Abstract
We present a case of a healthy 19-year-old female who developed infectious mononucleosis complicated by unilateral empyema.
CASE REPORT
A previously healthy 19-year-old female presented to her primary care physician with a 1-day history of severe sore throat. She denied fever, headache, rhinorrhea, dysphagia, neck stiffness, cough, shortness of breath, nausea, abdominal pain, or other significant symptoms. A throat swab for rapid assessment of group A Streptococcus antigen was negative, a serum heterophile antibody was positive, and her peripheral blood smear revealed the presence of atypical lymphocytes. She was diagnosed with infectious mononucleosis and prescribed acetaminophen with codeine as well as a 3-day course of prednisone.
Four days later, she presented again to her primary care physician complaining of a persistent sore throat as well as a new onset of fever, difficulty swallowing, shortness of breath, and left upper quadrant abdominal pain with significantly decreased appetite. She was admitted to her local hospital, where her exam was reportedly notable for left tonsillar enlargement with exudates, left anterior cervical lymphadenopathy, tachycardia, and splenomegaly. She was treated with intravenous fluids, ketorolac, and methylprednisolone. She was discharged to home on hospital day 2 on a 6-day taper of prednisone.
Over the following week, she remained ill with the symptoms described above. In addition, she developed progressive dyspnea on exertion to the extent that she could no longer climb 10 stairs without stopping. She presented again to her primary care physician complaining of worsening shortness of breath, fatigue, orthopnea, left-sided pleuritic chest pain, and dry heaves. She was readmitted to her local hospital, where she was afebrile, tachycardic to 135 beats per minute, normotensive, and tachypneic at 28 breaths per minute, with a pulse oximeter saturation of 77% on room air. Her exam was now notable for normalization of her oropharynx and lymphadenopathy but newly diminished breath sounds over the entire left hemithorax with dullness to percussion and egophony. Examination of her right hemithorax was within normal limits. Her lab results were notable for a white blood cell count of 20.1, with 37% neutrophils, 44% band forms, and 9% lymphocytes. Posteroanterior and lateral chest radiographs revealed a large left hydropneumothorax with near total opacification of the left hemithorax. She was started on ceftriaxone and metronidazole and transferred to our institution's surgical intensive care unit for further care.
Her past medical and surgical history was unremarkable. She took no chronic medications and had no known allergies. Her immunizations were up to date. She was a college student recently home on summer vacation. She smoked less than three cigarettes per day and consumed alcohol sparingly. She denied recreational drug use, recent travel outside of the Boston area (including no visits to Cape Cod or the adjacent islands), and any animal exposures. She was not sexually active. All of her family members were in good health.
Upon arrival at our institution, she remained tachycardic and tachypneic. Her exam was as stated above, with additional findings of supple neck, flat jugular venous pulse, and lack of trismus. Labs were notable for arterial blood gas: pH 7.38, PaCO2 44 mm Hg, PaO2 82 mm Hg on 100% FiO2 administered via a nonrebreather facemask. Her liver function tests were normal.
Our patient's initial diagnosis was made at the office of her primary care physician through the use of a Monospot assay (latex agglutination assay using horse erythrocytes), to which she tested positive, as well as by the presence of atypical lymphocytes noted on her peripheral smear. Upon arrival at our institution, a confirmatory assay for immunoglobulin M viral capsid antigen antibodies was sent, but the assay was unfortunately unable to be performed due to laboratory error. Further tests to confirm Epstein-Barr virus as the etiologic agent of her clinical picture of infectious mononucleosis (IM) were not pursued.
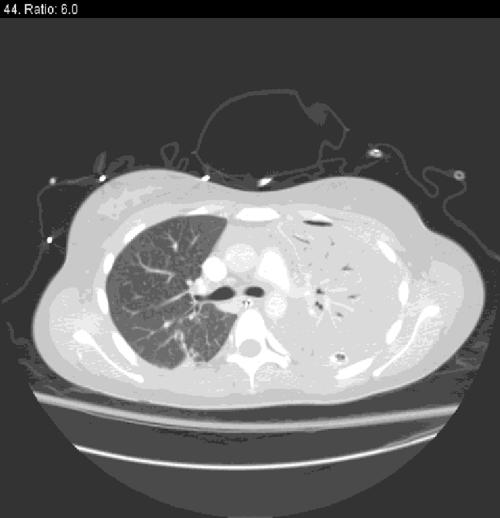
A chest tube was placed in her left pleural space, and it promptly drained 2,200 ml of foul-smelling purulent material that was sent for laboratory and microbiological evaluation. A computed tomography scan of her head, neck, and chest with intravenous contrast following chest tube placement was notable for nearly complete consolidation of the left lung, minimal aeration with air bronchograms, and a small residual left-sided effusion. Additionally, there was no evidence for a neck, peritonsillar, or retropharyngeal abscess, major vascular occlusion, mediastinitis, mediastinal lymphadenopathy, or esophageal rupture. Her right lung was entirely clear other than a small amount of basilar atelectasis (Fig. 1).
FIG. 1.
Computed tomography scan of the chest showing consolidation of the left lung and clear right lung with small basilar atelectasis.
Gram stain of the empyema fluid from her chest tube revealed abundant neutrophils and abundant gram-negative rods. Ultimately, the anaerobic culture grew Fusobacterium necrophorum and her antibiotic regimen was changed to penicillin and metronidazole for a 4-week course. Blood cultures drawn on her second admission to the outside hospital as well as on admission to our institution showed no growth.
Her hospital treatment consisted mainly of supportive care with intravenous fluids, supplemental oxygen, intermittent fibrinolytic therapy via chest tube, and antibiotics. At no time did she require intubation or vasoactive medications. On hospital day 2, she underwent a diagnostic bronchoscopy that failed to reveal clinically significant pathology. She required intensive care monitoring for 3 days due to significant supplemental oxygen requirement, after which she was transferred to a step-down unit. On hospital day 7, she had a diagnostic right-sided thoracentesis for evaluation of a small effusion that was negative for organisms. By hospital day 10, she was oxygenating well on room air and was tolerating oral intake.
She was discharged to home in satisfactory condition, while continuing antibiotic treatment for 4 weeks.
Discussion.
Common manifestations of IM that lead patients to seek medical attention are pharyngitis and tonsillar enlargement that can cause severe sore throat, dysphagia, and odynophagia (3). While corticosteroids have been used to treat airway compromise due to tonsillar inflammation, they may also be of some benefit for the pain associated with IM. However, Roy et al. have shown that, in a pediatric population, a single dose of dexamethasone for treatment of sore throat associated with mononucleosis may not be sufficient for resolution of symptoms (6). Furthermore, the use of glucocorticoids for symptomatic relief may predispose patients to bacterial superinfections (3). Indeed, our patient received a short course of steroids as an outpatient before the clinical deterioration that prompted her first admission to the hospital.
Epstein-Barr virus (the etiologic agent of classic infectious mononucleosis) frequently produces pharyngitis as part of the evolution of IM and may create an environment favorable for the development of anaerobic infections. Fusobacterium necrophorum is an anaerobic gram-negative bacillus that colonizes the oral cavity, female genital tract, and gastrointestinal tract and has been implicated in Lemierre's syndrome (postanginal sepsis) (7). This has been described as a complication of acute oropharyngeal infections in adolescents and young adults and classically has four findings: (i) primary infection of the oropharynx, (ii) secondary bacterial infection with at least one positive blood culture, (iii) thrombosis of the internal jugular vein (diagnosed either clinically or radiographically), and (iv) at least one distal focus of infection such as pneumonia or arthritis. The treatment of Lemierre's syndrome consists primarily of antimicrobial therapy directed against the Fusobacterium species, with possible anticoagulation for jugular venous thrombosis, and, as necessary, directed surgical evacuation of any abscesses.
Chirinos et al. have recently reviewed the literature and suggest that the typical presentation of Lemierre's syndrome has evolved from what was initially described in the preantibiotic era and now includes pharyngitis, a swollen neck, and noncavitating pulmonary infiltrates (2). The authors also indicate that the first clue to the diagnosis was a positive blood culture with an appropriate organism, such as Fusobacterium necrophorum, in 69.7% of cases, although negative cultures were seen in 12.8% of cases. As was the case with our patient, 79.8% of their patients had pulmonary manifestations, and 87.1% had the pharynx as the initial source of infection. However, our patient's case is more atypical in that she did not have the jugular vein thrombosis that was found in 71.5% of cases in their series. They also report a 6.4% overall mortality from Lemierre's syndrome when treated with appropriate antimicrobial therapy.
Although our patient developed some of the typical signs and symptoms of Lemierre's syndrome, her case fits into neither the classical description nor the updated description suggested by Chirinos et al. (2). Specifically, she developed no positive blood cultures nor did she suffer from jugular venous thrombosis. Another fact that was unique to our patient was the presence of unilateral empyema. Classically, empyemas are unilateral due to underlying pneumonia and are probably secondary to pulmonary aspiration of an organism that originates in the upper respiratory tract. On the other hand, pulmonary infiltrates and empyemas that complicate IM are frequently bilateral. These empyemas have been described to result from two different mechanisms: jugular venous thrombosis that generates septic emboli as part of classical Lemierre's syndrome or a retropharyngeal abscess that extends caudally into the mediastinum, creating mediastinitis and then extending into the pleural spaces (1, 4, 5). We did not identify either of these two mechanisms of empyema formation in our patient. The initial computed tomography scan showed the presence of unilateral empyema with complete consolidation of the underlying lung. We speculate that the subsequent bilateral pleural involvement in our patient was either secondary to passive contamination from one lung to the other or due to the bronchoscopy that was performed after her admission to the intensive care unit.
Conclusion.
The clinical course of infectious mononucleosis and its complications have changed since their initial description in the preantibiotic era. Lemierre's syndrome remains a rare but serious complication of IM whose clinical epidemiology may be evolving due to rapid initiation of antimicrobial therapy for pharyngeal infections, thus making the diagnosis even more challenging (2).
Our patient developed a variant of Lemierre's syndrome that was characterized by predominantly unilateral pulmonary infection with Fusobacterium necrophorum in the absence of identifiable septicemia, internal jugular vein thrombosis, or retropharyngeal abscess. We thus suggest a third mechanism by which an anaerobic empyema may develop as a sequela of infectious mononucleosis: primary aspiration of the Fusobacterium from the oropharynx leading to a dense unilateral pneumonia with resultant unilateral empyema. It is purely speculative whether our patient's treatment with glucocorticoids may have contributed to her subsequent development of post-IM anaerobic infection.
REFERENCES
- 1.Andrianakis, I. A., A. N. Kotanidou, M. T. Pitaridis, et al. 2002. Life-threatening bilateral empyema and medistinitis complicating infectious mononucleosis. Intensive Care Med. 28:663-664. [DOI] [PubMed] [Google Scholar]
- 2.Chirinos, J. A., D. M. Lichtstein, J. Garcia, et al. 2002. The evolution of Lemierre Syndrome. Medicine 81:458-465. [DOI] [PubMed] [Google Scholar]
- 3.Cohen, J. 1998. Epstein-Barr virus infections, including infectious mononucleosis, p. 1089-1091. In A. S. Fauci, E. Braunwald, K. J. Isselbacher, et al. (ed.), Harrison's principles of internal medicine, 14th ed. McGraw-Hill, New York, N.Y.
- 4.Kopec, S. E., R. S. Irwin, C. J. Mello, et al. 1997. Bilateral anaerobic empyemas complicating infectious mononucleosis. Chest 112:833-835. [DOI] [PubMed] [Google Scholar]
- 5.Ockrim, J., S. Kettlewell, and G. R. Gray. 2000. Lemierre's syndrome. J. R. Soc. Med. 93:480-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roy, M., B. Bailey, D. K. Amre, et al. 2004. Dexamethasone for the treatment of sore throat in children with suspected infectious mononucleosis. Arch. Pediatr. Adolesc. Med. 158:250-254. [DOI] [PubMed] [Google Scholar]
- 7.Venglarcik, J. 2003. Lemierre's syndrome. Pediatr. Infect. Dis. J. 22:921-923. [DOI] [PubMed] [Google Scholar]