Abstract
Hemolytic-uremic syndrome, the main cause of acute renal failure in early childhood, is caused primarily by intestinal infections from some Escherichia coli strains that produce Shiga toxins. The toxins released in the gut are targeted to renal endothelium after binding to polymorphonuclear leukocytes. The presence of Shiga toxins in the feces and the circulating neutrophils of 20 children with hemolytic uremic syndrome was evaluated by the Vero cell cytotoxicity assay and flow cytometric analysis, respectively. The latter showed the presence of Shiga toxins on the polymorphonuclear leukocytes of 13 patients, 5 of whom had no other microbiologic or serologic evidence of infection by Shiga toxin-producing Escherichia coli. A positive relationship was observed between the amounts of Shiga toxins released in the intestinal lumen and those released in the bloodstream. The toxins were detectable on the neutrophils for a median period of 5 days after they were no longer detectable in stools. This investigation confirms that the immunodetection of Shiga toxins on neutrophils is a valuable tool for laboratory diagnosis of Shiga toxin-producing Escherichia coli infection in hemolytic-uremic syndrome and provides clues for further studies on the role of neutrophils in the pathogenesis of this syndrome.
Hemolytic-uremic syndrome (HUS) is the most common cause of acute renal failure in children and is characterized by thrombocytopenia and microangiopathic hemolytic anemia (25). Most HUS cases occur as a complication of intestinal infections with Shiga toxin-producing Escherichia coli (STEC) (12, 13, 24).
STEC produce two main types of toxins, Shiga toxin 1 (Stx1) and Shiga toxin 2 (Stx2), which are composed of A and B subunits. The latter mediates the binding to glycolipid receptors (globotriaosylceramide) present on the surface of target cells (19). After endocytosis, an enzymatically active fragment (9) of the A subunit cleaves the bond connecting adenine to the sugar of 28S rRNA (8) and DNA (3, 4), thus causing the arrest of protein synthesis (8) and the formation of apurinic sites in the nucleus (4, 20). The final result of these intracellular injuries is the triggering of apoptosis (4, 17).
The pathogenetic process of STEC infection initially involves colonization of the gut (19). STEC serogroups mainly associated with HUS, like E. coli O157 and E. coli O26 (12, 24), adhere to the intestinal mucosa with a characteristic “attaching-and-effacing” mechanism (18). Afterwards, they release large amounts of Shiga toxins in the intestinal lumen, which damage villus epithelial cells and are absorbed into the circulation and targeted to the renal endothelium (19). The presence of free Shiga toxins in the intestinal lumen can be detected by either cell toxicity or immunological assays, and such a detection represents a useful tool for laboratory diagnosis of STEC infections (13, 24).
Shiga toxins, during their journey from gut to renal endothelium, bind to circulating polymorphonuclear leukocytes (PMN) through a low-affinity unknown receptor (22). PMN apparently do not internalize Shiga toxins, carrying them to renal endothelial cells that express high-affinity globotriaosylceramide receptors (22). Shiga toxins bound to circulating PMN can be detected by specific antibodies and flow cytometric techniques (21, 22). Recently, this finding has been exploited for the diagnosis of STEC infection in HUS patients (21, 23).
The aim of the present study was to detect the presence and measure the quantity of Shiga toxins over time in both the feces and the PMN of children with HUS in order to evaluate (i) the kinetic of the toxin in the organism during the course of natural disease and (ii) the time periods in which the Shiga toxin-based assays can be used for the diagnosis of STEC infections in HUS patients.
MATERIALS AND METHODS
Patients.
Since 1988, the etiology of HUS in Italy is routinely and continuously studied through the cooperation of clinical pediatric nephrology centers and a national reference laboratory (Istituto Superiore di Sanità, Rome, Italy) for the identification of STEC infection. The National Registry of HUS includes all national centers for dialysis for pediatric patients. Participants in the present study were 20 consecutive HUS patients (less than 15 years old) admitted during 2003 and 2004 to three pediatric hospitals (Ospedale Pediatrico Bambino Gesù, Rome; Ospedale Santobono, Naples; and Clinica Pediatrica De Marchi, Milan) participating in the Italian surveillance system of HUS (11, 24). A case of HUS was defined according to the following diagnostic criteria: acute microangiopathic hemolytic anemia, thrombocytopenia (platelet count, <100,000 mm3), and acute renal injury (11, 24). The definition has been in place in the Registry since its start, and it is consistent with the majority of the international HUS studies. Patients were prospectively enrolled in the study after informed consent was obtained. The first feces and blood specimens were collected as soon as possible after admission into the hospital. Further samples were collected when possible during the subsequent days. Feces and sera were stored at −20°C. Whole-blood samples for detection of Shiga toxins on PMN cells were stored at room temperature after treatment with EDTA and processed within 24 h of sampling.
Detection of free Shiga toxins in feces.
Stool specimens were examined for the presence of free Shiga toxins by the Vero cell cytotoxicity assay as described previously (5, 6). Briefly, feces were diluted 1:10 in phosphate-buffered saline, and fecal extracts were obtained by centrifugation and filtration of the supernatant through 0.45-μm membrane filters. Doubling dilutions of the filtrates were inoculated into Vero cell monolayers (0.02 ml into 0.2 ml of cell culture medium), and the titer of the cytotoxic activity was expressed as the reciprocal of the highest dilution inducing a cytopathic effect after 3 days of incubation. Shiga toxins were identified by neutralization tests done by adding an equal volume of rabbit antiserum to Stx1 and Stx2, separately or together, to the positive fecal extract (6).
Detection of Shiga toxins bound to PMN.
Shiga toxins bound to PMN were detected by flow cytometry as previously described (21). The assay has been validated by comparing control subjects and HUS patients in a blinded fashion (21). The problem of false-positive results due to binding of anti-Stx antibodies to neutrophil Fc receptors was addressed by including human serum in the assay (21). Briefly, white blood cells isolated after erythrocytic lysis from 100 μl of blood were incubated with anti-Stx mouse monoclonal antibodies in the presence of human serum. After incubation with fluorescein isothiocyanate-goat anti-mouse immunoglobulin G, flow cytometric analysis was utilized to reveal the cell-bound fluorescence. The flow cytometer was set to acquire and gate events by forward scatter versus 90° side scatter and by green fluorescence versus 90° side scatter. This set resulted in a prompt analysis of both morphology and fluorescence, allowing a clear evaluation of control and positive samples. The mean channel value (MCV) of fluorescence of the PMN population was chosen as an objective parameter to measure the extent of binding of Shiga toxins to PMN (21). The cutoff MCV was 0.3, and values between 0.3 and 0.6 were considered borderline (21). Two PMN-positive patients were randomly selected, and their leukocytes were assayed as described above with a negative control monoclonal antibody against CD3, an antigen absent on the PMN membrane. The results of the assay were negative, thus excluding the possibility of nonspecific binding.
Other laboratory investigations for diagnosis of STEC infection.
For STEC isolation, feces were streaked onto MacConkey agar plates, and colony sweeps were tested for Shiga toxin production by the Vero cell assay and for the presence of stx genes by PCR amplification (24). Serum samples were tested for antibodies to the lipopolysaccharide (LPS) of five major STEC serogroups (O157, O26, O103, O111, and O145) by enzyme-linked immunosorbent assay and immunoblotting, as previously described (6, 7).
Data analysis.
Personal, clinical, and laboratory data were stored and analyzed with Microsoft Excel.
RESULTS
Patients and sampling.
Twenty consecutive HUS patients were enrolled in the study. Their median age was 26 months (range, 6 to 93), and 15 of them were males. Seventeen cases had prodromal diarrhea, which had a median duration of 3 days (range, 1 to 18) and was bloody in 12 cases. A total of 63 stool specimens were collected from the 20 patients (range per patient, 1 to 7). In the patients with prodromal diarrhea, the median interval between onset of diarrhea and collection of the first specimen was 9 days (range, 6 to 19). In the three patients without diarrhea, the specimens were collected 4 days (range, 4 to 6) after hospitalization. A total of 50 blood specimens were collected from the 20 patients (range per patient, 1 to 5) for PMN analysis. In the patients with prodromal diarrhea, the median interval between the onset of diarrhea and collection of the first specimen was 10 days (range, 5 to 21). In the three patients without diarrhea, the specimens were collected 5 days (range, 3 to 7) after hospitalization. Twenty-three serum specimens were collected from 18 patients (range per patient, 1 to 3). In the patients with prodromal diarrhea, the median interval between the onset of diarrhea and collection of the first specimen was 9 days (range, 6 to 29).
Evidence of STEC infections.
Of the 20 patients enrolled, 18 underwent the complete diagnostic panel for STEC infection, while two of them were not examined for LPS antibodies. The results of the different assays are reported in Table 1 according to the presence of prodromal diarrhea. Evidence of STEC infection was observed in 18 cases (90%) (Table 1). In particular, STEC strains were isolated from three patients (15%); the strains belonged to serogroups O26, O121, and O157, respectively, and all possessed stx2 genes. Free Shiga toxins were detected in the feces of six patients (30%), including the three positive for STEC: four patients had Stx2, one had Stx1, and one had both toxins. There was concordance between the genotype of the infecting STEC strains (stx2) and the toxin detected in the stools (Stx2). LPS antibodies were detected in the sera of 9 of the 18 patients examined (50%). Antibodies to O26 were detected in six cases, antibodies to O157 were detected in two cases, and antibodies to O103 were detected in one case. The patient with STEC O157 in the stool had antibodies to the O157 LPS, while the patient with STEC O26 had no LPS antibodies.
TABLE 1.
Evidence of STEC infection in 20 HUS patients
| Diagnostic assay (no. of cases examined) | No. of positive HUS cases
|
All (%) (n = 20) | |
|---|---|---|---|
| With diarrhea (n = 17) | Without diarrhea (n = 3) | ||
| STEC isolation (20) | 2 | 1 | 3 (15) |
| Fecal Shiga toxins (20) | 4 | 2 | 6 (30) |
| LPS antibodies (18) | 8 | 1 | 9 (50) |
| Shiga toxins on PMN (20) | 11 | 2 | 13 (65) |
| Any evidence (20) | 15 | 3 | 18 (90) |
Flow cytometric analysis showed the presence of Shiga toxins on the circulating PMN from 13 of the 20 HUS cases (65%). Eight patients also had serological or microbiological evidence of STEC infection, but Shiga toxin was detected in the PMN of five patients who had no other evidence of STEC infection. Conversely, PMN were found to be negative in four patients with LPS antibodies and in one patient positive for STEC O157, fecal Shiga toxins, and O157 antibodies.
Presence of Shiga toxins in feces and blood.
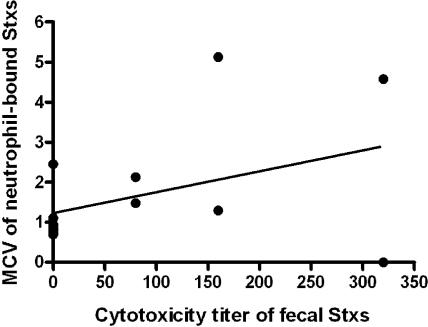
Figure 1 shows the amounts of Shiga toxins detected in PMN and stools from the 14 patients who were positive in at least one of the two assays. The data plotted for each case refer to the PMN and stool samples that showed the highest Shiga toxin concentration. The highest MCV observed for some samples were similar to the maximum Stx-binding capacity of PMN from healthy donors (MCV, ∼3), as previously determined in vitro by the same cytofluorimetric assay (21). Therefore, an MCV of less than 1 corresponded to a mean saturation of PMN receptors lower than 30% and was usually observed in patients with Shiga toxin-negative stool samples, with the exception of one patient, who had a high Shiga toxin titer in the feces. In all cases but one, MCV values higher than 1 corresponded to detectable levels of Shiga toxin in the feces.
FIG. 1.
Relationship between the amount of Shiga toxin (Stxs) detected in the feces and blood PMN of HUS patients. Data refer to 14 patients who were positive in at least one of the two assays. Shiga toxin titers in the feces are expressed as the reciprocal of the highest dilution inducing a cytopathic effect in Vero cells. The concentration of Shiga toxins in PMN is expressed as the MCV of fluorescence of the PMN population (21). The data plotted for each case refer to the PMN and stool samples that showed the highest Shiga toxin concentration. The straight line represents the linear regression fitted on MCV and cytotoxicity dilution titers.
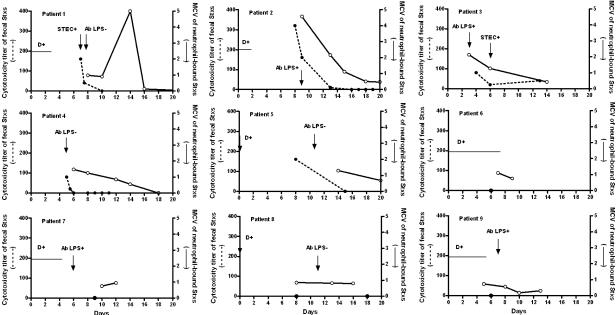
The time courses of Shiga toxin detection in feces and blood PMN from nine patients are compared in Fig. 2. The patients chosen to settle the synoptic panel were the most informative with regard to the number of diagnostic determinations performed. In patients 1 to 5, both of the Shiga toxin assays were positive for the first samples, and then the curve of intestinal detection showed a rapid fall, whereas a progressive slow decrease was observed in the curve of PMN detection. Subsequently, Shiga toxins were no longer detectable in the gut, although they were still present on PMN. The data from patients 6 to 9 confirmed this behavior, since they had negative stools but detectable amounts of PMN-bound Shiga toxins. In these nine patients, the time of Shiga toxin persistence on PMN with concomitant Shiga toxin-negative stools was calculated from the first positive determination to the last positive assay and gave a median value of 5 days (range, 3 to 9). The blood half-life of Shiga toxins calculated on the same patients was 4 days (median; range, 1 to 6).
FIG. 2.
Time courses of Shiga toxin (Stxs) detection in feces and blood PMN from nine HUS patients. The patients are numbered in decreasing rank of MCV of fluorescence and temporally aligned starting from the first day of prodromal diarrhea or from the day of HUS diagnosis. The results of other diagnostic tests for STEC infection are indicated. Ab, antibody.
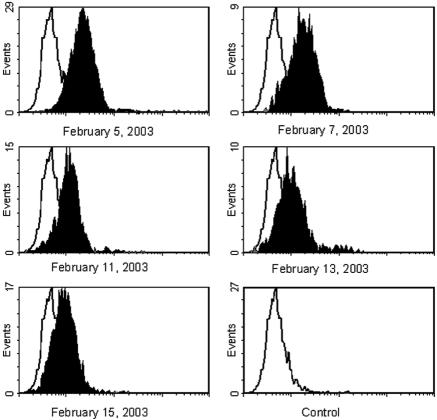
The cytogram profiles of different determinations performed on the PMN from a representative patient (Fig. 2, patient 4) are depicted in Fig. 3. The analysis showed a single Shiga toxin-positive PMN population that progressively reduced its MCV, reaching the basal fluorescence value of control samples after several days.
FIG. 3.
Cytogram profiles of different determinations performed on the PMN from a representative HUS patient.
DISCUSSION
The study of this group of HUS patients indicates that the Shiga toxins bound to circulating PMN are still detectable several days after the onset of prodromal diarrhea or even the diagnosis of HUS, thus confirming the data obtained previously by te Loo et al. (23).
In general, a positive relationship was observed between the Shiga toxin MCV on PMN and the amount of toxin detected in the stools, with the exception of one patient, who had a high titer of fecal Shiga toxin but a negative PMN. Accordingly, the lowest values of MCV (less than 1) were observed in the six PMN-positive patients with negative stools. These results suggest that the amount of Shiga toxin translocated through the gut mucosa to the bloodstream depends on the quantity of toxin released in the intestinal lumen.
The analysis of repeated samples allowed us to define the time course of Shiga toxin detection in feces and blood PMN. The results showed that the half-life of Shiga toxins in blood was around 4 days and that the toxins were still detectable in the PMN for a median period of 5 days after they were no longer detectable in stools.
The persistence of PMN-bound Shiga toxins in blood when the toxins are no longer detectable in feces is somehow surprising if the known properties of circulating neutrophils are considered. The mature PMN of the blood is a highly specialized nondividing cell with a short (1 or 2 days) life span (2). A very large number of PMN (1011 cells) leave the blood each day, replaced by the new mature cells that enter in the blood from the bone marrow, and the blood half-life of PMN is considered to be about 6 to 7 h (1). On this basis, the Shiga toxin-positive PMN that leave the blood and/or deliver Shiga toxins to endothelium should be replaced by new Shiga toxin-positive cells only until fresh toxin is produced in the gut and crosses the gut mucosa. Therefore, when the toxin is no longer present in the stools, one would expect that the Shiga toxin-positive PMN must disappear from the blood within the following 24 h.
On the contrary, our study consistently showed that Shiga toxins can be found in circulating PMN several days after the observation of the last positive stool sample. This finding does not seem related to differences in sensitivity between the Vero cytotoxicity assay and the flow cytometric analysis, respectively employed to reveal the presence of Shiga toxins in feces and in blood, with the limit of detection of the first assay (about 10 fmol) (16) being lower than that of the latter (about 25 fmol) (21).
The persistence of Shiga toxin-positive PMN could be explained by two different and partially concurrent hypotheses: (i) Shiga toxins on the PMN membrane might constitute a pool of molecules that pass rapidly between different PMN cells with a transfer of the toxin from older PMN to new mature neutrophils entering circulation from bone marrow, and (ii) Shiga toxins might induce a profound modification of the biology of PMN by inducing a prolongation of the blood half-life and of the functional life span of these cells.
The hypothesis of a transfer of Shiga toxins from older PMN to new mature cells is supported by the cytogram profiles observed in our study and shown in Fig. 3, in which the simultaneous presence of two peaks of fluorescence corresponding to Shiga toxin-positive and Shiga toxin-negative PMN was not observed.
The second hypothesis is in part sustained by data reported previously by Liu and colleagues (14, 15), which demonstrated an inhibitory effect of Stx2 on neutrophil apoptosis in vitro after 48 h. However, to our knowledge, studies on the effect of Shiga toxins on the spontaneous apoptosis of PMN at 5 days as well as investigations on the effect of Shiga toxins on PMN blood half-life are not available. Further studies on in vitro models are obviously needed to clarify this matter.
Although the present study was focused on HUS patients already suffering from endothelial renal damage, the mechanisms proposed to explain the behavior of PMN carrying Shiga toxins might also be operative in the early phase of the pathogenesis that precedes renal damage. It should be noted that the extension of PMN life span and blood half-life could contribute to the high level of neutrophilia often observed in HUS patients at presentation (26).
This study also confirms that the immunodetection of the Shiga toxins bound to circulating PMN has to be considered as a valuable tool for laboratory diagnosis of STEC infection in HUS patients. STEC infection was indeed detected by this method in 13 out of 20 patients examined, including 5 who were negative for the presence of antibodies to the LPS of major STEC serogroups and/or STEC and Shiga toxins in the stools. On the contrary, the PMN assay was negative in five patients with serologic and/or microbiologic evidence of STEC infection. The longer persistence of Shiga toxins in blood represents the major advantage of this method with respect to the detection of STEC/Shiga toxin in the stool. Conversely, the main drawbacks are that (i) it does not provide information on the serogroup of the infecting STEC strains, which is of great epidemiologic importance, and (ii) it is more difficult to perform with respect to commercially available immunoassays for the detection of Shiga toxins in the stool (10).
In conclusion, this study (i) indicates that Shiga toxins bound to circulating PMN of HUS patients are still detectable several days after the onset of prodromal diarrhea or even the diagnosis of the syndrome, (ii) confirms that their immunodetection is a valuable tool for laboratory diagnosis of STEC infection in HUS patients, and (iii) provides clues for further studies on the role of PMN in the pathogenesis of HUS.
Acknowledgments
This work was financed by the University of Bologna (funds for selected research topics) and COFIN 2004 (M.B.).
Footnotes
This paper is dedicated to the memory of Gianfranco Rizzoni, who created the Study Group on HUS of the Italian Society for Pediatric Nephrology.
REFERENCES
- 1.Athens, J. W. 1969. Granulocyte kinetics in health and disease, p. 135-155. In National Cancer Institute (ed.), Human tumor cell kinetics. National Cancer Institute, Bethesda, Md. [PubMed]
- 2.Bainton, D. F., J. L. Ullyot, and M. G. Farquhar. 1971. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J. Exp. Med. 134:1907-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbieri, L., P. Valbonesi, M. Brigotti, L. Montanaro, F. Stirpe, and S. Sperti. 1998. Shiga-like toxin I is a polynucleotide:adenosine glycosidase. Mol. Microbiol. 29:661-662. [DOI] [PubMed] [Google Scholar]
- 4.Brigotti, M., R. Alfieri, P. Sestili, M. Bonelli, P. G. Petronini, A. Guidarelli, L. Barbieri, F. Stirpe, and S. Sperti. 2002. Damage to nuclear DNA induced by Shiga toxin 1 and by ricin in human endothelial cells. FASEB J. 16:365-372. [DOI] [PubMed] [Google Scholar]
- 5.Caprioli, A., I. Luzzi, A. Gianviti, H. Russmann, and H. Karch. 1995. Pheno-genotyping of verotoxin 2 (VT2)-producing Escherichia coli causing haemorrhagic colitis and haemolytic uraemic syndrome by direct analysis of patients' stools. J. Med. Microbiol. 43:348-353. [DOI] [PubMed] [Google Scholar]
- 6.Caprioli, A., I. Luzzi, F. Rosmini, P. Pasquini, R. Cirrincione, A. Gianviti, M. C. Matteucci, and G. Rizzoni. 1992. Hemolytic-uremic syndrome and Vero cytotoxin-producing Escherichia coli infection in Italy. J. Infect. Dis. 166:154-158. [DOI] [PubMed] [Google Scholar]
- 7.Caprioli, A., I. Luzzi, F. Rosmini, C. Resti, A. Edefonti, F. Perfumo, C. Farina, A. Goglio, A. Gianviti, and G. Rizzoni. 1994. Community-wide outbreak of hemolytic-uremic syndrome associated with non-O157 verocytotoxin-producing Escherichia coli. J. Infect. Dis. 169:208-211. [DOI] [PubMed] [Google Scholar]
- 8.Endo, Y., K. Tsurugi, T. Yutsudo, Y. Takeda, T. Ogasawara, and K. Igarashi. 1988. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur. J. Biochem. 171:45-50. [DOI] [PubMed] [Google Scholar]
- 9.Garred, O., B. van Deurs, and K. Sandvig. 1995. Furin-induced cleavage and activation of Shiga toxin. J. Biol. Chem. 270:10817-10821. [DOI] [PubMed] [Google Scholar]
- 10.Gavin, P. J., and R. B. Thomson. 2004. Diagnosis of enterohemorrhagic Escherichia coli infection by detection of Shiga toxins. Clin. Microbiol. Newsl. 26:49-54. [Google Scholar]
- 11.Gianviti, A., A. E. Tozzi, L. De Petris, A. Caprioli, L. Rava, A. Edefonti, G. Ardissino, G. Montini, G. Zacchello, A. Ferretti, C. Pecoraro, T. De Palo, A. Caringella, M. Gaido, R. Coppo, F. Perfumo, N. Miglietti, I. Ratsche, R. Penza, G. Capasso, S. Maringhini, S. Li Volti, C. Setzu, M. Pennesi, A. Bettinelli, L. Peratoner, I. Pela, E. Salvaggio, G. Lama, S. Maffei, and G. Rizzoni. 2003. Risk factors for poor renal prognosis in children with hemolytic uremic syndrome. Pediatr. Nephrol. 18:1229-1235. [DOI] [PubMed] [Google Scholar]
- 12.Griffin, P. M., and R. V. Tauxe. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13:60-98. [DOI] [PubMed] [Google Scholar]
- 13.Karmali, M. A., M. Petric, C. Lim, P. C. Fleming, G. S. Arbus, and H. Lior. 1985. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J. Infect. Dis. 151:775-782. [DOI] [PubMed] [Google Scholar]
- 14.Liu, J., T. Akahoshi, T. Sasahana, H. Kitasato, R. Namai, T. Sasaki, M. Inoue, and H. Kondo. 1999. Inhibition of neutrophil apoptosis by verotoxin 2 derived from Escherichia coli O157:H7. Infect. Immun. 67:6203-6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu, J., Y. He, T. He, Z. Zhang, T. Akahoshi, H. Kondo, and S. Zhong. 2002. Prolongation of functional life-span of neutrophils by recombinant verotoxin 2. Chin. Med. J. 115:900-903. [PubMed] [Google Scholar]
- 16.Melton-Celsa, A. R., and A. O'Brien. 1998. Structure, biology, and relative toxicity of Shiga toxin family members for cells and animals, p. 121-128. In J. D. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. American Society for Microbiology, Washington, D.C.
- 17.Nakao, H., and T. Takeda. 2000. Escherichia coli Shiga toxin. J. Nat. Toxins 9:299-313. [PubMed] [Google Scholar]
- 18.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paton, J. C., and A. W. Paton. 1998. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 11:450-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sestili, P., R. Alfieri, D. Carnicelli, C. Martinelli, L. Barbieri, F. Stirpe, M. Bonelli, P. G. Petronini, and M. Brigotti. 2005. Shiga toxin 1 and ricin inhibit the repair of H2O2-induced DNA single strand breaks in cultured mammalian cells. DNA Repair (Amsterdam) 4:271-277. [DOI] [PubMed] [Google Scholar]
- 21.Tazzari, P. L., F. Ricci, D. Carnicelli, A. Caprioli, A. E. Tozzi, G. Rizzoni, R. Conte, and M. Brigotti. 2004. Flow cytometry detection of Shiga toxins in the blood from children with hemolytic uremic syndrome. Cytom. B Clin. Cytom. 61:40-44. [DOI] [PubMed] [Google Scholar]
- 22.te Loo, D. M., L. A. Monnens, T. J. van der Velden, M. A. Vermeer, F. Preyers, P. N. Demacker, L. P. van den Heuvel, and V. W. van Hinsbergh. 2000. Binding and transfer of verocytotoxin by polymorphonuclear leukocytes in hemolytic uremic syndrome. Blood 95:3396-3402. [PubMed] [Google Scholar]
- 23.te Loo, D. M., V. W. van Hinsbergh, L. P. van den Heuvel, and L. A. Monnens. 2001. Detection of verocytotoxin bound to circulating polymorphonuclear leukocytes of patients with hemolytic uremic syndrome. J. Am. Soc. Nephrol. 12:800-806. [DOI] [PubMed] [Google Scholar]
- 24.Tozzi, A. E., A. Caprioli, F. Minelli, A. Gianviti, L. De Petris, A. Edefonti, G. Montini, A. Ferretti, T. De Palo, M. Gaido, and G. Rizzoni. 2003. Shiga toxin-producing Escherichia coli infections associated with hemolytic uremic syndrome, Italy, 1988-2000. Emerg. Infect. Dis. 9:106-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trompeter, R. S., R. Schwartz, C. Chantler, M. J. Dillon, G. B. Haycock, R. Kay, and T. M. Barratt. 1983. Haemolytic-uremic syndrome: an analysis of prognostic features. Arch. Dis. Child. 58:101-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walters, M. D., I. U. Matthei, R. Kay, M. J. Dillon, and T. M. Barratt. 1989. The polymorphonuclear leucocyte count in childhood haemolytic uraemic syndrome. Pediatr. Nephrol. 3:130-134. [DOI] [PubMed] [Google Scholar]