Abstract
Restriction fragment length polymorphism (RFLP) analysis of the PCR-amplified proximal region of the gene encoding cytadherence accessory protein P110 (MG192) revealed DNA sequence divergences among 54 Mycoplasma genitalium clinical strains isolated from the genitourinary tracts of women attending a sexually transmitted disease-related health clinic, plus one from the respiratory tract and one from synovial fluid. Seven of 56 (12.5%) strains exhibited RFLPs following digestion of the proximal region with restriction endonuclease MboI or RsaI, or both. No sequence variability was detected in the distal portion of the gene.
Mycoplasma genitalium is an important, emerging sexually transmitted bacterial pathogen capable of eliciting a wide range of symptomatologies (18, 19, 33). In men M. genitalium has been identified as a causative agent of nongonococcal, chlamydia-negative urethritis (1, 11, 35, 38). In women with genitourinary symptoms, M. genitalium was detected by PCR in the cervix and vagina (4, 26). Recently, M. genitalium has been strongly associated with cervicitis, endometritis, salpingitis, pelvic inflammatory disease, and tubal factor infertility by the use of PCR or serological criteria (6, 7, 23, 26). Furthermore, M. genitalium was detected in rectal (34), respiratory tract (3), and synovial fluid (36) specimens and has been linked to a range of pathologies, such as arthritis, pneumonia, AIDS progression, chronic fatigue, autoimmune disorders, and encephalitis (33, 37, 39).
M. genitalium was first isolated in 1981 from two urethral swab specimens of male patients with urethritis (38), and similarly, four additional M. genitalium strains were obtained in 1996 (20). Additionally, single colonies of M. genitalium were cloned from two extragenital sites (3, 36). No other successful cultivations have been reported, nor have any strains been derived from women, until our recent isolations from vaginal and cervical specimens (2). In the past the absence of sufficient numbers of M. genitalium clinical strains has been a direct result of the highly fastidious nature of this mycoplasma, which possesses the smallest genome of any known self-replicating cell. Thus, meaningful molecular typing of representative clinical isolates has been prevented; but molecular typing would contribute important epidemiological and virulence-related information, like that reported for Mycoplasma pneumoniae (10, 17, 31). Molecular typing of M. pneumoniae is based upon sequence divergences in the gene encoding cytadhesin P1 (gene p1; locus MPN141 in The Institute for Genomic Research [TIGR] database). We showed that sequence-related regions of the M. pneumoniae p1 gene are located in multiple copies throughout the streamlined genome (10, 32). The majority of repetitive elements related to p1 appear to lack promoter regions and may serve as a pool for recombination, which could lead to in vivo antigenic variations and/or alterations in tissue tropism (27, 29, 32). Currently, typing of M. pneumoniae clinical strains is performed by restriction analysis of PCR-amplified regions of the p1 gene (8, 14, 25, 30).
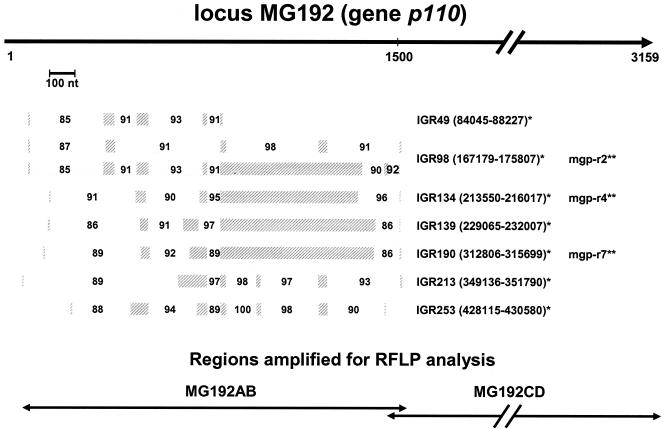
Immunological criteria have also been useful for the diagnosis of M. genitalium infections and associated pathologies (2). We observed in infected individuals an immunodominant response against two major antigens: the 140-kDa cytadhesin (P140; also reported as MgPa) and the 110-kDa adherence-accessory protein (P110) (2, 3, 36). Both proteins are encoded by adjacent genes (15, 16) that are organized in a single operon with two promoters, which further underlines their structural and functional linkages (24). Recently, genes encoding P140 and P110 have been reannotated as loci MG191 and MG192, respectively, with the gene designations mgpA and p110, respectively, in the database of TIGR. Although the precise function of the P110 protein has not yet been determined, its presence is required for maintenance of M. genitalium cytadherence capabilities (12, 13). As described earlier by us (9) and others (15, 27, 28), multiple incomplete copies of locus MG192/p110 are dispersed throughout the M. genitalium chromosome. We identified all homologous regions by downloading MG192/p110 sequence information from the database of TIGR (http://www.tigr.org) and performing a BLAST search (http://www.ncbi.nlm.nih.gov/BLAST/). The actual positions of the repetitive elements within the p110 reading frame as well as their positions in other locations in the genome are presented in Fig. 1. Noticeable from the BLAST search are the following: (i) only portions of the 5′-end (proximal) half of p110 occur as multiple copies, and (ii) while all p110 sequence-related repetitive elements are randomly located throughout the chromosome, they are always positioned at intergenic regions. This information identifies the proximal region of p110, in contrast to the distal region, as a suitable target for homologous recombination and establishment of strain molecular typing criteria. Therefore, we initially compared p110 in M. genitalium reference strain G37, isolated from urethral specimens of men, with that in strain TW10-5, which we isolated from respiratory tract specimens (Table 1).
FIG. 1.
Positions of multiple p110-related repetitive elements within the M. genitalium genome. Shaded bars represent positions within the proximal region of the p110 gene of p110-related multicopy elements, which are dispersed throughout the chromosome (BLAST analysis). The length of the highly homologous sequences (solid line, top) and the percentages of homology (values within open boxes) are also indicated, as are the intergenic regions (IGRs) and their locations in the chromosome (*, http://www.stdgen.lanl.gov). The proximal (MG192AB) and distal (MG192CD) regions amplified for RFLP analyses are presented at the bottom (see Table 2 for the primer sequences). **, actual positions of repetitive sequence elements mgp-r2, mgp-r4, and mgp-r7 (28) on the chromosome. nt, nucleotides.
TABLE 1.
Mycoplasma genitalium strains used for PCR-RFLP analysis of locus MG192/p110 gene
| Author (reference) | Strain designation | Source or description |
|---|---|---|
| Tully et al. (38) | G37 | Reference strain (ATCCa) |
| Baseman et al. (3) | TW10-5 | Isolated from respiratory tract |
| Baseman et al. (2) | 54 strains cultured from female genital tract specimens | |
| 750V | Vaginal specimen | |
| 762V | Vaginal specimen | |
| 769C | Cervical specimen | |
| 811C | Cervical specimen | |
| 1016C | Cervical specimen | |
| 1019V | Vaginal specimen | |
| Tully et al. (36) | UTMB-10G | Isolated from synovial fluid |
ATCC, American Type Culture Collection.
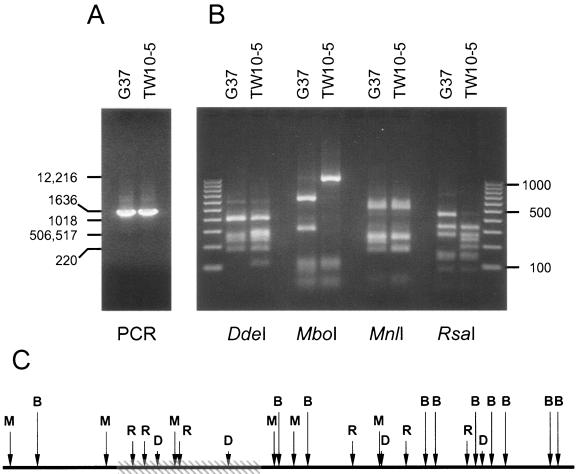
Mycoplasma cultures were grown to mid-log phase in SP-4 medium, and cells were harvested and washed with phosphate-buffered saline prior to isolation of genomic DNA by use of an Easy-DNA kit (Invitrogen, Carlsbad, Calif.). Chromosomal DNAs were quantified by determination of the optical density at 260 nm, and 100 ng was used in the amplification reactions. For analysis of the MG192/p110 proximal region, we designed primers MG192A (5′-CACTAGCCAATACCTTCCTTGTCAAAGAGG-3′) and MG192B (5′-CCATAACTATTCAAGGGCGTAGCG-3′) (Table 2), based upon the published genome sequence of reference strain G37 (http://www.tigr.org). The amplified product (MG192AB) contained nucleotides 68 to 1532 of the coding region (Table 2; Fig. 1). Amplification was performed with a Platinum Taq DNA polymerase high-fidelity system (Invitrogen), as follows: 94°C for 3 min and 35 cycles of 94°C for 30 s, 58°C for 30 s, and 68°C for 90 s. The PCR products generated were electrophoresed in 1% agarose to ensure that only a single product of the expected size was amplified from both templates (Fig. 2A). The amplified regions were subjected to RFLP analysis, as follows: a set of restriction endonucleases (AluI, DdeI, HaeIII, HpaII, HphI, MboI, MnlI, and RsaI) was selected based upon sequence analysis of the targeted region of the p110 gene by using Molecular Toolkit (http://arbl.cvmbs.colostate.edu/molkit/index.html). All restriction reactions were performed under the conditions suggested by the manufacturer (New England Biolabs, Inc.), and the restriction patterns of strains G37 and TW10-5 were compared in 2% agarose (GenePure 3:1 agarose; ISC BioExpress, Kaysville, Utah) alongside a 100-bp DNA ladder (Bio-Rad, Richmond, Calif.). When restriction endonucleases DdeI, MboI, MnlI, and RsaI were used, RFLPs were observed (Fig. 2B). Based upon the published sequence of the G37 genome, we were able to predict the positions of divergent regions within the portion of p110 analyzed (Fig. 2C). Both amplified products were cloned with TOPO (Invitrogen) and sequenced, which confirmed all restriction data and sequence differences detected between strain G37- and TW10-5-derived MG192AB regions (Fig. 2C).
TABLE 2.
Primers used for amplification of p110 (MG192) proximal (MG192AB) and distal (MG192CD) regions
| Locus or primer | Position in genome | Description or primer sequence | PCR product size (bp) |
|---|---|---|---|
| MG192 locus | 225907-229065 | Cytadherence accessory protein P110 | |
| Primers | |||
| MG192A | 225974-226003 | CACTAGCCAATACCTTCCTTGTCAAAGAGG | 1,465 |
| MG192B | 227438-227415 | CCATAACTATTCAAGGGCGTAGCG | |
| MG192C | 227415-227438 | CGCTACGCCCTTGAATAGTTATGG | 1,834 |
| MG192D | 229248-229221 | TCACTGATCTCTTCACTCAGTAAGTGCC |
FIG. 2.
PCR-RFLP analyses of p110 proximal regions in M. genitalium strains G37 and TW10-5. (A) The specific product, MG192AB (Fig. 1, bottom), was amplified from both strains and electrophoresed in 1% agarose (a 1-kb DNA ladder is indicated at the far left, with the numbers on both the left and the right being in base pairs). (B) The restriction patterns of amplified MG192AB regions obtained with DdeI, MboI, MnlI, and RsaI were separated and compared in 2% agarose alongside a 100-bp DNA ladder. (C) Predicted region of divergent sequence (cross-hatched box) within the MG192AB proximal region of p110 is indicated, along with the positions of the DdeI (D), MboI (B), MnlI (M), and RsaI (R) restriction sites, in the sequence generated from strain G37.
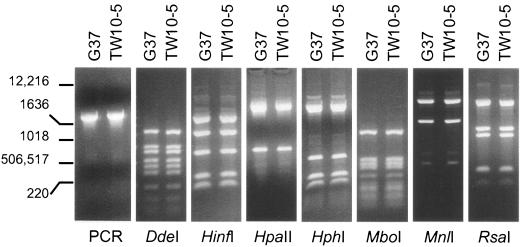
For amplification of the p110 distal region (MG192CD; Fig. 1, bottom), we designed primers MG192C (5′-CGCTACGCCCTTGAATAGTTATGG-3′) and MG192D (5′-TCACTGATCTCTTCACTCAGTAAGTGCC-3′) (Table 2), and the amplified product consisted of 1,651 bp of the p110-coding region and 184 bp of the adjacent downstream region. Amplification was performed under the same cycling conditions described earlier, and the amplified regions were subjected to restriction enzyme digestion (Fig. 3). No RFLPs were observed between the two strains (Fig. 3), indicating a high degree of conservation of the p110 distal region.
FIG. 3.
PCR-RFLP analysis of p110 distal regions in M. genitalium strains G37 and TW10-5. The specific product, MG192CD (Fig. 1, bottom), was amplified from both strains. The restriction patterns obtained by using DdeI, HinfI, HpaII, HphI, MboI, MnlI, and RsaI were analyzed as described in the Fig. 2 legend. The numbers on the left are in base pairs.
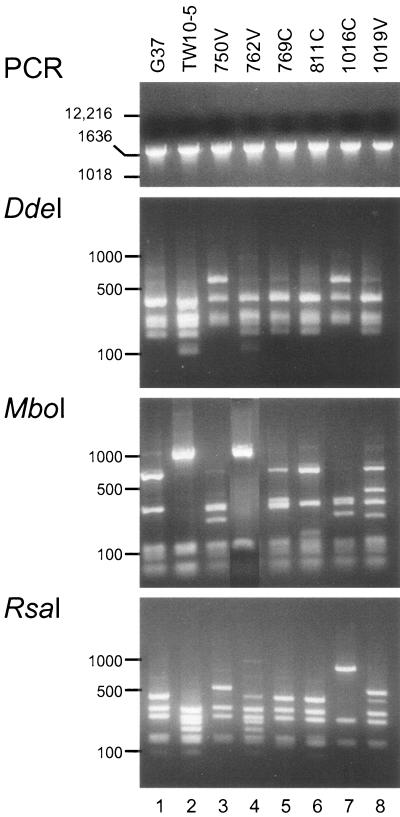
Next, we evaluated a unique set of 54 early-passage M. genitalium clinical strains derived from genital tract specimens from women undergoing physical examination as part of an NIAID-sponsored, institutional review board-approved study of the San Antonio Sexually Transmitted Diseases Cooperative Research Center (2, 5), along with strain UTMB-10B, which was isolated from synovial fluid (Table 1). Genomic DNAs were prepared and amplifications were performed as described above. The expected specific MG192AB products were amplified from all strains (Fig. 4, PCR panel) and subjected to restriction enzyme digestion. Within the set of strains originating from the female genital tract, we observed six distinct restriction pattern profiles (Fig. 4, panels DdeI, MboI, and RsaI). A list of all divergent strains and a summary of the restriction profiles appear in Table 3. Although similar restriction patterns were observed (Fig. 4; for example, the DdeI patterns of strains 750V and 1016C and the MboI patterns of strains TW10-5 and 762V), many restriction patterns were distinct (for example, the RsaI patterns of strains 750V and 1016C and the MboI patterns of strains G37, TW10-5, 750V, 769C, 811C, 1016C, and 1019V), suggesting considerable heterogeneity in the locus MG192/p110 sequence. It is likely that additional sequence divergences will be detected with other restriction enzymes and newly derived clinical strains. On the other hand, the M. genitalium strain designated UTMB-10G, which we previously isolated from synovial fluid (Table 1), exhibited no polymorphisms compared to the sequence of G37 (data not shown). Also, as suggested by our earlier analysis of the distal region of p110, restriction of amplified MG192CD regions for all strains (Table 2) revealed no RFLPs.
FIG. 4.
PCR-RFLPs detected in p110 proximal regions of seven M. genitalium clinical strains. PCR products of the expected size (panel PCR) were generated for all strains by using primers MG192A and MG192B (Table 2). The remaining panels present the restriction patterns of the designated strains (Table 1) by using the enzymes DdeI (panel DdeI), MboI (panel MboI), and RsaI (panel RsaI). The products were analyzed as described in the Fig. 2 legend. The numbers on the left are in base pairs.
TABLE 3.
Clinical strains and endonucleases that generate RFLPs compared to reference strain G37 restriction patterns
| Restriction endonuclease | RFLP for the following strainsa:
|
||||||
|---|---|---|---|---|---|---|---|
| TW10-5 | 750V | 762V | 769C | 811C | 1016C | 1019V | |
| AluI | + | + | |||||
| DdeI | + | + | + | + | |||
| HaeIII | + | + | |||||
| HinfI | + | ||||||
| HpaII | + | ||||||
| HphI | + | + | |||||
| MboI | + | + | + | + | + | ||
| MnlI | + | + | + | + | |||
| RsaI | + | + | + | + | + | + | |
The restriction patterns generated for all other strains tested showed no p110-related polymorphism compared to the restriction patterns generated for G37.
Currently, no method for the molecular typing of M. genitalium strains targets the p110 gene (19, 21, 22). The available information suggests high degrees of phenotypic and genotypic heterogeneity among a limited number of clinical M. genitalium isolates (2, 19, 21, 22). For example, partial sequences of the gene encoding the 140-kDa cytadhesin MgPa (locus MG191), which is characterized by sequence-related, incomplete repetitive elements located throughout the chromosome (9, 15, 27), revealed sequence heterogeneity in clinical strains (19, 20). In our search for a target for molecular typing of clinical M. genitalium strains, we selected the gene encoding cytadherence accessory protein P110 for several reasons. First, this protein is necessary for maintenance of tip-mediated cytadherence capabilities (12) and is one of the major immunodominant antigens detected in the sera of infected individuals (2, 39). Second, sequence analysis revealed that incomplete repetitive elements of p110 regions were distributed throughout the M. genitalium genome (9, 15, 28). Interestingly, only the 5′ half (proximal region) of the gene shared homologies with these repetitive sequences. Homologous recombination of these regions that results in protein P110 sequence and antigenic variations could provide M. genitalium with survival advantages through immune evasion of the host and sequence-driven modifications of biological function. In contrast, the distal half of p110 exhibited no meaningful sequence homologies elsewhere in the chromosome.
Using specific primers and experimental conditions that yielded PCR products of the expected size for all M. genitalium strains tested, we confirmed our hypothesis that sequence divergences would be detected in the proximal region of p110. In other words, 7 of 56 (12.5%) clinical strains tested exhibited RFLPs when their sequences were compared with that of the reference strain. We believe that these strain sequence variations are conserved, as suggested by the sequence stability of p110 over many passages in strains G37 and TW10-5 (Table 1; this is the focus of a separate study). It is possible that additional sequence divergences in both the proximal and the distal regions may exist, but they were not observed by use of our assay design. These data, combined with reported sequence variations of the cytadhesin P140-encoding gene (19, 20), reinforce the hypothesis that sequence variations in M. genitalium are a result of homologous recombination. For our set of M. genitalium strains, one generalization is apparent: all demonstrated RFLPs occur in the proximal region when the sequence is cut with MboI and/or RsaI. This provides the basis for the analysis of additional clinical strains from other geographical areas. In this regard, it should be feasible to amplify the MG192/p110 proximal region by the use of patient samples without culture of M. genitalium, which remains an extremely rare event.
Furthermore, by combining RFLP analyses of p110 and p140 sequence divergences, it should be possible to improve strain classification and relate that information to M. genitalium epidemiological typing, pathogenic potential, and clinical manifestations. Also, such information should be useful in providing an understanding of bacterial population dynamics in vivo and enhancing patient treatment.
Nucleotide sequence accession number.
The sequence obtained for strain TW10-5 was submitted to GenBank and can be found under accession number AY679761.
Acknowledgments
This work was supported by NIAID-NIH grants AI45429-06 and AI041010-06.
We acknowledge Marianna Cagle for isolating and subcloning single cells of M. genitalium clinical strains.
No author had any conflict of interest, either financial or personal, that may have biased his or her actions.
REFERENCES
- 1.Barbeyrac, B., C. Bernet-Poggi, F. Febrer, H. Renaudin, M. Dupon, and C. Bebear. 1993. Detection of Mycoplasma pneumoniae and Mycoplasma genitalium in clinical samples by polymerase chain reaction. Clin. Infect. Dis. 17:S83-S89. [DOI] [PubMed] [Google Scholar]
- 2.Baseman, J. B., M. Cagle, J. E. Korte, C. Herrera, W. G. Rasmussen, J. G. Baseman, R. Shain, and J. M. Piper. 2004. Diagnostic assessment of Mycoplasma genitalium in culture-positive women. J. Clin. Microbiol. 42:203-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baseman, J. B., S. F. Dallo, J. G. Tully, and D. L. Rose. 1988. Isolation and characterization of Mycoplasma genitalium strains from the human respiratory tract. J. Clin. Microbiol. 26:2266-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanchard, A., W. Hamrick, L. Duffy, K. Baldus, and G. H. Cassell. 1993. Use of the polymerase chain reaction for detection of Mycoplasma fermentans and Mycoplasma genitalium in the urogenital tract and amniotic fluid. Clin. Infect. Dis. 17(Suppl. 1):S272-S279. [DOI] [PubMed] [Google Scholar]
- 5.Blaylock, M. W., O. Musatovova, J. G. Baseman, and J. B. Baseman. 2004. Determination of infectious load of Mycoplasma genitalium in clinical samples of human vaginal cells. J. Clin. Microbiol. 42:746-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clausen, H. F., J. Fedder, M. Drasbek, P. K. Nielsen, B. Toft, H. J. Ingerslev, S. Birkelund, and G. Christiansen. 2001. Serological investigation of Mycoplasma genitalium in infertile women. Hum. Reprod. 16:1866-1874. [DOI] [PubMed] [Google Scholar]
- 7.Cohen, C. R., L. E. Manhart, E. A. Bukusi, S. Astete, R. C. Brunham, K. K. Holmes, S. K. Sinei, J. J. Bwayo, and P. A. Totten. 2002. Association between Mycoplasma genitalium and acute endometritis. Lancet 359:765-766. [DOI] [PubMed] [Google Scholar]
- 8.Cousin-Allery, A., A. Charron, B. de Barbeyrac, G. Fremy, J. Skov Jensen, H. Renaudin, and C. Bebear. 2000. Molecular typing of Mycoplasma pneumoniae strains by PCR-based methods and pulsed-field gel electrophoresis. Application to French and Danish isolates. Epidemiol. Infect. 124:103-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dallo, S. F., and J. B. Baseman. 1991. Adhesin gene of Mycoplasma genitalium exists as multiple copies. Microb. Pathog. 10:475-480. [DOI] [PubMed] [Google Scholar]
- 10.Dallo, S. F., J. R. Horton, C. J. Su, and J. B. Baseman. 1990. Restriction fragment length polymorphism in the cytadhesin P1 gene of human clinical isolates of Mycoplasma pneumoniae. Infect. Immun. 58:2017-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deguchi, T., and S. Maeda. 2002. Mycoplasma genitalium: another important pathogen of nongonococcal urethritis. J. Urol. 167:1210-1217. [DOI] [PubMed] [Google Scholar]
- 12.Dhandayuthapani, S., W. G. Rasmussen, and J. B. Baseman. 1999. Disruption of gene mg218 of Mycoplasma genitalium through homologous recombination leads to an adherence-deficient phenotype. Proc. Natl. Acad. Sci. USA 96:5227-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhandayuthapani, S., W. G. Rasmussen, and J. B. Baseman. 2002. Stability of cytadherence-related proteins P140/P110 in Mycoplasma genitalium requires MG218 and unidentified factors. Arch. Med. Res. 33:1-5. [DOI] [PubMed] [Google Scholar]
- 14.Dorigo-Zetsma, J. W., J. Dankert, and S. A. Zaat. 2000. Genotyping of Mycoplasma pneumoniae clinical isolates reveals eight P1 subtypes within two genomic groups. J. Clin. Microbiol. 38:965-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraser, C. M., J. D. Gocayne, O. White, M. D. Adams, R. A. Clayton, R. D. Fleischmann, C. J. Bult, A. R. Kerlavage, G. Sutton, J. M. Kelley, J. L. Fritchman, J. F. Weidman, K. V. Small, M. Sandusky, J. Fuhrmann, D. Nguyen, T. R. Utterback, D. M. Saudek, C. A. Phillips, J. M. Merrick, J.-F. Tomb, B. A. Dougherty, K. F. Bott, P.-C. Hu, T. S. Lucier, S. N. Peterson, H. O. Smith, C. A. Hutchison III, and J. C. Venter. 1995. The minimal gene complement of Mycoplasma genitalium. Science 270:397-403. [DOI] [PubMed] [Google Scholar]
- 16.Inamine, J. M., S. Loechel, A. M. Collier, M. F. Barile, and P. C. Hu. 1989. Nucleotide sequence of the MgPa (mgp) operon of Mycoplasma genitalium and comparison to the P1 (mpp) operon of Mycoplasma pneumoniae. Gene 82:259-267. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs, E., M. Vonski, K. Oberle, O. Opitz, and K. Pietsch. 1996. Are outbreaks and sporadic respiratory infections by Mycoplasma pneumoniae due to two distinct subtypes? Eur. J. Clin. Microbiol. Infect. Dis. 15:38-44. [DOI] [PubMed] [Google Scholar]
- 18.Jensen, J. S. 2004. Mycoplasma genitalium: the aetiological agent of urethritis and other sexually transmitted diseases. J. Eur. Acad. Dermatol. Venereol. 18:1-11. [DOI] [PubMed] [Google Scholar]
- 19.Jensen, J. S., E. Bjornelius, B. Dohn, and P. Lidbrink. 2004. Use of TaqMan 5′ nuclease real-time PCR for quantitative detection of Mycoplasma genitalium DNA in males with and without urethritis who were attendees at a sexually transmitted disease clinic. J. Clin. Microbiol. 42:683-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen, J. S., H. T. Hansen, and K. Lind. 1996. Isolation of Mycoplasma genitalium strains from the male urethra. J. Clin. Microbiol. 34:286-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kokotovic, B., N. F. Friis, J. S. Jensen, and P. Ahrens. 1999. Amplified-fragment length polymorphism fingerprinting of Mycoplasma species. J. Clin. Microbiol. 37:3300-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma, L., and D. H. Martin. 2004. Single-nucleotide polymorphisms in the rRNA operon and variable numbers of tandem repeats in the lipoprotein gene among Mycoplasma genitalium strains from clinical specimens. J. Clin. Microbiol. 42:4876-4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manhart, L. E., C. W. Critchlow, K. K. Holmes, S. M. Dutro, D. A. Eschenbach, C. E. Stevens, and P. A. Totten. 2003. Mucopurulent cervicitis and Mycoplasma genitalium. J. Infect. Dis. 187:650-657. [DOI] [PubMed] [Google Scholar]
- 24.Musatovova, O., S. Dhandayuthapani, and J. B. Baseman. 2003. Transcriptional starts for cytadherence-related operons of Mycoplasma genitalium. FEMS Microbiol. Lett. 229:73-81. [DOI] [PubMed] [Google Scholar]
- 25.Numazaki, K., M. Umetsu, and N. Adachi. 2003. Mycoplasma pneumoniae infection and its genotypical characterization in children of Hokkaido, Japan. In Vivo 17:421-424. [PubMed] [Google Scholar]
- 26.Palmer, H. M., C. B. Gilroy, E. J. Claydon, and D. Taylor-Robinson. 1991. Detection of Mycoplasma genitalium in the genitourinary tract of women by the polymerase chain reaction. Int. J. STD AIDS 2:261-263. [DOI] [PubMed] [Google Scholar]
- 27.Peterson, S. N., C. C. Bailey, J. S. Jensen, M. B. Borre, E. S. King, K. F. Bott, and C. A. Hutchison III. 1995. Characterization of repetitive DNA in the Mycoplasma genitalium genome: possible role in the generation of antigenic variation. Proc. Natl. Acad. Sci. USA 92:11829-11833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson, S. N., P. C. Hu, K. F. Bott, and C. A. Hutchison III. 1993. A survey of the Mycoplasma genitalium genome by using random sequencing. J. Bacteriol. 175:7918-7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rocha, E. P., and A. Blanchard. 2002. Genomic repeats, genome plasticity and the dynamics of Mycoplasma evolution. Nucleic Acids Res. 30:2031-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasaki, T., T. Kenri, N. Okazaki, M. Iseki, R. Yamashita, M. Shintani, Y. Sasaki, and M. Yayoshi. 1996. Epidemiological study of Mycoplasma pneumoniae infections in Japan based on PCR-restriction fragment length polymorphism of the P1 cytadhesin gene. J. Clin. Microbiol. 34:447-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su, C. J., S. F. Dallo, and J. B. Baseman. 1990. Molecular distinctions among clinical isolates of Mycoplasma pneumoniae. J. Clin. Microbiol. 28:1538-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su, C. J., S. F. Dallo, A. Chavoya, and J. B. Baseman. 1993. Possible origin of sequence divergence in the P1 cytadhesin gene of Mycoplasma pneumoniae. Infect. Immun. 61:816-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor-Robinson, D. 2002. Mycoplasma genitalium—an up-date. Int. J. STD AIDS 13:145-151. [DOI] [PubMed] [Google Scholar]
- 34.Taylor-Robinson, D., C. B. Gilroy, and F. E. Keane. 2003. Detection of several Mycoplasma species at various anatomical sites of homosexual men. Eur. J. Clin. Microbiol. Infect. Dis. 22:291-293. [DOI] [PubMed] [Google Scholar]
- 35.Totten, P. A., M. A. Schwartz, K. E. Sjostrom, G. E. Kenny, H. H. Handsfield, J. B. Weiss, and W. L. Whittington. 2001. Association of Mycoplasma genitalium with nongonococcal urethritis in heterosexual men. J. Infect. Dis. 183:269-276. [DOI] [PubMed] [Google Scholar]
- 36.Tully, J. G., D. L. Rose, J. B. Baseman, S. F. Dallo, A. L. Lazzell, and C. P. Davis. 1995. Mycoplasma pneumoniae and Mycoplasma genitalium mixture in synovial fluid isolate. J. Clin. Microbiol. 33:1851-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tully, J. G., J. W. Shih, R. H. Wang, D. L. Rose, and S. C. Lo. 1993. Titers of antibody to Mycoplasma in sera of patients infected with human immunodeficiency virus. Clin. Infect. Dis. 17(Suppl. 1):S254-S258. [DOI] [PubMed] [Google Scholar]
- 38.Tully, J. G., D. Taylor-Robinson, R. M. Cole, and D. L. Rose. 1981. A newly discovered mycoplasma in the human urogenital tract. Lancet i:1288-1291. [DOI] [PubMed] [Google Scholar]
- 39.Wang, R. Y., T. Grandinetti, J. W. Shih, S. H. Weiss, C. L. Haley, M. M. Hayes, and S. C. Lo. 1997. Mycoplasma genitalium infection and host antibody immune response in patients infected by HIV, patients attending STD clinics and in healthy blood donors. FEMS Immunol. Med. Microbiol. 19:237-245. [DOI] [PubMed] [Google Scholar]