Abstract
Candida spp. are common causes of bloodstream infections among hospitalized patients. Fluconazole (FLC) remains a first-line therapy for candidemia; and voriconazole (VRC), an expanded-spectrum triazole, was recently approved for the treatment of candidemia in nonneutropenic patients. In vitro studies have suggested that VRC has potent activity against Candida spp. with reduced susceptibilities to FLC. We present a case report of invasive candidiasis and candidemia due to a Candida glabrata isolate that developed resistance to all currently available triazole antifungals after a course of FLC treatment. This case prompted us to determine the frequency of cross-resistance among bloodstream Candida isolates collected during a recent 12-month period at a large, academic medical center. FLC MICs were determined for 125 of 153 isolates (81.7%). Thirty of 125 isolates (24%) were resistant or showed reduced susceptibilites to FLC (MICs ≥ 16 μg/ml). When 28 of these 30 isolates were tested for their VRC susceptibilities, 9 (32%) had MICs that were ≥2 μg/ml. Five of these nine isolates were C. glabrata, two isolates were Candida tropicalis, one isolate was Candida albicans, and one isolate was Candida parapsilosis. All five Candida krusei isolates tested had VRC MICs ≤0.5 μg/ml. These data have prompted the introduction of reflexive FLC susceptibility testing of first bloodstream Candida isolates at our institution. The case report and our data also suggest that VRC should be avoided as initial therapy in unstable patients with invasive candidiasis, particularly in the setting of prior azole exposure. Studies are needed to define the clinical significance of in vitro resistance to the newer antifungal agents.
Candida spp. account for 9% of nosocomial bloodstream infections (BSIs), making Candida the fourth most common cause of nosocomial BSIs in the United States (23). Candida glabrata has emerged as the second most common Candida species causing BSIs among hospitalized adults (11, 22). While it is generally regarded as less virulent than Candida albicans, C. glabrata presents a particular therapeutic challenge in that it either may be intrinsically resistant to triazole antifungals (20) or may acquire resistance during relatively short periods of exposure to these drugs (1, 2). Expanded-spectrum triazoles such as voriconazole (VRC) have been shown to have potent in vitro activities against most Candida spp., although the VRC MICs appear to be higher for C. glabrata and Candida krusei than for other species (4, 10). In a recently published clinical trial, the efficacy of VRC was comparable to that of amphotericin B followed by fluconazole (FLC) (sequential treatment) for the treatment of candidemia in nonneutropenic patients (6). The drug was recently approved by the U.S. Food and Drug Administration for the treatment of candidemia in nonneutropenic patients, which makes the evaluation of the in vitro VRC susceptibility profiles of Candida species of particular importance.
In the present study we describe a patient with intra-abdominal and bloodstream infections due to a C. glabrata isolate that developed resistance to triazole antifungals after a course of FLC treatment. We document the in vitro resistance of this strain to all available triazole antifungal agents, and we also provide a perspective on this resistance by analyzing in vitro FLC and VRC susceptibility data for Candida isolates recovered from blood cultures over a 12-month period at Johns Hopkins Hospital (JHH). The therapeutic implications of these results are discussed.
CASE REPORT
A 45-year-old woman in her usual state of health approximately 1 year prior to admission was found to have multiple hepatic masses. She was diagnosed with a pancreatic neuroendocrine tumor metastatic to the liver. A pylorus-preserving pancreaticoduodenectomy and radiofrequency ablation of hepatic metastases were performed 42 days prior to the current admission. The patient remained in the surgical intensive care unit (ICU) overnight following the surgery. She did not receive FLC prophylaxis in the ICU.
Thirty-four days prior to the current admission the patient developed a fever, with her temperature increasing to 39.2°C. Her abdominal wound was erythematous. A computed tomographic (CT) scan of the abdomen showed no evidence of abscesses. Blood cultures grew Enterococcus faecalis, and vancomycin treatment was begun. She defervesced; but a few days later she was again febrile, and a repeat CT scan revealed new, low-attenuation hepatic lesions compatible with abscesses. A blood culture grew Escherichia coli and E. faecalis, and gatifloxacin treatment was begun. The patient continued to experience fever and rigors. Ultrasound-guided drainage of the patient's hepatic abscesses was performed, and cultures of the abscess material grew E. faecalis, Escherichia hermannii, and C. glabrata. Blood cultures grew E. faecalis and Lactobacillus spp. Two catheters were placed percutaneously into the patient's hepatic abscesses for continued drainage, and broad-spectrum antibacterials were administered. FLC was begun at 800 mg per day intravenously (i.v.) 21 days prior to the current admission. She underwent manipulations of her existing drainage catheters and placement of a third catheter into an undrained hepatic abscess 18 days prior to the current admission. Cultures of fluid aspirated from this collection grew E. faecalis, a coagulase-negative Staphylococcus sp., and C. glabrata. She was discharged to home with the drainage catheters in place to complete a course of antimicrobial therapy, including FLC.
After approximately 2 weeks at home and 3 days after completing her course of antimicrobial therapy, she was readmitted to the hospital with fever and hypotension. Broad-spectrum antibacterial therapy was begun on admission (day 0); and caspofungin (CAS) therapy was initiated on hospital day +1, with a loading dose of 70 mg i.v., followed by 50 mg i.v. per day. Blood cultures were positive for Prevotella spp., and liver abscess drainage cultures grew a coagulase-negative Staphylococcus sp. and Stenotrophomonas maltophilia. Cultures of specimens collected from the hepatic abscess drains on day +4 grew a coagulase-negative Staphylococcus sp., a Lactobacillus sp., and C. glabrata. Cultures of blood samples collected on days +7 and +9 grew a coagulase-negative Staphylococcus sp., and blood samples for culture collected on days +9 and +11 grew C. glabrata. On hospital day +11 she was transferred to the surgical ICU with mental status changes. The abscess drainage catheters were changed, and a fourth catheter was inserted on day +12. Cultures of hepatic abscess samples collected on day +12 grew C. glabrata, a coagulase-negative Staphylococcus sp., and E. faecalis. CAS was discontinued, and amphotericin B deoxycholate (AMB) at 0.7 mg/kg of body weight/day was started on day +13, with a dose increase to 1.0 mg/kg/day on day +15. On hospital day +20 she was intubated and placed on vasopressors.
On hospital day +21, AMB was replaced by liposomal amphotericin B (L-AMB) at 5 mg/kg/day, and VRC was added at 6 mg/kg i.v. twice daily for two doses, followed by 4 mg/kg i.v. twice daily. She slowly improved, and by hospital day +27 she was extubated. On day +31 she was transferred out of the ICU. Cultures of blood, urine, respiratory secretions, and cerebrospinal fluid remained unremarkable. Fluid collected from the indwelling, percutaneous hepatic drains grew bacteria and C. glabrata. An abdominal CT scan on hospital day +35 showed a decrease in the size of one of the liver abscesses, although a 1-cm lesion suspicious for an abscess was noted in the spleen. She returned to the ICU from day +36 to day +45 because of gastrointestinal bleeding. She again stabilized and was transferred out of the ICU. L-AMB was discontinued on hospital day +52. VRC was continued.
On hospital day +53 she deteriorated, with recurrent hypotension and respiratory distress that required intubation and transfer back to the ICU. A repeat abdominal CT scan showed an increase in the size of one of the hepatic abscesses. Cultures of percutaneous drain fluid grew Candida albicans, C. glabrata, vancomycin-sensitive Enterococcus faecium, a viridans group streptococcus, and a coagulase-negative Staphylococcus sp. Volume overload and renal failure ensued, necessitating continuous venovenous hemodialysis. VRC administration was changed from intravenous to enteral in the setting of renal insufficiency. Blood cultures grew a vancomycin-resistant Enterococcus sp. Despite continued aggressive intensive care, the insertion of a new hepatic abscess drain, broad-spectrum antibacterial treatment, and the reinitiation of L-AMB treatment, the patient developed progressive multiorgan system failure. Care was withdrawn, and the patient died on hospital day +69.
MATERIALS AND METHODS
Yeast culture, identification, and storage.
Clinical specimens were processed according to the standard procedures of the JHH Clinical Mycology Laboratory. Yeasts were identified to the species level by using germ tube formation (Remel, Lenexa, KS), morphology in cornmeal agar with caffeic acid, phenoloxidase and urease tests, carbohydrate fermentation, and carbohydrate assimilation assays (API 20C AUX; bioMérieux, Marcy l'Etoile, France) when necessary. Candida isolates from normally sterile body sites, including blood, were stored in water at 4°C and/or in 5% glycerol at −70°C.
Antifungal susceptibility testing and typing of the C. glabrata strain isolated from the case patient.
The FLC susceptibility testing requested by the clinical team during the patient's hospitalization was performed by the JHH Clinical Mycology Laboratory using a broth macrodilution method consistent with the M27-A2 method (8), except for the use of FLC solution for intravenous injection instead of drug compound. Triazole susceptibility testing of the patient's isolates was also performed retrospectively in the JHH Clinical Mycology Laboratory by using the Sensititre YeastOne system, according to the manufacturer's instructions (Trek Diagnostic Systems, Inc., Cleveland, OH).
Selected C. glabrata isolates from the patient were sent by the JHH Clinical Mycology Laboratory to the Fungus Testing Laboratory of the University of Texas Health Science Center (UTHSC) in San Antonio for additional susceptibility testing, including testing for susceptibility to AMB and CAS.
Typing of the patient's C. glabrata isolates was performed by pulsed-field gel electrophoresis. DNA was prepared by techniques consistent with published methods (15). Electrophoresis was performed by using the GenePath system and Candida parameters (Bio-Rad Laboratories, Hercules, CA). The bands were visualized with the Gel Doc 1000 system (Bio-Rad) by using Quantity One Image Capture software (Bio-Rad) and were analyzed with the Molecular Analyst fingerprinting software (Bio-Rad).
Triazole susceptibility testing of bloodstream Candida isolates at JHH.
To determine the FLC susceptibility profile for Candida isolates causing bloodstream infection at JHH, patients with candidemia between June 2003 and July 2004 (excluding August and September 2003) were identified by using the Johns Hopkins Department of Pathology database. The Johns Hopkins Institutional Review Board reviewed and approved this study of bloodstream isolates as exempt research. One Candida isolate per episode of candidemia was selected, except in instances where a patient was infected with more than one Candida species. In these cases, given that it was available, one isolate from each species was selected. When possible, an isolate from the patient's first day of candidemia was tested. If an isolate from the first day of candidemia was not available, an isolate from a subsequent day of candidemia was used.
Isolates were passaged at least twice and subcultured on Sabouraud dextrose agar overnight before FLC susceptibility testing was performed by a broth microdilution method (hereafter referred to as the BMD assay) consistent with the M27-A2 method (8), except for the use of FLC solution for intravenous injection instead of drug compound. FLC 96-well plates were prepared by using dilutions of FLC 2-mg/ml solution for intravenous injection (Pfizer Roerig, Inc., New York, NY, and Abbott Laboratories, Abbott Park, IL). Plates stored at −70°C were considered acceptable for use until 6 months following the date of preparation. After inoculation of the wells with the yeast suspension, 100 μl of the same suspension was used to inoculate Chocolate II Agar plates (Becton Dickinson, Sparks, MD) as growth and purity controls. MIC results were read visually after 48 h of incubation and were considered acceptable for inclusion in the analysis if the colony counts on these plates were between approximately 50 and 250 and if there was no evidence of mold or bacterial contamination.
Isolates with FLC MICs of ≥16 μg/ml in the BMD assay were tested for susceptibilities to FLC, itraconazole (ITC), and VRC by using the Sensititre YeastOne system according to the manufacturer's suggested protocol (Trek Diagnostic Systems, Inc.). MIC results were read visually after 24 to 26 h of incubation and were considered acceptable for inclusion if Sabouraud dextrose agar purity control plates were without contamination and the colony counts on the growth control plate were between 15 and 80.
Reference isolates C. albicans ATCC 90028 and ATCC 90029 and quality control strains C. parapsilosis ATCC 22019 and C. krusei ATCC 6258 were used as controls in the BMD assay. At least two of these organisms were tested for each batch of FLC plates prepared for the BMD assay. C. parapsilosis ATCC 22019 and Candida krusei ATCC 6258 were tested with each batch of Sensititre YeastOne testing that was performed. The MIC results were considered acceptable for inclusion in the analysis if the MICs were within the accepted reference ranges (8; Trek Diagnostic Systems, Inc., YeastOne package insert). The acceptable microdilution FLC MIC range for C. albicans ATCC 90028 and ATCC 90029 was considered to be ≤0.125 μg/ml to 0.5 μg/ml.
Discrepant isolates were defined as isolates with FLC MIC results that differed by >2 dilutions in the BMD assay and the Sensititre YeastOne assay. For analysis purposes, a BMD assay MIC of >64 μg/ml was considered to be within 2 dilutions of a Sensititre YeastOne MIC of ≥32 μg/ml. Discrepant isolates were retested, and the final FLC MIC was determined by examining all MIC results. For an isolate’s MIC results to be considered interpretable, two of three (or, in some cases, three of four) MIC results had to be within 2 dilutions of one another. The final recorded FLC MIC was the highest BMD MIC of those MICs within 2 dilutions of one another. If repeated testing could not resolve the MIC difference, the isolate was excluded.
Definitions.
A candidemia episode was defined as the isolation of a Candida strain from at least one blood culture. Episodes of candidemia in a single patient were regarded as distinct if they were separated by at least a 30-day period during which time no blood cultures were positive for Candida.
Susceptibility to FLC was defined by the use of established interpretive breakpoints (8); specifically, susceptibility was defined by an MIC of ≤8 μg/ml, dose-dependent susceptibility was defined by an MIC of 16 to 32 μg/ml, and resistance was defined by an MIC of ≥64 μg/ml. Susceptibility to ITC was also defined by the use of established interpretive breakpoints (8): susceptible, an MIC of ≤0.125 μg/ml; dose-dependent susceptible, an MIC of 0.25 to 0.5 μg/ml; and resistant, an MIC of ≥1 μg/ml. Reduced susceptibility or resistance to VRC and posaconazole (PSC) was defined by an MIC of ≥2 μg/ml (16, 19).
Statistical analysis.
The proportions of Candida spp. isolates with reduced susceptibilities to FLC and VRC and with uninterpretable FLC MICs were compared by using Fisher's exact test. The Spearman rank correlation coefficient was used to measure the association between VRC and FLC MICs. In addition, simple linear regression was used to describe the correlation between log2-transformed VRC and FLC MICs. A slope of 1 from this analysis indicates that as the FLC MIC doubles, the VRC MIC doubles.
RESULTS
Case report patient.
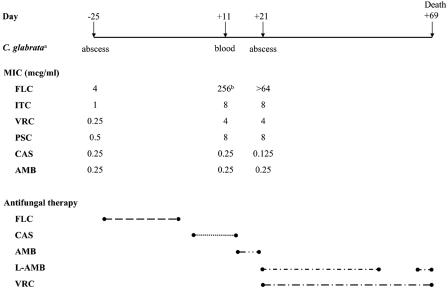
Seven C. glabrata isolates from the case report patient were available: an abscess isolate from day −25, an abscess isolate from day +4, two blood isolates from day +9, two blood isolates from day +11, and an abscess isolate from day +21. The antifungal susceptibility data from UTHSC for three isolates (day −25, pre-FLC; day +11, post-FLC; and day +21, post-FLC), as well as the antifungal therapy that the patient received, are presented in Fig. 1. The day +11 isolate was sent to UTHSC on day +26 of the patient's hospitalization, with the results reported on day +34; and the day −25 and day +21 isolates were sent retrospectively. These isolates were indistinguishable by pulsed-field gel electrophoresis performed in the JHH Clinical Microbiology Laboratory (data not shown).
FIG. 1.
Chronological presentation of C. glabrata isolates from case patient, triazole MICs, and antifungal therapy. Superscript letters: a, Only isolates sent to UTHSC for susceptibility testing are presented; b, Sensititre YeastOne MIC; all other results are from UTHSC.
The day −25 isolate was susceptible to FLC (MIC, 4 μg/ml). This isolate was resistant to ITC but was susceptible to both VRC (MIC, 0.25 μg/ml) and PSC (MIC, 0.5 μg/ml). The AMB MIC was 0.25 μg/ml, and the CAS MIC was 0.25 μg/ml. A bloodstream isolate obtained 2 weeks after FLC therapy, prior to AMB therapy, and during CAS therapy was resistant to FLC (testing was performed at JHH by using the Sensititre YeastOne system [Trek Diagnostic Systems, Inc.]), as well as ITC, VRC, and PSC. The AMB and CAS MICs were unchanged. The hepatic abscess isolate obtained on day +21 also demonstrated in vitro resistance to FLC, ITC, VRC, and PSC. The AMB MIC remained 0.25 μg/ml, and the CAS MIC was 0.125 μg/ml.
Species distribution and FLC susceptibilities of Candida spp. causing bloodstream infections at JHH.
To put this case in perspective, data from candidemia episodes occurring during the 12 months from June 2003 through July 2004 (excluding August and September 2003) at JHH were collected. A total of 147 separate episodes of candidemia occurred in 144 patients, and 153 Candida isolates were identified from these 147 episodes of candidemia. Six patients were infected with two different Candida species during the same episode of candidemia, and three patients had two separate episodes of candidemia. C. albicans was the most commonly isolated species (56 of 153 isolates; 37%), followed by C. glabrata (52 of 153 isolates; 34%), C. parapsilosis (22 of 153 isolates; 14%), C. tropicalis (15 of 153 isolates; 9.8%), C. krusei (7 of 153 isolates; 5%), and C. lusitaniae (1 of 153 isolates; 0.7%).
FLC MICs were determined for 125 of 153 isolates (81.7%) (Table 1), most of which (85.6%) were from the first day of candidemia. Seventeen isolates (11%) were not found, and three (2%) did not grow when they were subcultured from storage vials. Three isolates (one isolate each of C. albicans, C. tropicalis, and C. parapsilosis) had low colony counts on the growth control plate, and the purity control plate for one C. glabrata isolate had contamination with mold.
TABLE 1.
Candida spp. recovered from blood cultures over a 12-month period and in vitro FLC susceptibility profiles
| Candida sp. | No. of isolates
|
No. (%) of isolates with the following FLC susceptibility (MIC [μg/ml]):
|
|||
|---|---|---|---|---|---|
| Total | Tested | Susceptible (≤8) | Dose-dependent susceptible (16-32) | Resistant (≥64) | |
| C. albicans | 56 | 45 | 43 (96) | 1 (2) | 1 (2) |
| C. glabrata | 52 | 44 | 25 (57) | 11 (25) | 8 (18) |
| C. parapsilosis | 22 | 21 | 20 (95) | 0 | 1 (5) |
| C. tropicalis | 15 | 9 | 6 (67) | 1 (11) | 2 (22) |
| C. krusei | 7 | 5 | 0 | 3 (60) | 2 (40) |
| C. lusitaniae | 1 | 1 | 1 (100) | 0 | 0 |
| Total | 153 | 125 | 95 (76) | 16 (13) | 14 (11) |
Four isolates (3%; three C. tropicalis isolates and one C. albicans isolate) had MIC discrepancies that could not be resolved with repeated testing. Isolates of C. tropicalis were significantly more likely to have uninterpretable FLC MIC results than isolates of other Candida species (4 of 13 C. tropicalis isolates tested versus 4 of 120 non-C. tropicalis isolates tested; P = 0.003, Fisher's exact test).
Thirty of 125 isolates (24%) had FLC MICs of ≥16 μg/ml (Table 1); 14 (11%) had FLC MICs of ≥64 μg/ml. Of the 30 isolates with higher FLC MICs, 19 (63%) were C. glabrata, 5 (17%) were C. krusei, 3 (10%) were C. tropicalis, 2 (7%) were C. albicans, and 1 (3%) was C. parapsilosis. Of the 14 isolates with FLC MICs ≥64 μg/ml, 8 (57%) were C. glabrata, 2 were C. krusei (14%), 2 were C. tropicalis (14%), 1 was C. albicans (7%), and 1 was C. parapsilosis (7%).
Eleven of 153 Candida isolates (7%) were grown from cultures of blood collected in the JHH surgical ICUs, where FLC prophylaxis is routinely administered to patients with expected ICU stays of ≥3 days. Candida spp. known to have reduced FLC susceptibilities (C. glabrata and C. krusei) accounted for a greater proportion of bloodstream Candida isolates in the surgical ICUs than in other patient locations (8 of 11 isolates versus 51 of 142 isolates; P = 0.02, Fisher's exact test), but reduced FLC susceptibility was not more prevalent among the surgical ICU isolates tested than among isolates from other locations (3 of 7 isolates versus 27 of 118 isolates; P = 0.36, Fisher's exact test). In this analysis we were not able to distinguish infections incident to the surgical ICUs from prevalent infections.
ITC and VRC MICs for bloodstream Candida isolates with reduced FLC susceptibilities.
ITC MICs were determined for 28 of 30 isolates (93%) with FLC MICs ≥16 μg/ml. One C. tropicalis isolate tested in the BMD assay did not grow when it was subcultured for ITC and VRC susceptibility testing, and one C. glabrata isolate could not be located for ITC and VRC susceptibility testing. Twenty-one of 28 isolates (75%; including 17 of 18 C. glabrata isolates tested) were ITC resistant, with MICs ≥1 μg/ml. The remaining seven isolates (four C. krusei isolates, one C. glabrata isolate, one C. albicans isolate, and one C. parapsilosis isolate) demonstrated dose-dependent ITC susceptibilities, with MICs of 0.25 to 0.5 μg/ml.
VRC MICs were also determined for 28 isolates with FLC MICs ≥16 μg/ml (Table 2). Nine of 28 isolates (32%) had VRC MICs ≥2 μg/ml. Five of nine isolates with reduced VRC susceptibilities (56%) were C. glabrata. Two were C. tropicalis, one was C. albicans, and one was C. parapsilosis. No isolate of C. krusei, a species considered intrinsically FLC resistant, had reduced VRC susceptibility. FLC-resistant Candida species other than C. krusei were significantly more likely to have VRC MICs ≥2 μg/ml than C. krusei (9 of 11 isolates tested versus 0 of 5 isolates tested; P = 0.005, Fisher's exact test). All 11 non-C. krusei, FLC-resistant isolates had VRC MICs ≥1 μg/ml, 9 of 11 (82%) had VRC MICs ≥2 μg/ml, and 5 of 11 (45%) had VRC MICs ≥4 μg/ml.
TABLE 2.
VRC susceptibilities of Candida bloodstream isolates with reduced susceptibility to FLC (MIC ≥16 μg/ml)
| Candida sp. | No. of isolates
|
No. (%) of isolates with the following VRC susceptibility (MIC [μg/ml]):
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Tested | Susceptiblea
|
Resistanta
|
|||||||||
| ≤0.06 | 0.12 | 0.25 | 0.5 | 1.0 | 2.0 | 4.0 | 8.0 | 16 | >16 | |||
| C. albicans | 2 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| C. glabrata | 19 | 18 | 0 | 2 (11) | 3 (17) | 5 (28) | 3 (17) | 3 (17) | 2 (11) | 0 | 0 | 0 |
| C. parapsilosis | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| C. tropicalis | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| C. krusei | 5 | 5 | 0 | 0 | 3 (60) | 2 (40) | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 30 | 28 | 0 | 3 (11) | 6 (21) | 7 (25) | 3 (11) | 4 (14) | 3 (11) | 0 | 0 | 2 (7) |
Total percentages may exceed 100 due to rounding.
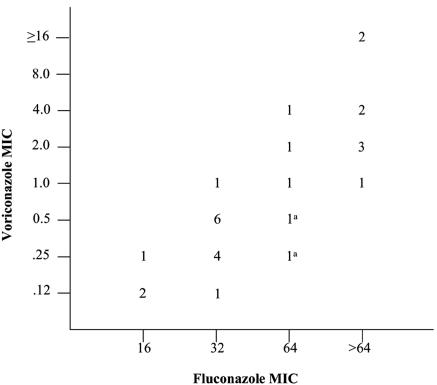
VRC MICs were correlated with FLC MICs (Fig. 2) when the C. krusei isolates were included (coefficient for log2[FLC MIC] = 1.56; 95% confidence interval = 1.13 to 2.00; Spearman correlation coefficient = 0.83) and when all five C. krusei isolates were excluded (coefficient for log2[FLC MIC] = 1.58; 95% confidence interval = 1.14 to 2.01; Spearman correlation coefficient = 0.88). Specifically, we estimated that as the FLC MIC doubles, the VRC MIC will double to quadruple.
FIG. 2.
Distribution of FLC and VRC MICs for Candida isolates with FLC MICs ≥16 μg/ml, including C. krusei. VRC and FLC MICs were correlated (log2[VRC MIC] = −9.18 + 1.56 log2[FLC MIC]; Spearman correlation coefficient = 0.83). a, Isolates of C. krusei with an FLC MIC of 64 μg/ml but a VRC MIC of <1 μg/ml.
DISCUSSION
We describe here a patient with a complex illness who developed polymicrobial sepsis, including intra-abdominal and bloodstream infections due to C. glabrata. This patient's C. glabrata isolate, which was initially susceptible in vitro to FLC, VRC, and PSC, developed cross-resistance to all currently available triazole antifungals after a course of FLC therapy and with no known prior exposure to expanded-spectrum triazoles. This case is similar to another reported recently, involving a patient with prosthetic valve endocarditis due to C. parapsilosis (7). Despite infection with an initially FLC- and VRC-susceptible organism, the patient in that report (7) went on to develop recurrent candidemia caused by an apparently identical strain of C. parapsilosis that was FLC and VRC resistant. This patient had been treated with a prolonged course of FLC but had never received therapy with VRC (7).
The rapidity with which our patient's C. glabrata isolate developed resistance to all currently available triazoles is supported by in vitro work by Borst and colleagues (2). They demonstrated that C. glabrata isolates obtained prior to the introduction of FLC developed stable FLC, ITC, and VRC resistance within only 4 days of exposure to FLC (2). They concluded that the rapid acquisition of azole resistance in C. glabrata can occur in the absence of prior exposure to these drugs and is associated with the increased expression of ABC transporter genes (2). Another study of azole resistance mechanisms among C. glabrata isolates from hematopoietic stem cell transplant recipients receiving FLC prophylaxis showed that MICs doubled, on average, every 31 days (1).
Although some studies have shown that C. albicans continues to account for more than half of all cases of candidemia (4, 14, 22), others have demonstrated that Candida species other than C. albicans in some areas of the United States now account for the majority of Candida bloodstream infections (5, 11). In our study, Candida species other than C. albicans accounted for almost two-thirds of the candidemia episodes, and C. glabrata alone accounted for just over one-third. Even more striking is the finding that 24% of the evaluable bloodstream Candida isolates during this period had in vitro FLC MICs ≥16 μg/ml, indicating at least reduced susceptibility, and 11% were resistant, with MICs ≥64 μg/ml. A limitation of our data is that approximately 13% of isolates were not found or did not grow and that susceptibility testing problems were encountered for another 6% of the isolates, which precluded their inclusion in the analyses. Most notably, we had difficulty with a subset of C. tropicalis isolates that had disparate MICs on repeated testing, possibly as a result of the trailing phenomenon, which may occur frequently with this species (10).
Our data suggest that with the exception of C. krusei, Candida isolates with FLC MICs ≥64 μg/ml are likely to be resistant in vitro to VRC. Although our study is limited by the small numbers of bloodstream Candida isolates from a single institution, our findings support the results obtained by Pfaller and colleagues, who showed that among 12,796 clinical Candida isolates, FLC MICs of ≥64 μg/ml predicted ravuconazole resistance, defined as a MIC of ≥2 μg/ml (16). Another study by Pfaller and colleagues demonstrated that while FLC resistance was uncommon among 3,932 clinical Candida isolates (2.9%), approximately half of the FLC-resistant isolates had VRC and PSC MICs of ≥2 μg/ml (17). This correlation between in vitro FLC and VRC resistance among Candida species highlights the clinical issues raised by our case presentation and may have important implications for antifungal therapy selection in patients with invasive candidiasis.
While FLC resistance and VRC resistance were relatively uncommon in the studies cited above, with approximately 3% of isolates having FLC MICs of ≥64 μg/ml and less than 2% of isolates having VRC MICs of ≥2 μg/ml (17), resistance to these drugs was more common in our study. At least 9 of the 125 bloodstream isolates in our study (approximately 7%) had VRC MICs of ≥2 μg/ml, highlighting the geographic differences in azole resistance that exist nationally and internationally (14, 18). Most of the resistant isolates in our study were C. glabrata; but a number of C. albicans, C. tropicalis, and C. parapsilosis isolates were also found to have reduced susceptibility or resistance to both drugs. By contrast, it is interesting that the few isolates of C. krusei in our study were susceptible to VRC, a finding which has been noted by others (17).
Because we did not collect clinical data, we were unable to ascertain whether extensive triazole experience among patients with candidemia at JHH could explain the prevalence of C. glabrata or the degree of reduced triazole susceptibility observed. FLC prophylaxis is routinely administered to JHH surgical ICU patients with an expected ICU stay of at least 3 days (13); it is also administered to liver and hematopoietic stem cell transplant recipients. A retrospective study of candidemia in the JHH surgical ICU before and after the institution of FLC prophylaxis showed that the incidence of candidemia was significantly lower in the period after FLC prophylaxis became routine, although C. glabrata was a more common cause of incident and prevalent candidemia in that period than in the period prior to prophylaxis implementation (21). In the current study, C. glabrata and C. krusei accounted for a greater proportion of bloodstream Candida isolates in the surgical ICUs than in other patient locations, but reduced FLC susceptibility was not more common among a small number of surgical ICU Candida isolates. Overall, only 7% of bloodstream Candida sp. isolates and 13% of C. glabrata isolates were obtained from cultures of blood drawn in the surgical ICUs. By contrast, 16% of all isolates and 21% of C. glabrata isolates were grown from cultures of blood collected in the medical ICU, where FLC prophylaxis is not routinely used. The prevalence of C. glabrata and the reduced triazole susceptibilities of isolates at JHH are likely due to a multitude of factors, including triazole exposure in different clinical settings, rather than solely due to a prophylaxis practice applied to a select group of patients in the two surgical ICUs in the hospital.
Our lack of clinical data also prevented us from evaluating the relationship between in vitro susceptibility and clinical outcome. Ostrosky-Zeichner and colleagues reported an overall response rate of 56% in patients with refractory invasive candidiasis who received VRC as salvage therapy (9). This response rate, as the authors note, is similar to the rates seen in studies of other antifungal drugs used as primary therapy, even though many patients had prior azole exposure (9). However, a large proportion of patients in this study (almost 20%) were infected with C. krusei, which may have lower VRC MICs than FLC-resistant isolates of other Candida spp. The response rate was lower (38%) in patients infected with C. glabrata, a species known to acquire azole resistance during therapy (9). Some patients included in that study were participants in studies conducted outside of the United States, where FLC resistance among clinical Candida isolates may be less prevalent (3, 14). In vitro susceptibility data from these patients' isolates were not available (9).
Current guidelines suggest that clinicians consider antifungals other than FLC as therapy for invasive candidiasis in patients who have had prior azole exposure and who are unstable or infected with species known to have reduced susceptibilities to FLC (12). Based on the frequency with which triazole resistance was detected among the Candida isolates at our institution, reflexive FLC susceptibility testing of patients' initial blood isolates is being instituted. With the wider availability of easy-to-use antifungal susceptibility testing formats that include expanded-spectrum triazoles, further studies evaluating the relationship between in vitro antifungal resistance and clinical outcome are critical to determine the most effective treatment approach for patients with invasive disease. Until such data are available, we believe that it may be prudent to avoid VRC as initial therapy for invasive candidiasis in unstable patients, particularly in the setting of C. glabrata infection and prior triazole exposure.
Acknowledgments
We thank Elizabeth Johnson, Department of Biostatistics, Johns Hopkins Bloomberg School of Public Health, for statistical support.
S.S.M. is supported by a K23 Mentored Patient-Oriented Research Career Development Award from NIAID (grant AI53601) and has received grant support from Pfizer. W.G.M. has received grant support from Pfizer and Merck. Merck provided financial support to W.G.M. for the FLC susceptibility testing of the JHH bloodstream isolates included in this study.
REFERENCES
- 1.Bennett, J. E., K. Izumikawa, and K. A. Marr. 2004. Mechanism of increased fluconazole resistance in Candida glabrata during prophylaxis. Antimicrob. Agents Chemother. 48:1773-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borst, A. M., M. T. Raimer, D. W. Warnock, C. J. Morrison, and B. A. Arthington-Skaggs. 2005. Rapid acquisition of stable azole resistance by Candida glabrata isolates obtained before the clinical introduction of fluconazole. Antimicrob. Agents Chemother. 49:783-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuenca-Estrella, M., D. Rodriquez, B. Almirante, J. Morgan, A. M. Planes, M. Almela, J. Mensa, F. Sanchez, J. Ayats, M. Gimenez, M. Salvado, D. W. Warnock, A. Pahissa, and J. L. Rodriguez-Tudela. 2005. In vitro susceptibilities of bloodstream isolates of Candida species to six antifungal agents: results from a population-based active surveillance programme, Barcelona, Spain, 2002-2003. J. Antimicrob. Chemother. 55:194-199. [DOI] [PubMed] [Google Scholar]
- 4.Diekema, D. J., S. A. Messer, A. B. Brueggemann, S. L. Coffman, G. V. Doern, L. A. Herwaldt, and M. A. Pfaller. 2002. Epidemiology of candidemia: 3-year results from the Emerging Infections and the Epidemiology of Iowa Organisms Study. J. Clin. Microbiol. 40:1298-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hajjeh, R. A., A. N. Sofair, L. H. Harrison, G. M. Lyon, B. A. Arthington-Skaggs, S. A. Mirza, M. Phelan, J. Morgan, W. Lee-Yang, M. A. Ciblak, L. E. Benjamin, L. T. Sanza, S. Huie, S. F. Yeo, M. E. Brandt, and D. W. Warnock. 2004. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J. Clin. Microbiol. 42:1519-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kullberg, B. J., J. D. Sobel, M. Ruhnke, P. G. Pappas, C. Viscoli, J. H. Rex, J. D. Cleary, E. Rubinstein, L. W. P. Church, J. M. Brown, H. T. Schlamm, I. T. Oborska, F. Hilton, and M. R. Hodges. 2005. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomized non-inferiority trial. Lancet 366:1435-1442. [DOI] [PubMed] [Google Scholar]
- 7.Moudgal, V., T. Little, D. Boikov, and J. A. Vasquez. 2005. Multiechinocandin- and multiazole-resistant Candida parapsilosis isolates serially obtained during therapy for prosthetic valve endocarditis. Antimicrob. Agents Chemother. 49:767-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 9.Ostrosky-Zeichner, L., A. M. L. Oude Lashof, B. J. Kullberg, and J. H. Rex. 2003. Voriconazole salvage treatment of invasive candidiasis. Eur. J. Clin. Microbiol. Infect. Dis. 22:651-655. [DOI] [PubMed] [Google Scholar]
- 10.Ostrosky-Zeichner, L., J. H. Rex, P. G. Pappas, R. J. Hamill, R. A. Larsen, H. W. Horowitz, W. G. Powderly, N. Hyslop, C. A. Kauffman, J. Cleary, J. E. Mangino, and J. Lee. 2003. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother. 47:3149-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pappas, P. G., J. H. Rex, J. Lee, R. J. Hamill, R. A. Larsen, W. Powderly, C. A. Kauffman, N. Hyslop, J. E. Mangino, S. Chapman, H. W. Horowitz, J. E. Edwards, and W. E. Dismukes. 2003. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin. Infect. Dis. 37:634-643. [DOI] [PubMed] [Google Scholar]
- 12.Pappas, P. G., J. H. Rex, J. D. Sobel, S. G. Filler, W. E. Dismukes, T. J. Walsh, and J. E. Edwards. 2004. Guidelines for the treatment of candidiasis. Clin. Infect. Dis. 38:161-189. [DOI] [PubMed] [Google Scholar]
- 13.Pelz, R. K., C. W. Hendrix, S. M. Swoboda, M. Diener-West, W., G. Merz, J. Hammond, and P. A. Lipsett. 2001. Double-blind, placebo-controlled trial of fluconazole to prevent candidal infections in critically ill surgical patients. Ann. Surg. 233:542-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfaller, M. A., D. J. Diekema, R. N. Jones, H. S. Sader, A. C. FLCit, R. J. Hollis, S. A. Messer, and the SENTRY Participant Group. 2001. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY Antimicrobial Surveillance Program. J. Clin. Microbiol. 39:3254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaller, M. A., J. Rhine-Chalberg, S. W. Redding, J. Smith, G. Farinacci, A. W. Fothergill, and M. G. Rinaldi. 1995. Variations in fluconazole susceptibility and electrophoretic karyotype among oral isolates of Candida albicans from patients with AIDS and oral candidiasis. J. Clin. Microbiol. 32:59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfaller, M. A., S. A. Messer, L. Boyken, C. Rice, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2004. Cross-resistance between FLC and ravuconazole and the use of fluconazole as a surrogate marker to predict susceptibility and resistance to ravuconazole among 12,796 clinical isolates of Candida spp. J. Clin. Microbiol. 42:3137-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfaller, M. A., S. A. Messer, L. Boyken, R. J. Hollis, C. Rice, S. Tendolkar, and D. J. Diekema. 2004. In vitro activities of voriconazole, posaconazole, and fluconazole against 4,169 clinical isolates of Candida spp. and Cryptococcus neoformans collected during 2001 and 2002 in the ARTEMIS global antifungal surveillance program. Diagn. Microbiol. Infect. Dis. 48:201-205. [DOI] [PubMed] [Google Scholar]
- 18.Pfaller, M. A., S. A. Messer, L. Boyken, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2003. Variation in susceptibility of bloodstream isolates of Candida glabrata to fluconazole according to patient age and geographic location. J. Clin. Microbiol. 41:2176-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, and D. J. Diekema. 2002. In vitro activities of ravuconazole and voriconazole compared with those of four approved systemic antifungal agents against 6,970 clinical isolates of Candida spp. Antimicrob. Agents Chemother. 46:1723-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanglard, D., and F. C. Odds. 2002. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect. Dis. 2:73-85. [DOI] [PubMed] [Google Scholar]
- 21.Swoboda, S. M., W. G. Merz, and P. A. Lipsett. 2003. Candidemia: the impact of antifungal prophylaxis in a surgical intensive care unit. Surg. Infect. 4:345-354. [DOI] [PubMed] [Google Scholar]
- 22.Trick, W. E., S. K. Fridkin, J. R. Edwards, R. A. Hajjeh, R. P. Gaynes, and the National Nosocomial Infections Surveillance System Hospitals. 2002. Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989-1999. Clin. Infect. Dis. 35:627-630. [DOI] [PubMed] [Google Scholar]
- 23.Wisplinghoff, H., T. Bischoff, S. M. Tallent, H. Seifert, R. P. Wenzel, and M. B. Edmond. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309-317. [DOI] [PubMed] [Google Scholar]