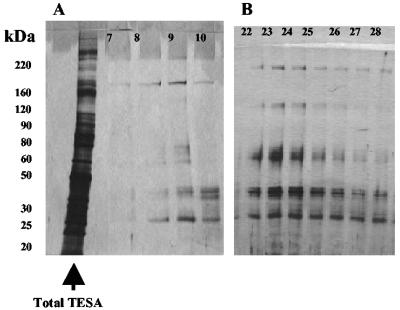
Purification of total TESA proteins by immunoaffinity chromatography.
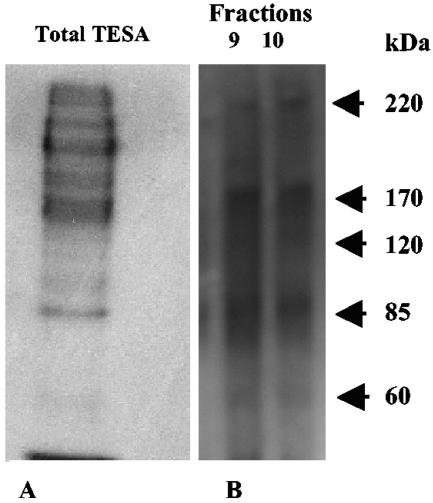
TESAIA proteins from the total strain Tulahuen and Brazil TESAs had essentially the same pattern of purified bands in the peak fraction (for Tulahuen, peak fraction 9; for Brazil, peak fraction 23) (Fig. 1). When the total TESA proteins were compared with the corresponding TESAIA proteins, far fewer polypeptide bands were observed by silver staining (Fig. 1A and B). Western blots of total TESA and TESAIA preparations with a pool of positive chagasic sera also revealed far fewer bands with prominent polypeptide bands at 220, 170, 120, 85, and 60 kDa for both strains (Fig. 2 [data for the Brazil strain are not shown]).
FIG. 1.
SDS-PAGE separation of the immunopurified proteins from total concentrated TESA and TESAIA visualized by silver staining. (A) Tulahuen strain (total concentrated TESA is in the leftmost lane; fractions 7 to 10; the peak is in fraction 9); (B) Brazil strain (fractions 22 to 28; the peak is in fraction 23).
FIG. 2.
Western blot analysis of sodium dodecyl sulfate-polyacrylamide gel electrophoresis-separated total Tulahuen strain TESA (A) and TESAIA (B) preparations of T. cruzi. The dot was probed with a pool of sera from patients with Chagas' disease.
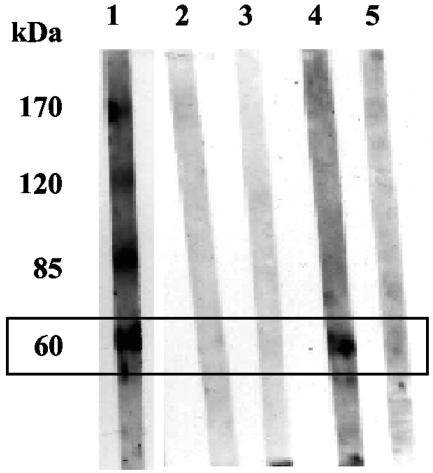
The TESAIA proteins were further evaluated by Western blotting with a panel of 17 serum samples from patients confirmed to have Chagas' disease by NCIL; 9 serum samples from healthy seronegative individuals, as confirmed by NCIL, from an area of endemicity; and 10 serum samples from patients with clinically confirmed mucocutaneous leishmaniasis. The last subjects had no clinical evidence of Chagas' disease and had strongly positive serologic test results (indirect immunofluorescence and indirect hemagglutination) for leishmaniasis. Polypeptides with molecular weights of 170,000 (17 of 17 samples), 120,000 (16 of 17 samples), 85,000 (17 of 17 samples), and 60,000 (15 of 17 samples) were recognized by the sera from patients with Chagas' disease. Sera from healthy individuals and subjects with leishmaniasis did not react with the 170-, 120-, or 85-kDa polypeptide bands; but 3 of 10 of the serum samples from the leishmaniasis patients reacted with the 60-kDa polypeptide band. This observation suggests that this polypeptide is partially responsible for the known serologic cross-reactivity between these two parasitic diseases. Further evaluation with a larger number of serum samples from patients with leishmaniasis is required to confirm this result (Fig. 3).
FIG. 3.
Western blot analysis of sodium dodecyl sulfate-polyacrylamide gel electrophoresis-separated proteins purified by affinity chromatography from total TESA of T. cruzi (Tulahuen strain). Lanes: 1, Chagas disease-positive sera; 2 and 3, sera from healthy patients; 4 and 5, Leishmania-positive sera (lane 4, reactive with the 60-kDa band; lane 5, nonreactive with the 60-kDa band). Arrow, 60-kDa band that is cross-reactive.
ELISA based upon TESAIA proteins.
We used a panel of 166 serum samples to perform a preliminary evaluation to compare the total TESA- and TESAIA-based ELISAs (Tulahuen strain). Seventy-four of 75 serum samples from subjects with confirmed Chagas' disease were reactive by the TESAIA-based assay when an OD cutoff value of 0.300 was used (sensitivity, 98.7%; 95% confidence interval, 96 to 100%). None of the sera from healthy individuals from a region of endemicity (n = 75) or from patients with leishmaniasis (n = 16) were reactive in this assay (specificity, 100%). The mean OD values for chagasic sera, the negative control sera, and Leishmania-positive sera were 1.043 ± 0.334 (range, 0.298 to 1.735), 0.166 ± 0.046 (range, 0.086 to 0.284), and 0.145 ± 0.048 (range, 0.083 to 0.242), respectively. The mean OD for the Leishmania-positive sera was considerably lower in the optimized TESAIA-based ELISA (0.145 ± 0.048) compared to the mean values obtained in the total TESA-based assays with antigens from either the Tulahuen strain (0.253 ± 0.481) or the Brazil strain (0.300 ± 0.481). These differences did not reach statistical significance, however, due to the wide variability seen in the total TESA-based assays. Compared to the results of the optimized ELISAs based on total TESAs, the optimized TESAIA assay had a slightly lower sensitivity (98.7% versus 100%) but a higher specificity (100%) than the total TESA assays based on antigens from strains Brazil (97.8%) and Tulahuen (96.7%).
DISCUSSION
The use of TESA proteins for the diagnosis of Chagas' disease has been reported, and their use has generally provided good results in terms of specificity and sensitivity (15, 18, 20, 27, 28). However, most studies have tested relatively small numbers of samples (18, 20, 28), and some have used TESA proteins from a single strain (27, 28). In the current study, we used a large panel of well-defined sera to evaluate ELISAs based on TESA proteins from two T. cruzi strains from different geographic regions (strains Brazil and Tulahuen). We demonstrated for the first time the utility of concentrating the most antigenic components of TESA, presumably by eliminating interfering low-molecular-weight peptides or proteins. In our hands, this simple manipulation dramatically increased the signal-to-noise ratio for the total TESA Brazil antigen in particular (∼15-fold). We also show, for the first time, that immunoaffinity chromatography purification of TESA proteins can significantly enhance specificity with only a slight loss of sensitivity. Finally, our observations implicate a 60-kDa protein in the known serologic cross-reactivity that confounds the evaluation of patients and the screening of blood in regions where both Chagas' disease and leishmaniasis are endemic.
We chose to test our concentrated total TESA ELISAs using samples obtained from a blood bank in a region where Chagas' disease is endemic (i.e., in a real-life situation in which a candidate assay for Chagas' disease might be used). Both the strain Brazil- and the strain Tulahuen-based assays had excellent sensitivities (100%) for the blood bank specimens confirmed to be positive and excellent specificities (99 to 100%) for samples from healthy control subjects from a region where Chagas's disease is endemic and from a region where Chagas's disease is not endemic. Furthermore, the tests maintained very reasonable specificities (94 to 99%) even when large numbers of samples from subjects with other parasitic diseases were tested. As has been reported previously (20, 28), sera from patients infected with Leishmania spp. were the most likely to cross-react in our TESA assays (2 of 20 presumed false-positive results for patients with cutaneous leishmaniasis in both the Brazil and the Tulahuen antigen-based assays). Moreover, our specificity estimate of ∼90% for samples from leishmaniasis patients is likely to be an overestimate since no samples from patients with visceral leishmaniasis were included in our panel. Our data suggest that the concentrated total TESAs from both the Brazil and the Tulahuen strains include conserved epitopes that are highly immunogenic, which meets one of the principal criteria for Chagas' disease tests suggested by the World Health Organization (32).
During the course of these studies, in addition to confirming the potential utility of the TESAs for the diagnosis of Chagas' disease, we were also able to address several practical issues relevant to the potential use of these assays in the field. Large amounts of TESA proteins could be harvested with simple and inexpensive manipulations of relatively small volumes of trypomastigote cultures (two 175-cm2 Vero cell monolayers yielded ∼5 ml of concentrated TESA proteins at ∼850 μg/ml). Pooled total and concentrated TESA proteins were stable for at least 18 months when they were frozen as aliquots at −80°C, with no loss of reactivity or increase in background “noise” (data not shown). The anticipated yield from only two infected 175-cm2 flasks would permit the coating of approximately 420 ELISA plates and the performance of 16,800 tests. The 32-fold concentration of total TESA proteins that we achieved with a 30,000-molecular-weight cutoff filter resulted in marked increases in the signal-to-noise ratio, simplifying assay interpretation and the establishment of optimal cutoff values for specificity. It is likely that this procedure eliminates low-molecular-weight proteins-peptides that may interfere with the assay.
To our knowledge, the potential impact of immunoaffinity purification of TESAs has not been reported previously. In our hands, this step eliminated almost all of the complexity present in the total TESA protein mixtures, reducing the preparation from many bands to essentially five bands with molecular masses of 220, 170, 120, 85, and 60 kDa. The purification procedure consisted of a single step using Sepharose coupled to pooled antibodies from patients with Chagas' disease. This approach resulted in a high yield of purified proteins (190 μg/ml from an initial 850-μg/ml concentration of TESAs) and had the advantage that the immunoaffinity column could be reused several times. Data for the TESAIA-based ELISA should be considered preliminary, however, because we performed only a small-scale purification that yielded enough material to test 166 of the 709 serum samples in our panel. A larger-scale purification of TESAIAs and broader field studies of the TESAIA-based ELISA with different strains of T. cruzi are planned.
Many TESAs have recently been recognized to be transialidases. These enzymes may be virulence factors; and they participate in binding to noninfected epithelial, fibroblast, and muscle mammalian cell lines (9, 24). They are strong immunogens that elicit polyclonal antibody responses and are implicated in the induction of both inflammation and autoimmunity (21, 30). A monoclonal antibody (monoclonal antibody 39) that recognizes parasite transialidases detects polypeptide bands ranging in size from 85 to 170 kDa in TESA proteins (23). Several of these transialidase bands have molecular masses similar to those of the polypeptides present in our TESAIA preparation. Silber and colleagues (17, 25) used chromatography to purify T. cruzi proteins from total parasite extracts for the diagnosis of Chagas' disease. They identified a 67-kDa lectin-like protein that binds to human erythrocyte membranes in a galactose-dependent fashion (25) and evaluated its potential as a diagnostic reagent (17). Although they reported a good sensitivity (98%) and a good specificity (98.11%) in an ELISA format, relatively small numbers of positive serum samples were tested, and only sera from healthy subjects were included as negative controls. Studies for the identification of the proteins present in our TESAIA preparation are under way.
To our knowledge, we are the first to implicate a 60-kDa protein in the serologic cross-reactivity between Leishmania-infected samples and samples from patients with Chagas' disease. Cross-reactivity in an ELISA based on TESAs has previously been reported for subjects with visceral and cutaneous leishmaniases (20). In that study, Nakazawa and colleagues (20) implicated polypeptides of ≤150 kDa as cross-reactive but did not carry the characterization any further. Our TESAIA also contains a 60-kDa protein, yet no cross-reactivity was observed in the 16 Leishmania-infected samples tested, including the samples presumed to be false positive in the total TESA-based ELISA. The mean ODs for the Leishmania-positive sera were higher in the total TESA-based ELISAs than in the TESAIA-based ELISA, suggesting that immunoaffinity chromatography purification may eliminate the background “noise” that interferes with assay specificity. Alternately, the improved specificity of the TESAIA-based ELISA, despite the presence of a 60-kDa band in the TESAIA preparation by Western blotting, raises the possibility that two proteins of similar molecular masses are present in the total, concentrated TESA. It is also possible that presumably linear epitopes of a 60-kDa protein recognized by Leishmania-infected sera in the Western blot format are not present when TESAIA is used in the ELISA format. Studies are under way to determine whether or not removal of the reactive 60-kDa protein(s) from the total TESA preparation by molecular exclusion chromatography can achieve improved specificity while maintaining high sensitivity.
In summary, we have demonstrated that concentrated total TESA proteins are simple and inexpensive to produce, are stable at −80°C for prolonged periods of time, and have an excellent sensitivity and an excellent specificity in an ELISA format. Using a simple protocol based on pooled chagasic antibodies, we have shown for the first time that immunoaffinity chromatography-purified TESA proteins can be used to significantly enhance the specificity of ELISA. These observations raise the possibility of testing by a sequential total TESA-based ELISA followed by a TESAIA-based ELISA as a simple and cost-effective strategy for screening of blood bank samples for Chagas' disease.
Acknowledgments
This work was supported by Health Canada grant HT070-010033 and was partially supported by a grant from FONACIT (grant LAB-2000001639), Caracas, Venezuela, to José Bubis. Doctoral scholarship support for M. Berrizbeitia was provided by the Universidad de Oriente, Venezuela.
We thank Herbert Tanowitz (Einstein University of Medicine, New York, NY) for providing the T. cruzi strains; Deisy Perdomo, Rafael Medina, and Lilian Spencer (Departamento de Biología Celular, Universidad Simón Bolívar, Caracas, Venezuela) for technical support; Mary Isabel Gonzatti (Departamento de Biología Celular, Universidad Simón Bolívar, Caracas, Venezuela) for letting us use the ELISA reader in her laboratory (TECAN); and Guy Beauchamp (Université de Montréal) for support with the statistical analysis.
REFERENCES
- 1.Almeida, I. C., D. T. Covas, L. M. Soussumi, and L. R. Travassos. 1997. A highly sensitive and specific chemiluminescent enzyme-linked immunosorbent assay for diagnosis of active Trypanosoma cruzi infection. Transfusion 37:850-857. [DOI] [PubMed] [Google Scholar]
- 2.Antas, P. R., E. N. Azevedo, M. R. Luz, N. Medrano-Mercado, A. C. Chaves, P. G. Vidigal, A. C. Volpini, A. J. Romanha, and T. C. Araujo-Jorge. 2000. A reliable and specific enzyme-linked immunosorbent assay for the capture of IgM from human chagasic sera using fixed epimastigotes of Trypanosoma cruzi. Parasitol. Res. 86:813-820. [DOI] [PubMed] [Google Scholar]
- 3.Araujo, F. G., and D. Guptill. 1984. Use of antigen preparations of the amastigote stage of Trypanosoma cruzi in the serology of Chagas' disease. Am. J. Trop. Med. Hyg. 33:362-371. [DOI] [PubMed] [Google Scholar]
- 4.Aznar, C., P. Liegeard, C. Mariette, S. Lafon, M. J. Levin, and M. Hontebeyrie. 1997. A simple Trypanosoma cruzi enzyme-linked immunoassay for control of human infection in nonendemic areas. FEMS Immunol. Med. Microbiol. 18:31-37. [DOI] [PubMed] [Google Scholar]
- 5.Berrizbietia, M., M. Ndao, M. Gottschalk, A. Ache, F. Vasquez, S. Lacouture, M. Medina, and B. J. Ward. 2004. Development and comparison of enzyme immunoassays for diagnosis of Chagas' disease using fixed forms of Trypanosoma cruzi (epimastigotes, amastigotes, and trypomastigotes) and assessment of antigen stability for the three assays. J. Clin. Microbiol. 42:1766-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford, M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Breniere, S. F., R. Carrasco, H. Miguez, J. L. Lemesre, and Y. Carlier. 1985. Comparisons of immunological tests for serodiagnosis of Chagas' disease in Bolivian patients. Trop. Geogr. Med. 37:231-238. [PubMed] [Google Scholar]
- 8.Carbonetto, C. H., E. L. Malchiodi, M. G. Chiaramonte, N. W. Zwirner, and R. A. Margni. 1989. Use of formalinized epimastigotes for the detection of anti-Trypanosoma cruzi antibodies using immunoenzyme technique. Rev. Argent. Microbiol. 21:79-83. [PubMed] [Google Scholar]
- 9.Cross, G. A., and G. B. Takle. 1993. The surface trans-sialidase family of Trypanosoma cruzi. Annu. Rev. Microbiol. 47:385-411. [DOI] [PubMed] [Google Scholar]
- 10.Curtis, W., and M. Chase. 1967. Methods in immunology and immunochemistry, vol. I. Academic Press, Inc., New York, N.Y.
- 11.de Hubsch, R. M., N. Chiechie, G. Comach, R. R. Aldao, and R. D. Gusmao. 1989. The dot immunoenzymatic assay on nitrocellulose (dot-ELISA) in the diagnosis of Chagas' disease. II. Seroepidemiologic study in 4 rural communities of Venezuela. Mem. Inst. Oswaldo Cruz 84:401-408. [DOI] [PubMed] [Google Scholar]
- 12.de Hubsch, R. M., N. Chiechie, G. Comach, R. Rangel Aldao, and R. D. Gusmao. 1988. Immunoenzyme assay using micro dot on nitrocellulose (dot-ELISA) in the diagnosis of Chagas' disease. I. Comparative study of 2 antigenic preparations of Trypanosoma cruzi. Mem. Inst. Oswaldo Cruz 83:277-285. [DOI] [PubMed] [Google Scholar]
- 13.Frasch, A. C. 2000. Functional diversity in the trans-sialidase and mucin families in Trypanosoma cruzi. Parasitol. Today 16:282-286. [DOI] [PubMed] [Google Scholar]
- 14.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 15.Kesper, N., Jr., K. A. de Almeida, A. M. Stolf, and E. S. Umezawa. 2000. Immunoblot analysis of trypomastigote excreted-secreted antigens as a tool for the characterization of Trypanosoma cruzi strains and isolates. J. Parasitol. 86:862-867. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 17.Marcipar, I. S., E. Welchen, C. Roodveldt, A. J. Marcipar, and A. M. Silber. 2003. Purification of the 67-kDa lectin-like glycoprotein of Trypanosoma cruzi, LLGP-67, and its evaluation as a relevant antigen for the diagnosis of human infection. FEMS Microbiol. Lett. 220:149-154. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto, T. K., P. C. Cotrim, J. F. da Silveira, A. M. Stolf, and E. S. Umezawa. 2002. Trypanosoma cruzi: isolation of an immunodominant peptide of TESA (trypomastigote excreted-secreted antigens) by gene cloning. Diagn. Microbiol. Infect. Dis. 42:187-192. [DOI] [PubMed] [Google Scholar]
- 19.Monteon, V. M., L. Guzman-Rojas, C. Negrete-Garcia, J. L. Rosales-Encina, and P. A. Lopez. 1997. Serodiagnosis of American trypanosomosis by using nonpathogenic trypanosomatid antigen. J. Clin. Microbiol. 35:3316-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakazawa, M., D. S. Rosa, V. R. Pereira, M. O. Moura, V. C. Furtado, W. V. Souza, M. N. Barros, F. G. Abath, and Y. M. Gomes. 2001. Excretory-secretory antigens of Trypanosoma cruzi are potentially useful for serodiagnosis of chronic Chagas' disease. Clin. Diagn. Lab. Immunol. 8:1024-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pestel, J., J. P. Defoort, H. Gras-Masse, D. Afchain, A. Capron, A. Tartar, and A. Ouaissi. 1992. Polyclonal cell activity of a repeat peptide derived from the sequence of an 85-kilodalton surface protein of Trypanosoma cruzi trypomastigotes. Infect. Immun. 60:715-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinho, R. T., R. C. Pedrosa, P. Costa-Martins, and L. R. Castello-Branco. 1999. Saliva EIA: a method for the diagnosis of chronic Chagas' disease in endemic areas. Acta Trop. 72:31-38. [DOI] [PubMed] [Google Scholar]
- 23.Pinho, R. T., M. A. Vannier-Santos, C. R. Alves, A. P. Marino, L. R. Castello Branco, and J. Lannes-Vieira. 2002. Effect of Trypanosoma cruzi released antigens binding to non-infected cells on anti-parasite antibody recognition and expression of extracellular matrix components. Acta Trop. 83:103-115. [DOI] [PubMed] [Google Scholar]
- 24.Schenkman, S., D. Eichinger, M. E. Pereira, and V. Nussenzweig. 1994. Structural and functional properties of Trypanosoma trans-sialidase. Annu. Rev. Microbiol. 48:499-523. [DOI] [PubMed] [Google Scholar]
- 25.Silber, A. M., I. S. Marcipar, C. Roodveldt, P. Cabeza Meckert, R. Laguens, and A. J. Marcipar. 2002. Trypanosoma cruzi: identification of a galactose-binding protein that binds to cell surface of human erythrocytes and is involved in cell invasion by the parasite. Exp. Parasitol. 100:217-225. [DOI] [PubMed] [Google Scholar]
- 26.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Umezawa, E. S., M. S. Nascimento, N. Kesper, Jr., J. R. Coura, J. Borges-Pereira, A. C. Junqueira, and M. E. Camargo. 1996. Immunoblot assay using excreted-secreted antigens of Trypanosoma cruzi in serodiagnosis of congenital, acute, and chronic Chagas' disease. J. Clin. Microbiol. 34:2143-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Umezawa, E. S., M. S. Nascimento, and A. M. Stolf. 2001. Enzyme-linked immunosorbent assay with Trypanosoma cruzi excreted-secreted antigens (TESA-ELISA) for serodiagnosis of acute and chronic Chagas' disease. Diagn. Microbiol. Infect. Dis. 39:169-176. [DOI] [PubMed] [Google Scholar]
- 29.Umezawa, E. S., M. A. Shikanai-Yasuda, and A. M. Stolf. 1996. Changes in isotype composition and antigen recognition of anti-Trypanosoma cruzi antibodies from acute to chronic Chagas' disease. J. Clin. Lab. Anal. 10:407-413. [DOI] [PubMed] [Google Scholar]
- 30.Weston, D., B. Patel, and W. C. Van Voorhis. 1999. Virulence in Trypanosoma cruzi infection correlates with the expression of a distinct family of sialidase superfamily genes. Mol. Biochem. Parasitol. 98:105-116. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization. 1999. Chagas' disease, Chile. Wkly. Epidemiol. Rec. 74:9-16. [Google Scholar]
- 32.Zingales, B., A. Gruber, C. B. Ramalho, E. S. Umezawa, and W. Colli. 1990. Use of two recombinant proteins of Trypanosoma cruzi in the serological diagnosis of Chagas' disease. Mem. Inst. Oswaldo Cruz 85:519-522. [DOI] [PubMed] [Google Scholar]