Abstract
The diagnosis of disseminated toxoplasmosis in a 14-year-old allogeneic bone marrow recipient with graft-versus-host disease was determined by the detection of Toxoplasma gondii tachyzoites in sputum smears. Sputum analysis is a valuable alternative in the clinical assessment of pulmonary toxoplasmosis, especially when conventional invasive techniques are not practicable.
CASE REPORT
A 14-year-old girl had been transplanted with human lymphocyte antigen-identical allogeneic hematopoietic stem cells from an unrelated donor because of a T-cell lymphoma. Her pretransplant serological tests were positive for Toxoplasma gondii (immunoglobulin G [IgG] = 60 IU/ml, IgM negative). The donor's Toxoplasma serology was negative. The recipient received polyclonal immunoglobulin for passive immunoprophylaxis and cyclosporine and methylprednisolone for graft-versus-host disease (GvHD) prophylaxis. She developed acute GvHD, and mycophenolate mofetil was added to her previous therapy on day 97 posttransplantation. On day 113, she presented with fever, cough, dyspnea, pancytopenia, and elevated levels of C-reactive protein. There were interstitial infiltrates in both lungs seen in chest computed tomography images (CT). All diagnostic tests for cytomegalovirus, adenovirus, and Pneumocystis, Aspergillus, and Legionella spp. were negative. The patient's condition worsened despite probabilistic anti-infective treatment with ceftriaxone, teicoplanin, and caspofungin. A central tricytopenia prompted repeated transfusions. On day 123, she developed headache and vomiting. The fundus examination was normal. The brain CT showed a hypodense area on the right side of the thalamus. Her respiratory status deteriorated with severe hypoxemia requiring oxygen therapy.
Diff-Quick-stained cytospin-prepared sputum smears (Microscopy Hemacolor MERCK catalog no. 65044-93) showed tachyzoites suggestive of Toxoplasma gondii (Fig. 1). T. gondii was detected with specific direct immunofluorescence tests on both bone marrow and sputum specimens. Real-time PCR targeting a repetitive 529-bp DNA fragment of T. gondii (GenBank accession no. AF487550) was positive in both blood and sputum samples.
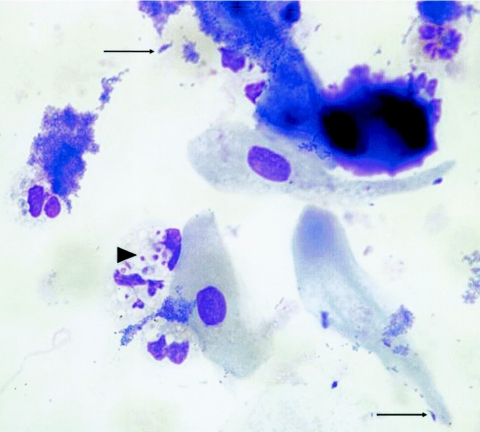
FIG. 1.
Diff-Quick staining of a sputum specimen showing abundant intracellular (arrowhead) and some typically crescent-shaped extracellular (arrows) T. gondii tachyzoites, approximately 10 μm in length. Magnification, ×400 (original magnification, ×1,000).
A treatment combining pyrimethamine (50 mg daily after a 100-mg loading dose) and sulfadiazine (1,250 mg four times daily) was initiated, and the patient steadily improved. Blood and sputum specimen collected after 14 days of treatment were negative for T. gondii PCR. There was progressive hematological reconstruction (as evidenced by blood numeration) and a reduction in size of the brain lesion on the CT. The girl was discharged from hospital after 20 days of pyrimethamine-sulfadiazine treatment.
Toxoplasma gondii is a protozoan parasite of cats and other Felidae. Humans and other homeotherm animals serve as intermediate hosts (15). In immunocompetent hosts, the acute phase is usually asymptomatic. In any case, bradyzoites remain trapped within tissues inside cysts, which can persist in the brain, heart, liver, kidney, and muscles. The immune system controls cyst disruption. As cyst disruption occurs, the released bradyzoites rapidly convert to tachyzoites. Tachyzoites are seen in primary or reactivated infection; their detection is the hallmark of active infection (4).
Disseminated toxoplasmosis is a rare but often fatal opportunistic infection of immunodeficient patients, albeit it has infrequently been reported in immunocompetent patients (2). It can occur in patients with AIDS or in recipients of solid organ or bone marrow transplants (BMT). It usually develops as a primo-infection in seronegative solid organ transplant recipients when the donor was seropositive; i.e., the infection results from the reactivation of Toxoplasma cysts enclosed within a contaminated transplanted organ (10). On the other hand, toxoplasmosis after BMT usually occurs in seropositive recipients and is more common when the donor is seronegative, the donor's naive immune system apparently contributing to the reactivation of toxoplasmosis in BMT recipients. However, a few cases in BMT have been reported in patients with negative pretransplant serology for Toxoplasma, suggesting a transmission of infection via donor marrow and blood products (3). In addition to positive toxoplasmosis serology before BMT, other risk factors of disseminated toxoplasmosis are allogeneic transplantations and severe GvHD. The prognosis of toxoplasmosis in BMT recipients is poor, with a case fatality rate that can reach 92% (14) (timely diagnosis and appropriate treatment can reduce this to 40%) (9).
The prevalence of toxoplasmosis in BMT recipients varies greatly (0.3 to 5%) (9). The main reason lies in the within-countries disparity in population seroprevalence for T. gondii; this ranges for instance from about 3 to 68% (11) in the United States and from 60 to 70% in France (7). The prevalence of toxoplasmosis is probably underestimated since symptoms are nonspecific and diagnosis in BMT recipients is difficult; about one-half of the cases have been diagnosed postmortem (9). Although serology is of little use for diagnosis after BMT, PCR, which can be performed in blood, cerebrospinal fluid, and bronchoalveolar lavage fluid (BAL) samples, is more useful (1). PCR is also useful to monitor treatment (9). BAL is usually the specimen of choice for the diagnosis of pulmonary toxoplasmosis. Sputum analysis lacks sensitivity in comparison with BAL examination for the diagnosis of pulmonary toxoplasmosis (4), although the diagnosis in this case was based on a sputum specimen. The major limitation of BAL is that it must be obtained during a bronchoscopy, which is not always feasible in patients with respiratory distress. Sputum is often more easily available, even from severely ill patients. Direct visualization of tachyzoites in smears is faster than PCR, and detection of tachyzoites in the sputum can lead to a rapid and accurate diagnosis. Misidentification of T. gondii with other pathogens of similar morphology by untrained microscopists and/or when the parasite density is low should be controlled using concomitantly performed specific techniques such as direct immunofluorescence and PCR. PCR has proven its value in the surveillance of BMT patients (6); it may detect a smaller quantity of parasite in the sample. Nevertheless, one drawback of this high sensitivity is that the clinical interpretation of a positive PCR is not always straightforward, since it might be due to a controlled toxoplasmosis reactivation in asymptomatic patients (1). Quantitative real-time PCR might be useful in differentiating between infection and disease. The data from Martino et al. suggest that preemptive therapy based on the results of quantitative real-time PCR might be effective in preventing death associated to T. gondii infections in BMT patients (6).
To our knowledge, this is the first report where the diagnosis of disseminated toxoplasmosis was based on the visualization of T. gondii tachyzoites in sputum smears. This diagnosis led to the prompt initiation of adequate treatment, with complete recovery of the patient. Pretransplant Toxoplasma serology should be tested in both recipient and donor to identify patients at risk of infection. Chemoprophylaxis should be considered in patients with risk factors of disseminated toxoplasmosis reactivation such as positive pretransplant serology, allogeneic transplant, and GvHD and its treatment. Prophylaxis with trimethoprim-sulfamethoxazole (TMP-SMZ) in AIDS patients has proven effective against T. gondii reactivation (12) and can be used after allo-BMT. A high dosage of TMP-SMZ appears to be more effective for the prevention of toxoplasmosis than a low one (13). In part because TMP-SMZ might reduce hematopoiesis, prophylactic treatment against Toxoplasma in BMT recipients is rarely used nowadays even in high-risk patients, and no evidence-based data support this practice (5, 13, 16). Nevertheless, the empirical observation that patients who do not receive chemoprophylaxis developed disseminated toxoplasmosis is in keeping with the AIDS data (6). Thus, systematic chemoprophylaxis targeted at high-risk patients would effectively prevent reactivation of toxoplasmosis in BMT recipients (8). The use of sputum examination should be advocated as an additional diagnosis technique of pulmonary toxoplasmosis since the occurrence of T. gondii tachyzoites in sputum allows early diagnosis and instigation of therapy.
Acknowledgments
We thank Michel Thuriaux for helpful comments and for checking the English of the manuscript.
REFERENCES
- 1.Bretagne, S., J. M. Costa, F. Foulet, L. Jabot-Lestang, F. Baud-Camus, and C. Cordonnier. 2000. Prospective study of toxoplasma reactivation by polymerase chain reaction in allogeneic stem-cell transplant recipients. Transpl. Infect. Dis. 2:127-132. [DOI] [PubMed] [Google Scholar]
- 2.Carme, B., F. Bissuel, D. Ajzenberg, R. Bouyne, C. Aznar, M. Demar, S. Bichat, D. Louvel, A. M. Bourbigot, C. Peneau, P. Neron, and M. L. Darde. 2002. Severe acquired toxoplasmosis in immunocompetent adult patients in French Guiana. J. Clin. Microbiol. 40:4037-4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandrasekar, P. H., F. Momin, et al. 1997. Disseminated toxoplasmosis in marrow recipients: a report of three cases and a review of the literature. Bone Marrow Transplant 19:685-689. [DOI] [PubMed] [Google Scholar]
- 4.Dawis, M. A., E. J. Bottone, A. Vlachos, and M. H. Burroughs. 2002. Unsuspected Toxoplasma gondii empyema in a bone marrow transplant recipient. Clin. Infect. Dis. 34:e37-e39. [DOI] [PubMed] [Google Scholar]
- 5.de Medeiros, B. C., C. R. de Medeiros, B. Werner, G. Loddo, R. Pasquini, and L. F. Bleggi-Torres. 2001. Disseminated toxoplasmosis after bone marrow transplantation: report of 9 cases. Transpl. Infect. Dis. 3:24-28. [DOI] [PubMed] [Google Scholar]
- 6.Martino, R., S. Bretagne, H. Einsele, J. Maertens, A. J. Ullmann, R. Parody, U. Schumacher, C. Pautas, K. Theunissen, C. Schindel, C. Munoz, N. Margall, and C. Cordonnier. 2005. Early detection of Toxoplasma infection by molecular monitoring of Toxoplasma gondii in peripheral blood samples after allogeneic stem cell transplantation. Clin. Infect. Dis. 40:67-78. [DOI] [PubMed] [Google Scholar]
- 7.Martino, R., S. Bretagne, M. Rovira, A. J. Ullmann, J. Maertens, T. Held, E. Deconinck, and C. Cordonnier. 2000. Toxoplasmosis after hematopoietic stem transplantation: report of a 5-year survey from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 25:1111-1114. [DOI] [PubMed] [Google Scholar]
- 8.Martino, R., and C. Cordonnier. 2003. Toxoplasmosis following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 31:617-618. [DOI] [PubMed] [Google Scholar]
- 9.Mele, A., P. J. Paterson, H. G. Prentice, P. Leoni, and C. C. Kibbler. 2002. Toxoplasmosis in bone marrow transplantation: a report of two cases and systematic review of the literature. Bone Marrow Transplant. 29:691-698. [DOI] [PubMed] [Google Scholar]
- 10.Ortonne, N., P. Ribaud, V. Meignin, C. Sarfati, H. Esperou, A. Devergie, E. Gluckman, G. Socie, and A. Janin. 2001. Toxoplasmic pneumonitis leading to fatal acute respiratory distress syndrome after engraftment in three bone marrow transplant recipients. Transplantation 72:1838-1840. [DOI] [PubMed] [Google Scholar]
- 11.Remington, J. S., and J. O. Klein (ed.). 2001. Toxoplasmosis, p. 89-195. In Infectious diseases of the fetus and newborn infant. The W. B. Saunders Co., Philadelphia, Pa.
- 12.Ribera, E., A. Fernandez-Sola, C. Juste, A. Rovira, F. J. Romero, L. Armadans-Gil, I. Ruiz, I. Ocana, and A. Pahissa. 1999. Comparison of high and low doses of trimethoprim-sulfamethoxazole for primary prevention of toxoplasmic encephalitis in human immunodeficiency virus-infected patients. Clin. Infect. Dis. 29:1461-1466. [DOI] [PubMed] [Google Scholar]
- 13.Roemer, E., I. W. Blau, N. Basara, M. G. Kiehl, M. Bischoff, S. Gunzelmann, D. Kirsten, H. Sanchez, E. L. Wocker, and A. A. Fauser. 2001. Toxoplasmosis, a severe complication in allogeneic hematopoietic stem cell transplantation: successful treatment strategies during a 5-year single-center experience. Clin. Infect. Dis. 32:E1-E8. [DOI] [PubMed] [Google Scholar]
- 14.Sing, A., L. Leitritz, A. Roggenkamp, H. J. Kolb, A. Szabados, V. Fingerle, I. B. Autenrieth, and J. Heesemann. 1999. Pulmonary toxoplasmosis in bone marrow transplant recipients: report of two cases and review. Clin. Infect. Dis. 29:429-433. [DOI] [PubMed] [Google Scholar]
- 15.Weinrach, D. M., and A. Oviedo. 2001. Toxoplasmosis in a bone marrow transplant patient. Arch. Pathol. Lab. Med. 125:707. [DOI] [PubMed] [Google Scholar]
- 16.Zver, S., P. Cernelc, U. Mlakar, and J. Pretnar. 1999. Cerebral toxoplasmosis: a late complication of allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 24:1363-1365. [DOI] [PubMed] [Google Scholar]