Abstract
The study of the metabolome presents numerous challenges, first among them being the cataloging of its constituents. A step in this direction will be the development of tools to identify metabolites that share common structural features. The importance of sulfated molecules in cell–cell communication motivated us to develop a rapid two-step method for identifying these metabolites in microorganisms, particularly in pathogenic mycobacteria. Sulfurcontaining molecules were initially identified by mass spectral analysis of cell extracts from bacteria labeled metabolically with a stable sulfur isotope (34SO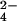 ). To differentiate sulfated from reduced-sulfur-containing molecules, we employed a mutant lacking the reductive branch of the sulfate assimilation pathway. In these sulfur auxotrophs, heavy sulfate is channeled exclusively into sulfated metabolites. The method was applied to the discovery of several new sulfated molecules in Mycobacterium tuberculosis and Mycobacterium smegmatis. Because a sulfur auxotrophic strain is the only requirement of the approach, many microorganisms can be studied in this manner. Such genetic engineering in combination with stable isotopic labeling can be applied to various metabolic pathways and their products.
). To differentiate sulfated from reduced-sulfur-containing molecules, we employed a mutant lacking the reductive branch of the sulfate assimilation pathway. In these sulfur auxotrophs, heavy sulfate is channeled exclusively into sulfated metabolites. The method was applied to the discovery of several new sulfated molecules in Mycobacterium tuberculosis and Mycobacterium smegmatis. Because a sulfur auxotrophic strain is the only requirement of the approach, many microorganisms can be studied in this manner. Such genetic engineering in combination with stable isotopic labeling can be applied to various metabolic pathways and their products.
The modification of primary and secondary metabolites by the addition or removal of sulfate can have a profound influence on their biological properties (1–5). Typically, sulfated molecules are directed outside the cell, where they serve as modulators of cell–cell interactions. As a notable example pertinent to human health, sulfation of the glycans of endothelial CD34 engenders high-affinity binding with the leukocyte adhesion molecule L-selectin, facilitating an interaction that eventually leads to the recruitment of lymphocytes into peripheral lymph nodes (6). Similarly, sulfation of tyrosyl residues found on the chemokine receptor CCR5 is a modification required for binding of HIV gp120 and therefore efficient viral entry (7).
The roles of sulfated compounds in prokaryotes and other microbes are less clear. In one well-characterized case, however, sulfation acts as a modulator of cell–cell communication, similar to its role in eukaryotes. The nitrogen-fixing bacterium Sinorhizobium meliloti utilizes a sulfated glycolipid as a secondary messenger to induce root nodulation in its plant host alfalfa (4, 8). Mutants lacking the sulfotransferase that installs this sulfate ester are unable to induce root nodulation in alfalfa but gain the ability to colonize the roots of vetch. That sulfation of a single glycolipid plays such a vital role in nitrogen fixation has far-reaching implications for the agricultural community and presents a possible target for chemical or genetic engineering.
Sulfation may also be relevant to the process of bacterial pathogenesis (9). Several mycobacteria, including the human pathogens Mycobacterium tuberculosis and Mycobacterium avium, are known to produce sulfated compounds. One example is Sulfatide-1 (SL-1, Fig. 1A), a sulfated glycolipid from M. tuberculosis that is notable for the correlation of its abundance to strain virulence (10, 11). Other sulfated metabolites include 2-sulfo-6-deoxytalose within the cell wall glycopeptidolipid (GPL) of M. avium, a structure found up-regulated in a drug-resistant strain isolated from a patient with AIDS (12). GPL sulfation has also been detected in the opportunistic pathogen Mycobacterium fortuitum (13). These findings are suggestive of an important biological role for sulfation in mycobacteria.
Fig 1.
Structure of SL-1 (A) and mycothiol (B). The SL-1 structure shown is as originally proposed by Goren and colleagues (10, 35).
Given the interesting properties of sulfated molecules, we sought to develop a sensitive and general method for their discovery from microbial metabolomes. Conventional studies using radioactive sulfate as a metabolic label are limited, because it is impossible to distinguish between compounds containing reduced sulfur (r-sulfur, i.e., compounds with thiols or thioethers) and those possessing sulfate esters (14, 15). Furthermore, direct chemical analysis of radiolabeled metabolites requires their laborious chromatographic separation.
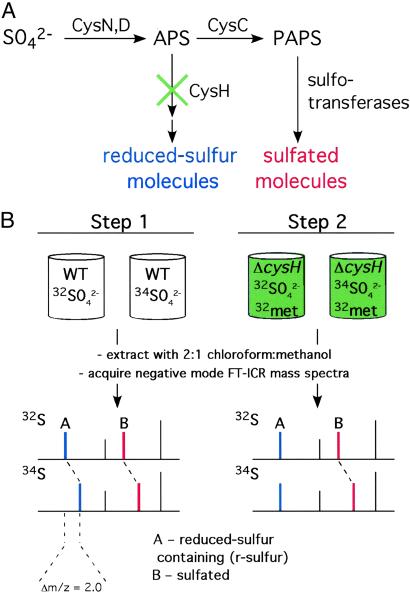
The method we developed utilizes Fourier transform–ion cyclotron resonance mass spectrometry (FT-ICRMS) to identify sulfated metabolites by virtue of their metabolic labeling with a stable sulfur isotope (34SO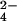 ). Sulfated molecules can be unambiguously distinguished from those possessing r-sulfur by performing the experiment with mutants lacking the reductive branch of the sulfate assimilation pathway (Fig. 2). MS is the preferred technique for this study, because its potential for metabolome-wide parallel analysis obviates the need for purification of individual metabolites and therefore any information, a priori, about the structure or physical properties of the compound. In this report, we applied the method to the identification and characterization of sulfated molecules from mycobacteria. We discovered two sulfated compounds in Mycobacterium smegmatis, an organism previously not known to produce sulfated molecules. One of these molecules was a sulfated disaccharide that was previously thought to be restricted to M. tuberculosis. In addition, we identified biosynthetic precursors to SL-1 and discovered a novel sulfated compound in M. tuberculosis.
). Sulfated molecules can be unambiguously distinguished from those possessing r-sulfur by performing the experiment with mutants lacking the reductive branch of the sulfate assimilation pathway (Fig. 2). MS is the preferred technique for this study, because its potential for metabolome-wide parallel analysis obviates the need for purification of individual metabolites and therefore any information, a priori, about the structure or physical properties of the compound. In this report, we applied the method to the identification and characterization of sulfated molecules from mycobacteria. We discovered two sulfated compounds in Mycobacterium smegmatis, an organism previously not known to produce sulfated molecules. One of these molecules was a sulfated disaccharide that was previously thought to be restricted to M. tuberculosis. In addition, we identified biosynthetic precursors to SL-1 and discovered a novel sulfated compound in M. tuberculosis.
Fig 2.
General method for the identification of sulfated metabolites in microbes. (A) The sulfate assimilation pathway begins with the conversion of inorganic sulfate (SO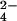 ) to APS by ATP sulfurylase (CysN,D). APS lies at a branchpoint in the pathway in mycobacteria (17). APS kinase (CysC) phosphorylates APS to form 3′-phosphoadenosine 5′-phosphosulfate (PAPS), the sulfate donor for sulfotransferases. These enzymes transfer the sulfuryl group onto a variety of substrates in the cell, forming sulfated molecules (red). In the reductive branch of the pathway, APS is reduced by APS reductase (CysH), eventually leading to the production of r-sulfur-containing molecules (blue). These can be distinguished from sulfated molecules (red) by using a mutation (green “×”) that blocks the reductive branch of the pathway. (B) MS can be used with heavy sulfur isotope labeling and the mutant in A (ΔcysH) to identify and distinguish r-sulfur and sulfated molecules. Sulfur-containing molecules are identified by MS in wild-type cells by virtue of their incorporation of 34S upon growth in 34SO
) to APS by ATP sulfurylase (CysN,D). APS lies at a branchpoint in the pathway in mycobacteria (17). APS kinase (CysC) phosphorylates APS to form 3′-phosphoadenosine 5′-phosphosulfate (PAPS), the sulfate donor for sulfotransferases. These enzymes transfer the sulfuryl group onto a variety of substrates in the cell, forming sulfated molecules (red). In the reductive branch of the pathway, APS is reduced by APS reductase (CysH), eventually leading to the production of r-sulfur-containing molecules (blue). These can be distinguished from sulfated molecules (red) by using a mutation (green “×”) that blocks the reductive branch of the pathway. (B) MS can be used with heavy sulfur isotope labeling and the mutant in A (ΔcysH) to identify and distinguish r-sulfur and sulfated molecules. Sulfur-containing molecules are identified by MS in wild-type cells by virtue of their incorporation of 34S upon growth in 34SO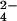 –containing media (Step 1). Sulfated (red) molecules can be distinguished from r-sulfur-containing molecules (blue) by performing the same experiment using a ΔcysH strain (Step 2).
–containing media (Step 1). Sulfated (red) molecules can be distinguished from r-sulfur-containing molecules (blue) by performing the same experiment using a ΔcysH strain (Step 2).
Materials and Methods
Strains, Growth Analysis, and Metabolite Extraction.
M. smegmatis mc2155 (16) and M. tuberculosis H37Rv (American Type Culture Collection 27294) were the wild-type strains used in this study. The M. smegmatis ΔcysH strain has been reported previously (17). Sulfur auxotrophy of the M. tuberculosis ΔcysH strain was confirmed by growth in sulfur-free medium. Details relating to the construction of M. tuberculosis ΔcysH will be reported elsewhere.
For MS analysis, a 2-ml culture of each strain was grown in modified Hartman deBonte media containing either 2 mM Na232SO4 or Na234SO4 (ICON Isotopes, Summit, NJ) and 1 mM methionine when required for growth, as the sole sulfur sources (18). Once cells had reached stationary phase, they were pelleted by centrifugation and washed with PBS. The resulting cell pellet was extracted with 0.5 ml of 2:1 chloroform:methanol by vigorous shaking for 2 h at room temperature. The organic phase was clarified by centrifugation and removed for MS analysis. Authentic SL-1 was obtained from Colorado State University (National Institutes of Health, National Institute of Allergy and Infectious Diseases Contract N01 AI-75320).
MS.
Spectra were acquired on a Bruker Apex II FT-ICR MS (Bruker Daltonics, Billerica, MA) equipped with a 7T actively shielded magnet. Cell extracts were sprayed from 2:1 chloroform: methanol at 3 μl/min on a pneumatically assisted electrospray source (Analytica, Branford, CT) in either positive or negative ion mode. Ions were accumulated in an external hexapole between 0.5 and 2 sec to yield strong signal before being transferred to the ion cyclotron resonance cell for mass analysis (19). For tandem MS (MS/MS) analysis, ions of interest were isolated by using a correlated harmonic excitation field isolation sweep (20). In certain cases, cleanup shots were used to eject ions that were not ejected from the initial sweep. Once isolated, ions of interest were collisionally activated by sustained off-resonance irradiation at 500 Hz above the cyclotron frequency for 250 ms, using Ar as the collision gas (21). Each spectrum is an average of either 16 or 32 transients composed of 256,000 or 512,000 data points acquired by using xmass, Version 5.01 (Bruker, Billerica, MA).
Synthesis of Trehalose-2-Sulfate.
Triethylammonium 4,6:4′,6′-dibenzylidene-α,α-trehalose 2-sulfate was prepared according to the procedure of Vasella and coworkers (22). This compound (10 mg, 0.13 mmol) was treated with 2.0 ml of trifluoroacetic acid in 2.0 ml of MeOH. After stirring for 15 min, the solution was concentrated under reduced pressure. The residue was basified with 0.50 ml Et3N and concentrated again.
Results
Identification of R-Sulfur and Sulfated Compounds.
Searching for novel sulfated compounds by MS presented a number of challenges. For example, the chemical nature, abundance, and expected mass of these putative sulfated compounds were not known. Previous studies have shown the utility of isotopic labeling for deconvoluting complex biological samples during MS analysis (23–26). We sought to expand on these techniques for the purposes of discovering metabolites with a specific chemical modification, the sulfate ester. Toward this end, we designed a two-step method that utilizes 34S as a stable isotopic label, and a specific gene knockout (ΔcysH) in the sulfate assimilation pathway to uniquely identify the sulfated constituents of a metabolome (see the legend to Fig. 2 for details of the sulfate assimilation pathway).
In the first step (Fig. 2B), wild-type cells were grown in minimal media containing either Na232SO4 or Na234SO4 as the sole sulfur source. In the cells grown with Na234SO4-containing media, the masses of compounds containing either sulfur or sulfate were expected to shift by 1.996 × n Da, where n is the number of sulfur atoms. Extracts from labeled cultures were analyzed using a FT-ICRMS equipped with an electrospray ionization source. This arrangement has many advantages, including gentle ionization to yield intact ions, high resolution, high mass accuracy, and MS/MS capabilities (27).
To differentiate candidate compounds containing sulfate from those containing r-sulfur, a second step (Fig. 2B) was used that eliminates the sulfur reduction pathway. Specifically, the same isotope incorporation experiment was performed in a mutant strain, ΔcysH, which lacks the ability to reduce adenosine-5′-phosphosulfate (APS) to sulfite, the first committed step in the biosynthesis of r-sulfur (17). Thus, when a ΔcysH strain was grown in 32S-methionine (32met) + Na234SO4-containing media, only sulfated compounds incorporated the heavy sulfur isotope. This method is general and applicable to any simple organism for which a mutant can be made in the early stages of the reductive branch of the sulfate assimilation pathway. Such mutant strains of many commonly studied microorganisms already exist, as they are easily isolated as sulfur auxotrophs (28–31).
Demonstration of the Method in M. tuberculosis and M. smegmatis: SL-1 and Mycothiol.
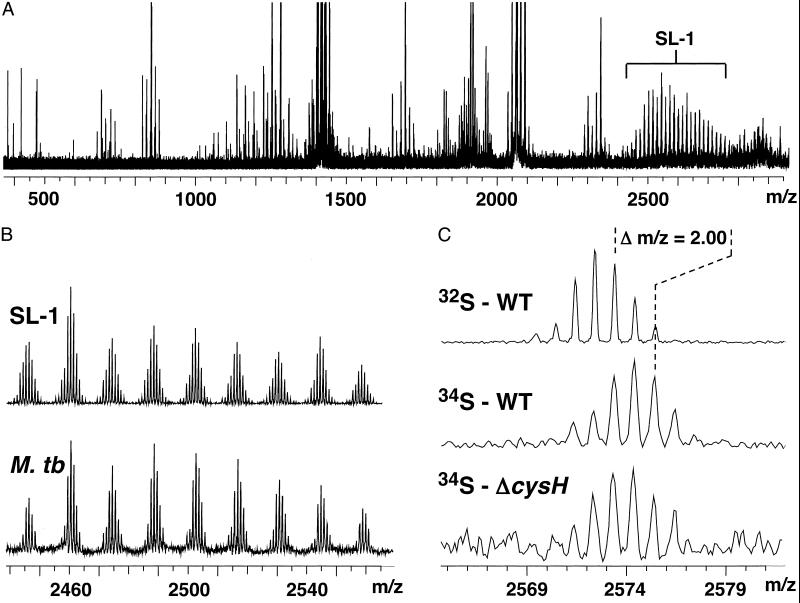
Comparison of negative-ion mode mass spectra from Na232SO4 and Na234SO4-labeled extracts of M. smegmatis and M. tuberculosis revealed a number of sulfur-containing compounds (Table 1). Owing to the high mass accuracy of FT-ICRMS, even in total crude extracts containing >1,500 individual components (Fig. 3A), the expected Δm/z between 32S and 34S of 1.996 could be readily distinguished from other 2-Da nominal differences. A total of five and nine sulfur-containing compounds were identified in M. tuberculosis and M. smegmatis, respectively. Compounds containing sulfate were distinguished from those containing r-sulfur by acquiring similar spectra from Na232SO4 and Na234SO4-labeled extracts of M. tuberculosis ΔcysH and M. smegmatis ΔcysH (Table 1).
Table 1.
Summary of sulfated and r-sulfur-containing molecules found in M. tuberculosis and M. smegmatis
| m/z 32S | m/z 34S | Shift in ΔcysH | HSO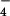 ion observed in MS/MS ion observed in MS/MS |
Form of sulfur | Metabolite annotations | |
|---|---|---|---|---|---|---|
| M. tuberculosis | 421.07 | 423.06 | Y | Y | Sulfate | Trehalose-2-sulfate |
| 485.14 | 487.13 | N | N | r-sulfur | Mycothiol | |
| 881.57 | 883.57 | Y | Y | Sulfate | None | |
| 1,277.97 | 1,279.97 | Y | — | Sulfate | SL-1 precursor | |
| 2,543.22 | 2,545.21 | Y | Y | Sulfate | SL-1 | |
| M. smegmatis | 421.07 | 423.06 | Y | Y | Sulfate | Trehalose-2-sulfate |
| 485.14 | 487.13 | N | N | r-sulfur | Mycothiol | |
| 521.12 | 523.12 | N | N/A | r-sulfur | None | |
| 583.13 | 585.12 | N | N/A | r-sulfur | None | |
| 597.14 | 599.14 | N | N/A | r-sulfur | None | |
| 632.20 | 634.20 | N | N/A | r-sulfur | None | |
| 639.19 | 641.18 | Y | Y | Sulfate | None | |
| 753.23 | 755.22 | N | N/A | r-sulfur | None | |
| 1,009.25 | 1,013.24 | N | N/A | r-sulfur | Mycothiol dimer |
Unless otherwise noted, masses given are monoisotopic and from negative ion mode acquisitions. All ions were singly charged. The resolution was >30,000 for all reported masses.
Name or structure assigned to the metabolite.
Assignment made in this study.
Ion containing a reduced form of sulfur, either a thioether or a thiol.
Due to low abundance, we were unable to perform MS/MS on this ion.
Calculations show this ion likely corresponds to a specific diacylated trehalose-2-sulfate (see text).
Monoisotopic mass of a major lipoform. It should be noted that the calculated mass of the originally proposed SL-1 structure does not match with our measured mass of the SL-1 standard. The standard used for our analysis is of the same batch used to elucidate the structure of SL-1.
Not applicable; MS/MS not performed on r-sulfur-containing compounds, with the exception of mycothiol.
This ion observed in positive mode.
Two mycothiol molecules linked by a disulfide bond, potassiated adduct.
Fig 3.
Incorporation of 34S into SL-1 can be detected in crude M. tuberculosis extracts. (A) Typical mass spectrum of M. tuberculosis extracts. The SL-1 lipoform envelope (bracketed) is readily observed in these samples. (B) Mass spectra of a purified SL-1 standard and a crude M. tuberculosis extract. (C) Zoom-in of one SL-1 lipoform showing that the mass of the molecule shifts by 2.00 Da in WT M. tuberculosis grown in 32SO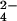 (Top) vs. 34SO
(Top) vs. 34SO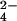 (Middle). This mass shift is also observed in M. tuberculosis ΔcysH grown in 34SO
(Middle). This mass shift is also observed in M. tuberculosis ΔcysH grown in 34SO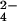 + 32met (Bottom), confirming that the compound is sulfated.
+ 32met (Bottom), confirming that the compound is sulfated.
In order to validate the method, we sought to identify known examples of sulfated and r-sulfur-containing compounds. Mentioned above, M. tuberculosis is known to produce the sulfated glycolipid, SL-1 (Fig. 1A). To identify SL-1, we compared mass spectra taken from M. tuberculosis extracts to a purified SL-1 standard. Ions with masses matching those of authentic SL-1 were prominent in the spectrum acquired from M. tuberculosis extracts (Fig. 3 A and B). Due to variations in the length of its four acyl groups [± (−CH2−)n], SL-1 is observed as an envelope of lipoforms centered around m/z 2,600, with individual lipoforms differing by 14 mass units (Figs. 1A and 3B). We next examined the extract from M. tuberculosis grown in 34SO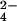 . In this sample, the SL-1 ions shifted in mass by the expected 2.00 Da, indicating the presence of a single sulfur atom in the structure of the molecule (Fig. 3C). The masses of SL-1 lipoforms from extracts taken from M. tuberculosis ΔcysH cultures labeled with 34SO
. In this sample, the SL-1 ions shifted in mass by the expected 2.00 Da, indicating the presence of a single sulfur atom in the structure of the molecule (Fig. 3C). The masses of SL-1 lipoforms from extracts taken from M. tuberculosis ΔcysH cultures labeled with 34SO also shifted by 2.00 Da, as predicted for a sulfated molecule (Fig. 3C). MS/MS experiments of one SL-1 lipoform yielded the HSO
also shifted by 2.00 Da, as predicted for a sulfated molecule (Fig. 3C). MS/MS experiments of one SL-1 lipoform yielded the HSO product ion, providing further evidence that the selected ion was sulfated (data not shown).
product ion, providing further evidence that the selected ion was sulfated (data not shown).
To demonstrate the method for r-sulfur-containing compounds, we focused our attention on mycothiol, an abundant cysteine derivative found in actinomycetes that is involved in neutralizing potentially damaging cellular electrophiles (Fig. 1B) (32–34). Using exact mass measurements (m/z 485.1437, within 2 ppm of calculated mass) and MS/MS experiments (data not shown), we showed that the mycothiol ion was present in both M. tuberculosis and M. smegmatis extracts. Mycothiol behaved as predicted for a r-sulfur-containing molecule; its mass shifted by 2.00 Da in wild-type extracts labeled with 34SO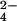 , and it was found only in the 32S form in extracts prepared from ΔcysH strains grown in 34SO
, and it was found only in the 32S form in extracts prepared from ΔcysH strains grown in 34SO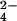 and 32met (Table 1).
and 32met (Table 1).
Sulfated and R-Sulfur-Containing Molecules in M. smegmatis.
Analysis of extracts prepared from 32SO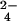 - and 34SO
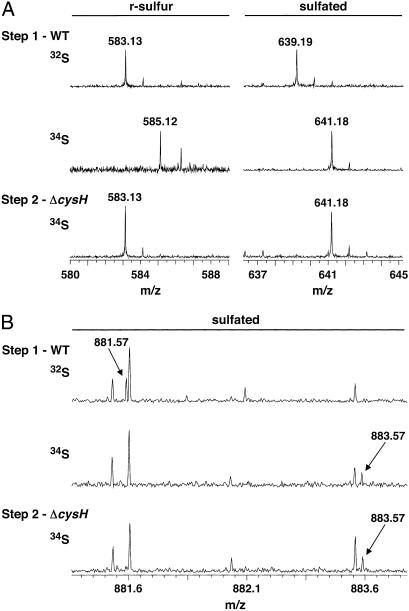
- and 34SO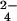 -labeled M. smegmatis resulted in the identification of a number of sulfur-containing molecules (Table 1). When the same labeling experiment was performed in M. smegmatis ΔcysH, two of the molecules, m/z 421.07 and 639.19, were found to be sulfated (Table 1 and Fig. 4A). In both cases, subjecting the 34S form of these ions to MS/MS yielded the H34SO
-labeled M. smegmatis resulted in the identification of a number of sulfur-containing molecules (Table 1). When the same labeling experiment was performed in M. smegmatis ΔcysH, two of the molecules, m/z 421.07 and 639.19, were found to be sulfated (Table 1 and Fig. 4A). In both cases, subjecting the 34S form of these ions to MS/MS yielded the H34SO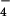 product ion (m/z 99.0), providing further evidence of their sulfation. The 34S form of these ions was used for MS/MS analysis, because it conveniently separates the HSO
product ion (m/z 99.0), providing further evidence of their sulfation. The 34S form of these ions was used for MS/MS analysis, because it conveniently separates the HSO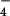 and H2PO
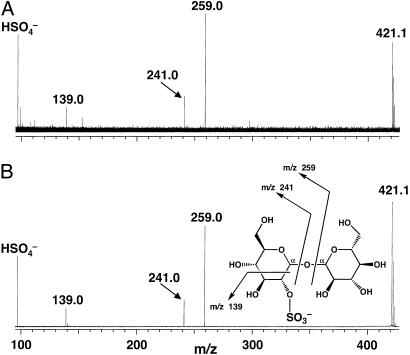
and H2PO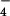 isobars, thereby removing ambiguity in the assignment of sulfate. Since no sulfated molecules have ever been characterized from M. smegmatis, we chose to examine one of them, m/z 421.07, in more detail. Exact mass measurements of this ion gave m/z 421.0661, which is within 1 ppm of the expected mass of a sulfated disaccharide. To further test this possibility, we performed MS/MS on m/z 421.07 (Fig. 5A). Several product ions characteristic of a sulfated disaccharide were observed. The product ions at m/z 259.0 and m/z 241.0 correspond to cleavage on either side of the glycosidic bond with charge retention on the sulfate. The ion at m/z 97.0 corresponds to an elemental composition of HSO
isobars, thereby removing ambiguity in the assignment of sulfate. Since no sulfated molecules have ever been characterized from M. smegmatis, we chose to examine one of them, m/z 421.07, in more detail. Exact mass measurements of this ion gave m/z 421.0661, which is within 1 ppm of the expected mass of a sulfated disaccharide. To further test this possibility, we performed MS/MS on m/z 421.07 (Fig. 5A). Several product ions characteristic of a sulfated disaccharide were observed. The product ions at m/z 259.0 and m/z 241.0 correspond to cleavage on either side of the glycosidic bond with charge retention on the sulfate. The ion at m/z 97.0 corresponds to an elemental composition of HSO , whereas the ion observed at m/z 139.0 results from a cross-ring cleavage, yielding an ion with an elemental composition of [C2H3O5S]–. We propose that the sulfated disaccharide in M. smegmatis corresponds to trehalose-2-sulfate, the core structure of M. tuberculosis SL-1. As further proof of structure, we compared MS/MS spectra from m/z 421.07 and a chemically synthesized trehalose-2-sulfate standard (Fig. 5B). An identical set of product ions was found in both samples, strongly supporting the identity of m/z 421.07 as trehalose-2-sulfate. Assignment of the major product ions of m/z 421.07, based on the structure of trehalose-2-sulfate, is shown in the inset of Fig. 5B.
, whereas the ion observed at m/z 139.0 results from a cross-ring cleavage, yielding an ion with an elemental composition of [C2H3O5S]–. We propose that the sulfated disaccharide in M. smegmatis corresponds to trehalose-2-sulfate, the core structure of M. tuberculosis SL-1. As further proof of structure, we compared MS/MS spectra from m/z 421.07 and a chemically synthesized trehalose-2-sulfate standard (Fig. 5B). An identical set of product ions was found in both samples, strongly supporting the identity of m/z 421.07 as trehalose-2-sulfate. Assignment of the major product ions of m/z 421.07, based on the structure of trehalose-2-sulfate, is shown in the inset of Fig. 5B.
Fig 4.
Identification of previously unknown sulfated compounds in M. tuberculosis and M. smegmatis. (A) Mass spectra of M. smegmatis extracts showing that m/z 583.13 contains r-sulfur, whereas m/z 639.19 is sulfated. The m/z 583.13 ion (Left) shifts to m/z 585.12 in WT (Step 1) cells grown in 34SO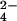 . The ion does not incorporate 34S when ΔcysH is grown in 34SO
. The ion does not incorporate 34S when ΔcysH is grown in 34SO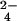 and 32met (Step 2). The m/z 639.19 ion (Right) shifts to m/z 641.18 when WT (Step 1) and ΔcysH strains (Step 2) are grown in 34SO
and 32met (Step 2). The m/z 639.19 ion (Right) shifts to m/z 641.18 when WT (Step 1) and ΔcysH strains (Step 2) are grown in 34SO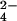 . (B) Mass spectra of M. tuberculosis extracts showing m/z 881.57 is a sulfated compound. The m/z 881.57 ion shifts to m/z 883.57 when WT (Step 1) and ΔcysH strains (Step 2) are grown in 34SO
. (B) Mass spectra of M. tuberculosis extracts showing m/z 881.57 is a sulfated compound. The m/z 881.57 ion shifts to m/z 883.57 when WT (Step 1) and ΔcysH strains (Step 2) are grown in 34SO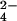 .
.
Fig 5.
MS/MS analysis of m/z 421.07 and a synthesized trehalose-2-sulfate standard. (A) Product ions of the m/z 421.07 ion. (B) Product ions of the trehalose-2-sulfate standard. (Inset) The assignment of product ions from A and B based on the structure of trehalose-2-sulfate.
Discovery of New Sulfated Molecules in M. tuberculosis.
Based on heavy sulfur incorporation in both wild-type and ΔcysH 34SO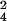 −-labeling experiments, three additional sulfated compounds were identified in M. tuberculosis, corresponding to m/z 421.07, 881.57, and 1,277.97 (Table 1). As shown in Fig. 4B, the m/z 881.57 ion differs by only 0.03 and 0.07 Da from its closest isobar in the 32S and 34S-forms, respectively. This example underscores the need for high-resolution data when searching a complex metabolome for mass shifts within unknown ions. The m/z 421.07 ion, also observed in M. smegmatis, corresponds to trehalose-2-sulfate (discussed above), the disaccharide core of SL-1. The possibility that m/z 1,277.97 and 881.57 were also related to SL-1, as either metabolic precursors or ionization fragments, was also explored. If trehalose-2-sulfate is the first intermediate in the biosynthesis of SL-1, as observation of this free sulfated disaccharide in M. tuberculosis extracts would suggest, it is likely that m/z 1,277.97 is a downstream intermediate in the same pathway. The [M–H]− ion at m/z 1,277.97 agrees with the deprotonated mass of trehalose-2-sulfate acylated with a palmitoyl group (C16 fatty acid) and a single hydroxyphthioceranoyl group (m/z 1,277.93, Fig. 1A). The observation of these proposed intermediates offers a possible biosynthetic route to SL-1, in which sulfation of the trehalose core precedes lipid modification.
−-labeling experiments, three additional sulfated compounds were identified in M. tuberculosis, corresponding to m/z 421.07, 881.57, and 1,277.97 (Table 1). As shown in Fig. 4B, the m/z 881.57 ion differs by only 0.03 and 0.07 Da from its closest isobar in the 32S and 34S-forms, respectively. This example underscores the need for high-resolution data when searching a complex metabolome for mass shifts within unknown ions. The m/z 421.07 ion, also observed in M. smegmatis, corresponds to trehalose-2-sulfate (discussed above), the disaccharide core of SL-1. The possibility that m/z 1,277.97 and 881.57 were also related to SL-1, as either metabolic precursors or ionization fragments, was also explored. If trehalose-2-sulfate is the first intermediate in the biosynthesis of SL-1, as observation of this free sulfated disaccharide in M. tuberculosis extracts would suggest, it is likely that m/z 1,277.97 is a downstream intermediate in the same pathway. The [M–H]− ion at m/z 1,277.97 agrees with the deprotonated mass of trehalose-2-sulfate acylated with a palmitoyl group (C16 fatty acid) and a single hydroxyphthioceranoyl group (m/z 1,277.93, Fig. 1A). The observation of these proposed intermediates offers a possible biosynthetic route to SL-1, in which sulfation of the trehalose core precedes lipid modification.
By contrast, m/z 881.57 appears to be structurally unrelated to SL-1. To further characterize this compound, we subjected the 34S form to MS/MS analysis. This revealed the H34SO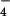 product ion (m/z 99.0) and a product ion corresponding to the loss of 34SO
product ion (m/z 99.0) and a product ion corresponding to the loss of 34SO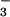 (m/z 801.6, Table 1, and data not shown). MS/MS therefore provided direct chemical evidence that m/z 881.57 was sulfated. Exact mass measurements and additional preliminary MS/MS experiments showed that the ion is likely to have both lipid and peptide components. Further structural details of m/z 881.57 are forthcoming and will be reported elsewhere.
(m/z 801.6, Table 1, and data not shown). MS/MS therefore provided direct chemical evidence that m/z 881.57 was sulfated. Exact mass measurements and additional preliminary MS/MS experiments showed that the ion is likely to have both lipid and peptide components. Further structural details of m/z 881.57 are forthcoming and will be reported elsewhere.
Discussion
We developed a simple two-step procedure for discovering sulfated metabolites from complex cellular extracts. The application of this method to M. tuberculosis and M. smegmatis resulted in a number of significant findings, including the first examples of sulfated molecules in M. smegmatis, observation of a biosynthetic precursor to SL-1, and the identification of a novel sulfated molecule in M. tuberculosis. The ability of the method to differentiate between sulfated and r-sulfur-containing compounds was demonstrated by using SL-1 and mycothiol as appropriate internal controls.
Labeling with 34SO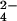 can also be used for MS-based discovery of sulfated proteins, polysaccharides, and small molecules in mammalian systems. In higher organisms, the experiment is simplified by the absence of sulfate reduction pathways; heavy sulfate is only incorporated into sulfated molecules. We anticipate that this metabolic labeling/MS approach will be amenable to studies of protein sulfation and the sulfated glycosaminoglycans that populate the extracellular matrix (23).
can also be used for MS-based discovery of sulfated proteins, polysaccharides, and small molecules in mammalian systems. In higher organisms, the experiment is simplified by the absence of sulfate reduction pathways; heavy sulfate is only incorporated into sulfated molecules. We anticipate that this metabolic labeling/MS approach will be amenable to studies of protein sulfation and the sulfated glycosaminoglycans that populate the extracellular matrix (23).
In addition to its use in the discovery of sulfated molecules, this method provides a framework and strategy for discovering other types of secondary metabolites with relatively little information about their chemical structure. If a known metabolic pathway is required for the biosynthesis of a compound, mutations that block undesired branchpoints in this pathway can be made in order to channel the isotope to the target molecule class. This technology, coupled with advances in MS, will aid in studies of metabolic flux, the identification of new biosynthetic pathways, and the annotation of cellular metabolomes.
Acknowledgments
J.D.M. was supported by a Ford Foundation Predoctoral Fellowship. S.J.W. was supported by a Howard Hughes Medical Institute Fellowship of the Life Sciences Research Foundation. Work in the authors' laboratories is supported by grants to C.R.B. (AI51622) and J.A.L. (GM47356) from the National Institutes of Health.
Abbreviations
SL-1, Sulfatide-1
r-sulfur, compounds containing reduced sulfur
APS, adenosine-5′-phosphosulfate
MS/MS, tandem MS
FT-ICRMS, Fourier transform–ion cyclotron resonance MS
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kehoe J. W. & Bertozzi, C. R. (2000) Chem. Biol. 7, R57-R61. [DOI] [PubMed] [Google Scholar]
- 2.Bowman K. G. & Bertozzi, C. R. (1999) Chem. Biol. 6, R9-R22. [DOI] [PubMed] [Google Scholar]
- 3.Hemmerich S. & Rosen, S. D. (2000) Glycobiology 10, 849-856. [DOI] [PubMed] [Google Scholar]
- 4.Roche P., Debelle, F., Maillet, F., Lerouge, P., Faucher, C., Truchet, G., Denarie, J. & Prome, J. C. (1991) Cell 67, 1131-1143. [DOI] [PubMed] [Google Scholar]
- 5.Hanin M., Jabbouri, S., Quesada-Vincens, D., Freiberg, C., Perret, X., Prome, J. C., Broughton, W. J. & Fellay, R. (1997) Mol. Microbiol. 24, 1119-1129. [DOI] [PubMed] [Google Scholar]
- 6.Hemmerich S., Butcher, E. C. & Rosen, S. D. (1994) J. Exp. Med. 180, 2219-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farzan M., Mirzabekov, T., Kolchinsky, P., Wyatt, R., Cayabyab, M., Gerard, N. P., Gerard, C., Sodroski, J. & Choe, H. (1999) Cell 96, 667-676. [DOI] [PubMed] [Google Scholar]
- 8.Ehrhardt D. W., Atkinson, E. M., Faull, K. F., Freedberg, D. I., Sutherlin, D. P., Armstrong, R. & Long, S. R. (1995) J. Bacteriol. 177, 6237-6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mougous J. D., Green, R. E., Williams, S. J., Brenner, S. E. & Bertozzi, C. R. (2002) Chem. Biol. 9, 767-776. [DOI] [PubMed] [Google Scholar]
- 10.Goren M. B., Brokl, O. & Das, B. C. (1976) Biochemistry 15, 2728-2735. [DOI] [PubMed] [Google Scholar]
- 11.Goren M. B., Brokl, O. & Schaefer, W. B. (1974) Infect. Immun. 9, 142-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khoo K. H., Jarboe, E., Barker, A., Torrelles, J., Kuo, C. W. & Chatterjee, D. (1999) J. Biol. Chem. 274, 9778-9785. [DOI] [PubMed] [Google Scholar]
- 13.Lopez Marin L. M., Laneelle, M. A., Prome, D., Laneelle, G., Prome, J. C. & Daffe, M. (1992) Biochemistry 31, 11106-11111. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy C. (1976) Infect. Immun. 14, 1241-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsukamura M., Mizuno, S. & Toyama, H. (1984) Microbiol. Immunol. 28, 965-974. [DOI] [PubMed] [Google Scholar]
- 16.Snapper S. B., Melton, R. E., Mustafa, S., Kieser, T. & Jacobs, W. R., Jr. (1990) Mol. Microbiol. 4, 1911-1919. [DOI] [PubMed] [Google Scholar]
- 17.Williams S. J., Senaratne, R. H., Mougous, J. D., Riley, L. W. & Bertozzi, C. R. (2002) J. Biol. Chem. 277, 32606-32615. [DOI] [PubMed] [Google Scholar]
- 18.Smeulders M. J., Keer, J., Speight, R. A. & Williams, H. D. (1999) J. Bacteriol. 181, 270-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Senko M. W., Hendrickson, C. L., Pasa-Tolic, L., Marto, J. A., White, F. M., Guan, S. & Marshall, A. G. (1996) Rapid Commun. Mass Spectrom. 10, 1824-1828. [DOI] [PubMed] [Google Scholar]
- 20.deKonig L. J. & Nibbering, N. M. (1997) Int. J. Mass Spectrom. Ion Proc. 165, 209-219. [Google Scholar]
- 21.Gauthier J., Trautman, T. R. & Jacobson, D. B. (1991) Anal. Chim. Acta 246, 211-225. [Google Scholar]
- 22.Langston S., Bernet, B. & Vasella, A. (1994) Helv. Chim. Acta 77, 2341-2353. [Google Scholar]
- 23.Kuberan B., Lech, M., Zhang, L., Wu, Z. L., Beeler, D. L. & Rosenberg, R. D. (2002) J. Am. Chem. Soc. 124, 8707-8718. [DOI] [PubMed] [Google Scholar]
- 24.Gygi S. P., Rist, B., Gerber, S. A., Turecek, F., Gelb, M. H. & Aebersold, R. (1999) Nat. Biotechnol. 17, 994-999. [DOI] [PubMed] [Google Scholar]
- 25.Oda Y., Huang, K., Cross, F. R., Cowburn, D. & Chait, B. T. (1999) Proc. Natl. Acad. Sci. USA 96, 6591-6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao X., Freas, A., Ramirez, J., Demirev, P. A. & Fenselau, C. (2001) Anal. Chem. 73, 2836-2842. [DOI] [PubMed] [Google Scholar]
- 27.Marshall A. G., Hendrickson, C. L. & Jackson, G. S. (1998) Mass Spectrom. Rev. 17, 1-35. [DOI] [PubMed] [Google Scholar]
- 28.Sassetti C. M., Boyd, D. H. & Rubin, E. J. (2001) Proc. Natl. Acad. Sci. USA 98, 12712-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu-Hsieh B. A. & Howard, D. H. (1992) Infect. Immun. 60, 698-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donadio S., Shafiee, A. & Hutchinson, C. R. (1990) J. Bacteriol. 172, 350-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delic-Attree I., Toussaint, B., Garin, J. & Vignais, P. M. (1997) Mol. Microbiol. 24, 1275-1284. [DOI] [PubMed] [Google Scholar]
- 32.Newton G. L., Unson, M. D., Anderberg, S. J., Aguilera, J. A., Oh, N. N., delCardayre, S. B., Av-Gay, Y. & Fahey, R. C. (1999) Biochem. Biophys. Res. Commun. 255, 239-244. [DOI] [PubMed] [Google Scholar]
- 33.Spies H. S. & Steenkamp, D. J. (1994) Eur. J. Biochem. 224, 203-213. [DOI] [PubMed] [Google Scholar]
- 34.Fahey R. C. (2001) Annu. Rev. Microbiol. 55, 333-356. [DOI] [PubMed] [Google Scholar]
- 35.Goren M. B. (1990) Handbook Lip. Res. 6, 363-461. [Google Scholar]