Abstract
Activation of protein kinase C (PKC) increases vesicular secretion in many cell types. We determined the calcium dependence of secretion and the size of the readily releasable pool of secretory granules in pituitary gonadotropes by photorelease of caged-calcium. The calcium affinity for exocytosis was roughly doubled by activation of PKC by a phorbol ester, whereas the size of the readily releasable pool was not greatly increased. The effect was due to activation of PKC, because it was blocked by a PKC inhibitor and was not mimicked by an inactive phorbol ester analogue. A similar increase in calcium sensitivity was induced by preincubation with gonadotropin-releasing hormone, the physiological releasing hormone. These findings provide direct evidence for physiological regulation of secretion by enhancement of Ca2+-sensing steps. Because exocytosis depends on the third- to fourth-power of intracellular free Ca2+ concentration, this mechanism ensures a powerful up-regulation of hormone release and may explain how PKC can stimulate exocytosis without an increase of Ca2+ above the resting level.
Keywords: PKC, Ca2+ sensitivity, vesicle pools
Neurons and endocrine cells release neurotransmitters and hormones by highly regulated exocytosis of secretory vesicles or granules. Factors implicated in this regulation include Ca2+, protein kinase C (PKC), protein kinase A, calmodulin, Munc13-1, and others (1–5). Here we focus on regulation by PKC. Activation of PKC by phorbol esters or diacylglycerols enlarges the readily releasable pool (RRP) of vesicles in chromaffin cells (3) and in hippocampal neurons (6). On the other hand, less direct work suggests that activation of PKC may increase the calcium sensitivity of secretion rather than RRP size in chick ciliary ganglion (7) and the calyx of Held (8). We now provide direct measurements supporting this latter conclusion in pituitary gonadotropes.
Intracellular Ca2+ is the principal regulator of stimulus–secretion coupling in many types of cells (9, 10), but there are examples of Ca2+-independent secretion (11), i.e., secretion that does not require elevated levels of Ca2+. They include secretion from epithelial cells stimulated by activating PKC or protein kinase A (12, 13), from mast cells dialyzed intracellularly with guanosine-5′-O-(3-thiotriphosphate) (GTPγS) (14, 15), or from insulin-secreting cells activated through latrophilin (16). Similarly, in pituitary gonadotropes, the resting secretion rate can be increased 10-fold by applying phorbol-12-myristate-13-acetate (PMA, an activator of PKC) with no detected Ca2+ elevation (17). In these cases, where exocytosis can be initiated independent of Ca2+, Ca2+ alone can trigger exocytosis as well. An important question then is whether or not these various triggering molecules are just increasing the Ca2+ sensitivity of exocytosis so that significant membrane fusion occurs even at resting intracellular free Ca2+ concentration ([Ca2+]i) levels. The normal steep dependence of exocytosis on [Ca2+]i means that even a small change in Ca2+ sensitivity could have large effects on the overall rate. Our work suggests that this model applies for gonadotropes.
Materials and Methods
Cell Culture and Solutions.
Isolated gonadotropes were prepared as described (18). The anterior pituitary was removed from 4- to 6-week-old male Sprague–Dawley rats that had been castrated at week 2 by the local supplier. Cells were used 2–4 days after dispersion. Experiments were done at 20–24°C in a bath solution containing 140 mM NaCl, 2.5 mM KCl, 1.3 mM CaCl2, 1 mM MgCl2, 10 mM Hepes, and 10 mM glucose (pH 7.4). At the end of each experiment, we confirmed that we were recording from a gonadotrope by challenging the cell with gonadotropin-releasing hormone (GnRH, 5 nM) and verifying a rapid, hormonally induced increase of [Ca2+]i.
Membrane Capacitance (Cm) Measurement.
Conventional whole-cell recordings used sylgard-coated 2–3 MΩ pipettes. Series resistance ranged from 4 to 12 MΩ. An EPC-9 patch-clamp amplifier was used together with PULSE+LOCK-IN software. A 977-Hz, 20-mV peak-to-peak sinusoidal voltage stimulus was superimposed on a holding potential of −80 mV. Currents were filtered at 2.9 kHz and sampled at 15.6 kHz.
Generation and Measurement of [Ca2+]i Ramps.
To determine the [Ca2+]i sensitivity of exocytosis, ramp [Ca2+]i increases were generated by 10-s steady UV (380 nm) illumination (TILL Monochromator, TILL Photonics, Gräfelting, Germany) of caged-Ca2+, nitrophenyl-EGTA (NP-EGTA). The same light was also used to monitor [Ca2+]i increases by the low-affinity Ca2+ indicator, fura-6F. [Ca2+]i was calculated as follows: [Ca2+]i = Kd × (Fmax − F)/(F − Fmax/β), where β = Fmax/Fmin and was determined to be 13.3 in our system. Kd of fura-6F is 5.3 μM as published by Molecular Probes. Because the basal [Ca2+]i is buffered at ≈200 nM, far below the Kd of fura-6F, the initial fluorescence value can be used as Fmax. We neither measured the apparent Kd of fura-6F under our conditions nor compensated for photobleaching.
Flash Photolysis.
Flashes of UV light and fluorescence-excitation light were generated as described (19) except that we used a TILL flash lamp. The NP-EGTA-containing internal solutions consisted of 110 mM CsCl, 5 mM NP-EGTA, 2 mM NaCl, 4 mM CaCl2, 2 mM MgATP, 0.3 mM GTP, 0.2 mM fura-6F, and 35 mM Hepes. Its basal [Ca2+]i was measured to be ≈200 nM by fura-2. Pipette solutions were adjusted to pH 7.2 with HCl or CsOH. [Ca2+]i was calculated from the fluorescence ratio (20). All experiments were performed at room temperature. To get [ΔCm/Δt]max, we divided the change of capacitance at time τ/2 by Δt = τ/2, where τ is the time constant from the exponential fit of the burst.
Results
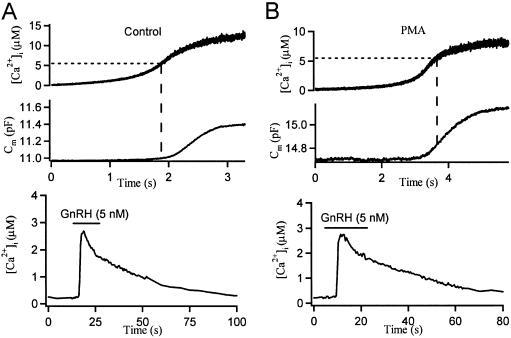
Identifying mechanisms for stimulation of exocytosis by PKC is complicated by the range of possible targets that may contribute in concert. Using pituitary gonadotropes, we simplified the task by photoreleasing intracellular Ca2+ from a caged-Ca2+ compound as the stimulus. By simultaneously monitoring Ca2+, with optical indicators, and exocytosis, using Cm as a measure of plasma membrane area, we could measure the size of the RRP and the Ca2+ sensitivity of exocytosis directly. Our first experiments used continuous UV illumination to uncage and ramp up [Ca2+]i slowly, giving in effect an intracellular Ca2+ titration of exocytosis. Because the RRP is released much faster than it is refilled, we focused on the initial period of rapid exocytosis. The steady UV illumination released Ca2+ from caged-Ca2+ (NP-EGTA) continuously, increasing [Ca2+]i gradually by ≈10 μM within seconds (Fig. 1A). The plasma membrane area (recorded as Cm) remained constant at the beginning when [Ca2+]i was low but at a certain point took off rapidly, adding 0.2–0.4 pF (20–40 μm2) of membrane as [Ca2+]i was rising. Then with depletion of the RRP, the change of Cm slowed to a rate that was limited by the resupply of secretory granules to the RRP. In the control cell shown, Cm just begins to rise at a [Ca2+]i of 5.5 μM (Fig. 1A, dashed line), whereas in the cell pretreated with 100 nM PMA, Cm is already in the middle of its rapid rising phase. To confirm that we were recording from gonadotropes, we challenged cells with GnRH (5 nM), at the end of the experiment, producing a rapid hormonally induced increase of [Ca2+]i (Fig. 1 Lower). This increase was not the normal oscillatory response of gonadotropes because the internal solution contained 5 mM NP-EGTA, with a very high buffering capacity. In fura-2-AM loaded cells without NP-EGTA, we did observe GnRH-induced [Ca2+]i oscillations (data not shown).
Fig 1.
PMA lowers the calcium threshold for secretion in gonadotropes. Steady UV illumination generates ramp [Ca2+]i increases with intracellular caged Ca2+ and measures [Ca2+]i by using a Ca2+ indicator, fura-6F. Simultaneous time courses of [Ca2+]i and Cm for a single control gonadotrope (A) and for a PMA-treated (for 2–3 min) gonadotrope (B). Note at the same [Ca2+]i level, Cm only begins to increase in the control cell, whereas it already takes off in the PMA-treated cell. At the end of the experiments, the cells were challenged by 5 nM GnRH to verify them as gonadotropes (Lower).
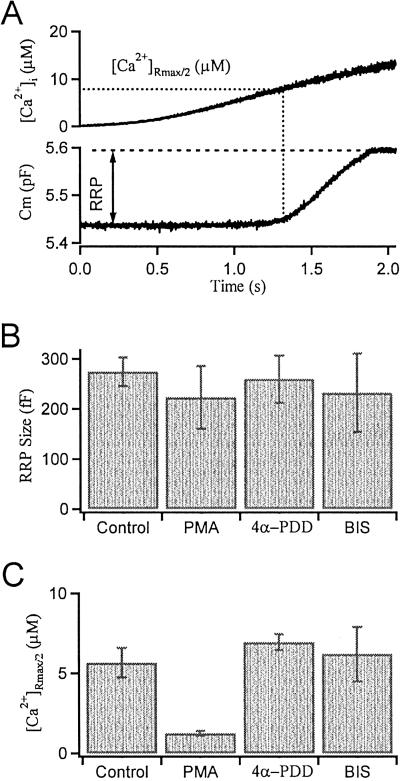
To quantify the effects of PMA, we took the [Ca2+]i level at the half-maximal rate of exocytosis as our index of the calcium sensitivity of exocytosis, where the rate was the slope of a polynomial fitted to the Cm traces (Fig. 2A). We also fitted a straight line to the last slow phase of the Cm increase and extrapolated it back to the time when the half-maximal rate was reached. The difference (ΔCm) between this extrapolated level and the baseline Cm was our measure of RRP size. By using a kinetic model of exocytosis that requires three Ca2+-binding steps before fusion (21), computer simulation demonstrated that this method of back extrapolation approximates the real RRP size within 10–20 fF (1–2 μm2). Unexpectedly, PMA did not increase the RRP size measured this way (Fig. 2B). Instead it greatly reduced the [Ca2+]i level needed to evoke a half-maximal rate of exocytosis ([Ca2+]Rmax/2) (from 5.66 ± 0.92 μM, mean ± SEM, in control gonadotropes to 1.29 ± 0.12 μM; Fig. 2C). This effect of PMA was not mimicked by 4α-phorbol-12,13-didecanoate (4α-PDD) (1 μM) and was blocked by the PKC inhibitor bisindolylmaleimide (BIS) (500 nM in both the bath and the patch pipette). The RRP size was not affected by 4α-PDD or BIS.
Fig 2.
Summary of PMA actions on exocytosis in gonadotropes. (A) Simultaneous time courses of [Ca2+]i and Cm for a single control gonadotrope illustrating how the RRP size and the [Ca2+]i threshold are determined by ramp [Ca2+]i experiments. (B) Pretreatments with PMA (100 nM), 4α-PDD (1 μM), and BIS (500 nM) have no significant effect on the RRP size (defined in text as ΔCm). (C) The calcium threshold (measured as [Ca2+]Rmax/2) is lowered by PMA, but not by 4α-PDD or by PMA in combination with BIS.
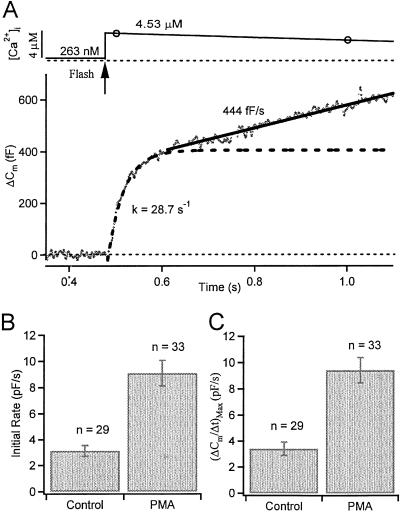
To validate conclusions drawn from our calcium-ramp experiments, we used flash photolysis of caged-Ca2+ to raise Ca2+ in a step-like manner. The Ca2+ step elicits a burst of exocytosis followed by a slow continuous component (Fig. 3A). We fitted the initial time course of the exocytotic burst by a second-order polynomial and took the slope of this line 20 ms after the flash as our measure of the initial rate of exocytosis (22). This initial rate tripled with 100 nM PMA (control, 3.13 ± 0.41 pF/s; PMA, 9.12 ± 0.97 pF/s; Fig. 3B), despite a smaller mean postflash [Ca2+]i level compared with controls (control, 5.73 ± 0.42 μM; PMA, 4.22 ± 0.27). Similarly, the maximum rate of secretion was increased (control, 3.40 ± 0.51 pF/s; PMA, 9.40 ± 0.96 pF/s; Fig. 3C).
Fig 3.
PMA increases the initial rate of exocytosis in gonadotropes. (A) Simultaneous measurements of [Ca2+]i level and membrane area Cm after a UV flash (arrow) uncages Ca2+ within 2 ms. Open circles are measured calcium points (continuously measured every 0.5 s). Cm increases in an early burst (fitted with single exponential with the rate constant, k = 1/τ indicated) followed by a sustained component (fitted with a straight line of the indicated slope). (B and C) PMA increases the initial rate of secretion (measured 20 ms after the flash) and the maximum rate.
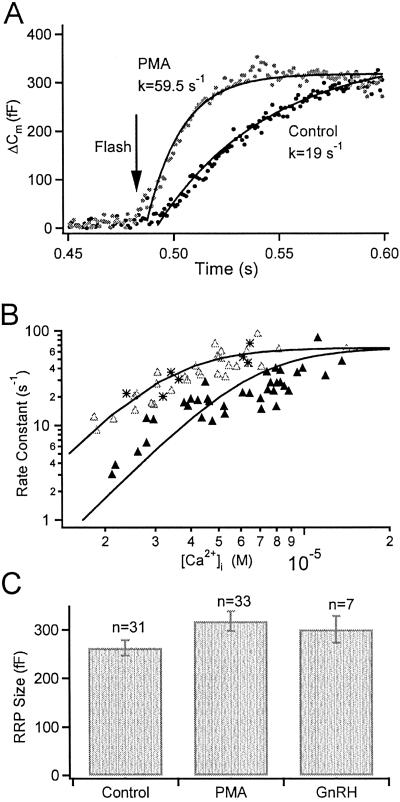
To compare the kinetics of exocytosis at similar [Ca2+]i levels, we averaged burst responses for experiments with postflash [Ca2+]i values (measured 20 ms after the flash) between 4 and 6 μM (mean [Ca2+]i values: control, 4.77 ± 0.19 μM, n = 10; PMA, 4.72 ± 0.16 μM, n = 15). The exocytotic burst of chromaffin cells must be fitted by two exponentials (23), but for gonadotropes it is reasonably fitted by one (Fig. 4A). The burst is speeded by PMA (in Fig. 4A the rate constant increases from 19 s−1 to 60 s−1). Plotting the rate constants against postflash [Ca2+]i levels gives the calcium dependence of fusion of the RRP, which shifts toward lower Ca2+ concentrations with PMA (Fig. 4B). The curves are the equation: Rate = R/(1 + (Kd/[Ca2+]i)3), where R is the maximum rate and Kd is the [Ca2+]i level where the rate reaches the half maximum. Restraining R to a common saturating rate, the fitted Kd were 6.71 ± 0.36 μM (control) and 3.39 ± 0.20 μM (PMA), consistent with the Ca2+-ramping experiments. Extrapolating the fitted curves predicts that PMA treatment would increase the release rate by 7.7-fold at the 100–200 nM resting [Ca2+]i level, in agreement with our previous observations that PMA induces spontaneous secretion from gonadotropes (17).
Fig 4.
PMA facilitates exocytosis in gonadotropes by increasing the calcium sensitivity of release without significantly altering RRP size. (A) Averaged Cm responses for control (filled symbols) and PMA (100 nM, open symbols) treated gonadotropes, summarizing experiments that had postflash [Ca2+]i values between 4 and 6 μM. Superimposed curves are single exponential fits with the rate constants indicated. (B) PMA increases the Ca2+ sensitivity of release (all experiments plotted individually). Rate constants for exponential fits of the exocytotic burst are plotted against calcium levels for control (filled triangles), PMA-treated (open triangles, 100 nM for 2–3 min), and GnRH-treated (asterisks, 5 nM for 2 min) gonadotropes, respectively. The continuous curves represent the equation: Rate = R/(1 + (Kd/[Ca2+]i)3). (C) RRP size is unchanged by PMA or GnRH preincubation.
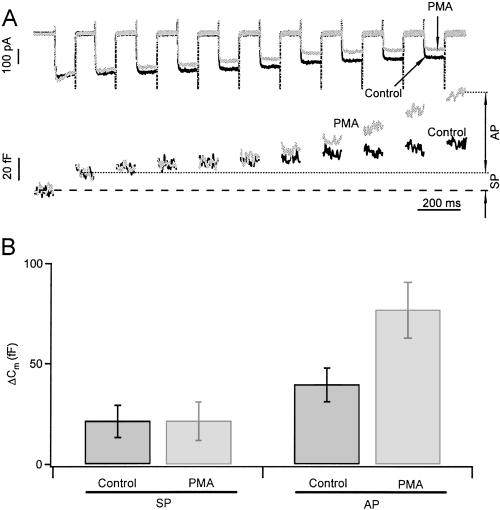
In contrast to many other excitable cells, in gonadotropes the physiological stimulus for secretion is usually not depolarization. Gonadotropes normally secrete in response to GnRH, which induces intracellular Ca2+ release via the phospholipase C and inositol trisphosphate pathway (24). Nevertheless, we were able to demonstrate the effect of PMA on exocytosis induced by depolarizing stimuli (Fig. 5). We used whole-cell patch clamp to monitor calcium channel current and Cm simultaneously. Trains of depolarizations elicited calcium influxes and small Cm increases. An initial small increase of Cm (≈20 fF) occurring within the first 100-ms depolarization to 0 mV probably corresponds to the fusion of readily releasable secretory granules close to the calcium channels. We will call these granules the synchronous pool. A subsequent, much slower increase of Cm may correspond to readily releasable granules that are farther away from calcium channels. We will call those granules the asynchronous pool. PMA (100 nM) treatment increased the amplitude of the asynchronous pool by a factor of 2.1 without altering that of the synchronous pool (Fig. 5). The increase of the asynchronous pool was not due to more calcium entry, because PMA actually decreased the calcium currents slightly (see Fig. 5A). However, the increase of the asynchronous pool with no change of the synchronous pool would be consistent with the following hypothesis: The RRP has not increased and the number of granules docked near calcium channels has not changed, but because the calcium sensitivity of the RRP has increased, the calcium spreading from each open calcium channel has a larger effective domain of action and can trigger fusion of more distant granules.
Fig 5.
PMA augments the size of the asynchronous pool (AP) but not the synchronous pool (SP) of secretory granules. (A) Calcium currents and Cm response to a train of ten 100-ms depolarizing pulses from −70 to 0 mV. Traces displayed are from the same cell, before and 3 min after PMA treatment. The change of Cm in response to the first depolarization defines the SP size and the subsequent increase in Cm defines the AP. PMA increased AP by a factor of 2 (despite a reduction on calcium currents), but left SP unaltered. (B) Summary of the effect of PMA on SP and AP from six cells.
GnRH, the natural secretagogue for gonadotropes, not only generates IP3 and Ca2+ elevations, but also activates PKC. Is this PKC activation physiologically relevant for sensitizing exocytosis? Indeed, 2-min pretreatments with GnRH (5 nM) increased the rate of the flash-induced exocytotic bursts (asterisks, Fig. 4B). Neither GnRH nor PMA affected RRP size (Fig. 4C).
Discussion
We provide direct evidence that activating PKC leads to a 2-fold rise in the apparent Ca2+ affinity of the exocytotic machinery without noticeable effect on the RRP size in gonadotropes. Previous studies of the chick ciliary ganglion synapse (7) and of the rat calyx of Held (8) suggested the same conclusion in those cells by using less direct methods. In contrast, PKC increases the RRP size in chromaffin cells (3) and hippocampal neurons (6). In addition, PKC activation increases the Ca2+ sensitivity of exocytosis in chromaffin cells by increasing the percentage of vesicles that are in a highly Ca2+-sensitive state (25). It is tempting to speculate how PKC activation increases the Ca2+ sensitivity for secretion in gonadotropes. In one hypothesis, the Ca2+ sensor of gonadotropes contains some isoform of synaptotagmin whose Ca2+ sensitivity can be modulated by phosphorylation. Alternatively, considering the calcium sensor and the soluble N-ethylmaleimide-sensitive fusion attachment protein receptor (SNARE) complex as an integrated fusion machine (26), one could envision that the fusion machine in gonadotropes is held in a low-affinity state by certain clamping proteins. Phosphorylation of the clamping proteins might release the clamp and sensitize secretion. It would be of great interest to know the nature of PKC action on the Ca2+ sensitivity of fusion and to identify the substrates of PKC.
It is somewhat puzzling that PKC has no effect on RRP size. As suggested in previous work, PKC increases the RRP size in chromaffin cells and hippocampal neurons. This effect has been attributed to the facilitation of a recruitment step to refill RRP. Speeding of refilling seems equally necessary in gonadotropes. Because the Ca2+ sensitivity of release is increased by PKC, the spontaneous release rate at resting [Ca2+]i should be profoundly facilitated as we expect from the cubic law of Ca2+ dependence of exocytosis. If the rate of RRP refilling were not increased as well by PKC, the RRP would be expected to shrink, which is not the case in our experiments. Thus, PKC seems to act by two distinctive mechanisms to modulate exocytosis: first, by accelerating the refilling of the RRP, possibly in all cell types, and second, by sensitizing the Ca2+ sensor of the exocytotic machinery in a few cell types.
The finding that PKC sensitizes the exocytotic machinery to Ca2+ provides an explanation for Ca2+-independent stimulation–secretion coupling in various cells (11): secretion without a calcium elevation. The 2-fold increase in the Ca2+ sensitivity of exocytosis that we find predicts an 8-fold increase in the extrapolated release rate at resting [Ca2+]i level, similar to what we have previously reported in gonadotropes (13). Similar direct measures of the Ca2+ sensitivity of release could now be applied to other systems where there is Ca2+-independent stimulation of secretion. In gonadotropes, sensitization of exocytosis by PKC should increase the efficacy of GnRH-stimulated secretion of gonadotropins from the pituitary during physiological GnRH pulses, which last 2–6 min (27). Sensitization may also extend the period of secretion beyond the time when the cytoplasmic Ca2+ falls to near resting levels.
It has been demonstrated that the synchronous pool (also called the immediately releasable pool) of granules constitutes only a small fraction (≈10%) of the RRP in gonadotropes (this study) and in other endocrine cells including adrenal chromaffin cells (28) and pancreatic beta cells (29). Because the vast majority of the RRP is far away from the calcium channels, any mechanism that modulates the release probability of these granules could have great importance in regulating hormone release. Enhancing the Ca2+ sensitivity of RRP would enable exocytosis at sites relatively distant from Ca2+ channels at intermediate or resting [Ca2+]i levels, and it may be a widespread pathway for secretory control.
Acknowledgments
We thank Drs. Wolfhard Almers, Kevin Gillis, Duk-Suh Koh, Erwin Neher, and Jane M. Sullivan for critical reading and helpful suggestions on the manuscript, and Xiahua Liang and Xiangping Xu for technical support. This work is supported by National Institutes of Health Grants AR17803 and HD12629, National Science Foundation of China Grants 30025023 and 30130230 (to T.X.), and National Basic Research Program of China (973) Grant G1999054000 (to T.X.).
Abbreviations
RRP, readily releasable pool
PMA, phorbol-12-myristate-13-acetate
Cm, membrane capacitance, GnRH, gonadotropin-releasing hormone
NP-EGTA, nitrophenyl-EGTA
[Ca2+]i, intracellular free Ca2+ concentration
4α-PDD, 4α-phorbol-12,13-didecanoate
BIS, bisindolylmaleimide
See commentary on page 16522.
References
- 1.Chen Y. A., Duvvuri, V., Schulman, H. & Scheller, R. H. (1999) J. Biol. Chem. 274, 26469-26476. [DOI] [PubMed] [Google Scholar]
- 2.Evans G. J. O., Wilkinson, M. C., Graham, M. E., Turner, K. M., Chamberlain, L. H., Burgoyne, R. D. & Morgan, A. (2001) J. Biol. Chem. 276, 47877-47885. [DOI] [PubMed] [Google Scholar]
- 3.Gillis K. D., Mossner, R. & Neher, E. (1996) Neuron 16, 1209-1220. [DOI] [PubMed] [Google Scholar]
- 4.Smith C., Moser, T., Xu, T. & Neher, E. (1998) Neuron 20, 1243-1253. [DOI] [PubMed] [Google Scholar]
- 5.Ashery U., Varoqueaux, F., Voets, T., Betz, A., Thakur, P., Koch, H., Neher, E., Brose, N. & Rettig, J. (2000) EMBO J. 19, 3586-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevens C. F. & Sullivan, J. M. (1998) Neuron 21, 885-893. [DOI] [PubMed] [Google Scholar]
- 7.Yawo H. (1999) J. Physiol. (London) 515, 169-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu X. S. & Wu, L. G. (2001) J. Neurosci. 21, 7928-7936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douglas W. W. (1968) Br. J. Pharmacol. 34, 453-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz B. & Miledi, R. (1967) J. Physiol. (London) 189, 535-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hille B., Billiard, J., Babcock, D. F., Nguyen, T. & Koh, D. S. (1999) J. Physiol. (London) 520, 23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott C. E., Abdullah, L. H. & Davis, C. W. (1998) Am. J. Physiol. 275, C285-C292. [DOI] [PubMed] [Google Scholar]
- 13.Koh D. S., Moody, M. W., Nguyen, T. D. & Hille, B. (2000) J. Gen. Physiol. 116, 507-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomperts B. D. (1983) Nature 306, 64-66. [DOI] [PubMed] [Google Scholar]
- 15.Almers W. & Neher, E. (1987) J. Physiol. 386, 205-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang J., Ushkaryov, Y., Grasso, A. & Wollheim, C. B. (1998) EMBO J. 17, 648-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Billiard J., Koh, D. S., Babcock, D. F. & Hille, B. (1997) Proc. Natl. Acad. Sci. USA 94, 12192-12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaftan E. J., Xu, T., Abercrombie, R. F. & Hille, B. (2000) J. Biol. Chem. 275, 25465-25470. [DOI] [PubMed] [Google Scholar]
- 19.Xu T., Naraghi, M., Kang, H. & Neher, E. (1997) Biophys. J. 73, 532-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grynkiewiez G., Poenie, M. & Tsien, R. (1985) J. Biol. Chem. 260, 3440-3450. [PubMed] [Google Scholar]
- 21.Heinemann C., Chow, R. H., Neher, E. & Zucker, R. S. (1994) Biophys. J. 67, 2546-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tse F. W., Tse, A., Hille, B., Horstman, H. & Almers, W. (1997) Neuron 18, 121-132. [DOI] [PubMed] [Google Scholar]
- 23.Xu T., Binz, T., Niemann, H. & Neher, E. (1998) Nat. Neurosci. 1, 192-200. [DOI] [PubMed] [Google Scholar]
- 24.Tse A., Tse, F. W., Almers, W. & Hille, B. (1993) Science 260, 82-84. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y., Udayasankar, S., Dunning, J., Chen, P. & Gillis, K. D. (2002) Proc. Natl. Acad. Sci. USA 99, 17060-17065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorensen J. B., Matti, U., Wei, S. H., Nehring, R. B., Voets, T., Ashery, U., Binz, T., Neher, E. & Rettig, J. (2002) Proc. Natl. Acad. Sci. USA 99, 1627-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moenter S. M., Brand, R. M., Midgley, A. R. & Karsch, F. J. (1992) Endocrinology 130, 503-510. [DOI] [PubMed] [Google Scholar]
- 28.Voets T., Neher, E. & Moser, T. (1999) Neuron 23, 607-615. [DOI] [PubMed] [Google Scholar]
- 29.Barg S., Ma, A., Eliasson, L., Galvanovskis, J., Gopel, S., Obermuller, S., Platzer, J., Renstrom, E., Trus, M., Atlas, D., et al. (2001) Biophys. J. 81, 3308-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]