Abstract
Throat swabs are regarded as the “gold standard” for diagnosing streptococcal pharyngitis and for surveillance research. Culturing throats in remote tropical settings is logistically difficult, and these settings are commonly burdened by high rates of streptococcal disease. The survival of streptococci on swabs may depend on whether they are of “throat” or “skin” type, as determined by emm pattern typing. The aims of this study were to compare the recovery rates of beta-hemolytic streptococci (BHS) using three different transport methods and to determine whether the recovery rates correlated with the emm pattern type. Monthly duplicate throat swabs were taken from occupants of selected households in three remote Aboriginal communities. Paired swabs were separated and handled in one of three ways: (i) direct inoculation onto culture media with cold-box transport (plated), (ii) sealed in a bag with a silica gel desiccant and cold-box transport (desiccant), and (iii) transport at ambient temperature and humidity (ambient). emm pattern typing was done by standard methods. Over 23 months, 4,842 throat swabs were taken, and 4,122 were paired. BHS were recovered on 11.5% of the 4,842 occasions (group A, 4.5%; group C, 1.7%; group G, 5.4%). Results from paired swabs showed the plated method was superior to desiccant and desiccant was better than ambient. Pooled data indicated that plated and desiccant were equivalent, and both were significantly better than ambient. There was no correlation between the emm pattern type and recovery of group A streptococci by different methods. In tropical and remote settings, cold-box transport with desiccant and subsequent inoculation of culture plates in the laboratory is a practical alternative to direct plating.
There are two reasons for swabbing the throat to recover beta-hemolytic streptococci (BHS)—for diagnosis of streptococcal pharyngitis as part of clinical management, and for research purposes. Even in the best of circumstances, throat swabs and cultures have limitations; these include variability of sampling, lack of standardized laboratory methods, difficulties with interpretation in an asymptomatic carrier, and a relatively long turnaround time (12, 17, 24). Tropical and remote settings pose particular challenges for getting throat swabs to laboratories; these include high temperatures and humidity, the frequent need to collect specimens in the outdoors, limited local infrastructure (including refrigeration), difficult communications, and long distances for transportation of materials and specimens (35). Laboratory facilities are often the least accessible in places where the burdens of streptococcal disease and postinfectious sequelae are highest.
The development of rapid antigen tests for group A beta-hemolytic streptococci (GAS) in the 1980s promised to facilitate rapid diagnosis. However, antigen testing may not be practical or cost-effective in many resource-poor settings with high rates of BHS infection (22). Newer tests, including gene probe technology and real-time PCR, are both sensitive and specific (8), but still more costly.
The collection and transport of throat swabs in the field pose more difficulties than collection of swabs from skin lesions. Beta-hemolytic streptococci, mainly GAS, are usually abundant in skin sores and survive the journey to the laboratory without too much difficulty, whereas BHS survival on throat swabs is less certain (1, 10, 32, 37). Direct inoculation of culture plates at the time of collection, or close to the time of collection, remains the “gold standard.” However, a delay of 6 h before being plated appears not to significantly effect the recovery of isolates; even at 24 h in a cool or temperate climate, the loss may be minimal (7, 31).
Methods used for recovery of BHS from throat swabs have also included transport media (Amies and Stuart's), enrichment media, and impregnated filter paper systems.
Transportation of swabs in the dry state, with a silica gel desiccant, was shown to improve recovery of BHS from throat swabs 35 years ago (29), but this was in a temperate climate. A subsequent study suggested the method may also be of value in the tropics, although the numbers were small (35). It has been proposed that, when throat swabs are dried, the reduction of more delicate and potentially inhibitory throat flora favors the survival and subsequent identification of BHS (31). A relatively new innovation, the ziplock plastic bag, plus the availability of inexpensive sachets of silica gel desiccant made transportation of throat swabs, sealed with desiccant, a potentially simple, affordable, and effective alternative to on-site inoculation of culture plates in remote tropical settings.
We decided to investigate the recovery rates of BHS from throat swabs taken in remote tropical communities, comparing three methods: (i) direct inoculation of culture plates on site with refrigerated transport back to the laboratory, (ii) throat swabs sealed in the presence of a silica gel desiccant and transported in a cold box to the laboratory with subsequent inoculation of culture plates, and (iii) transportation of swabs to the laboratory at ambient temperature and humidity as collected, that is, without desiccant or refrigeration (23, 30, 35).
Strains of GAS have been differentiated into five pattern types, based on the emm and emm-like genes, that appear to correlate with tissue tropism. Pattern types A to C are associated with the throat, pattern D with the skin, and pattern E with both sites (3). Our hypothesis was that differences in recovery rates of GAS using different methods may be partly determined by the emm pattern type. That is, recovery rates of “throat” strains, adapted to a moist environment, would be better on culture plates, whereas “skin” strains, adapted to a dry environment, would be better suited to ambient conditions. The primary aim of this study was to compare the recovery rates of three different methods for transporting throat swab specimens to the laboratory. An additional aim was to establish whether there was a correlation between the emm pattern type and recovery of GAS strains in the laboratory using the different methods.
MATERIALS AND METHODS
Study site and consultation.
We conducted a prospective study of households in three remote Aboriginal communities in the Top End of the Northern Territory, Australia. The households were selected because they had at least one resident with a history of acute rheumatic fever. This region has a mean daily maximum temperature of 32°C (32.5°C in January and 30.1°C in July); the mean annual rainfall is close to 1,300 cm, 85% falling in the months December to March. The land area of the Top End is 265,000 square miles, about the same as the state of Texas.
The study protocol was approved by the local ethics committee. Community leaders and heads of 54 identified households were approached by study investigators, and household members were invited to take part. All households agreed. Individual informed consent was then obtained before enrollment.
Collection and handling of throat swabs.
Study households were visited by study staff each month for 23 months. Participants were asked if they had symptoms of pharyngitis, and the throat was examined. Swabs were taken from the throats of all household occupants present at the time of each visit. Duplicate throat swabs were collected by simultaneously holding two cotton-tipped swab sticks together and sweeping the pharynx and tonsils. The swabs were placed back in the original packaging and kept out of the heat of the sun for transportation back to the community clinic within 2 hours. The duplicate-swab method was chosen because families did not consider repeated swabbing of the same person at one visit to be acceptable.
At the community clinic, paired swabs were processed in one of four ways. (i) Immediate inoculation of culture plates and refrigeration, followed by cold-box transportation, was compared with transport at ambient temperature and humidity (plated versus ambient). One swab of each pair was used to inoculate a nonselective culture medium, Columbia horse blood agar (HBA), and a selective medium of horse blood agar containing colistin and nalidixic acid (CNA) (Oxoid Australia Pty Ltd., Victoria, Australia). The plates were sealed and refrigerated for subsequent transportation in a cold box at approximately 4°C. The other swab was left at ambient temperature and humidity in the original packaging. All specimens were packed and transported by air back to the Menzies School of Health Research in Darwin, usually within 24 h. (ii) Immediate inoculation of culture plates and refrigeration was compared with cold-box transport in the presence of desiccant (plated versus desiccant). One swab was used to inoculate media (HBA and CNA) in the community clinic and transported as described above. The other swab was sealed in an airtight bag (20 swabs per bag) with a packet of silica gel desiccant and transported back to the laboratory in a cold box. (iii) Transport with desiccant in a cold box was compared with transport at ambient temperature and humidity (desiccant versus ambient). One swab was stored and transported to the laboratory at ambient temperature and humidity, and the other was sealed with desiccant and transported in the cold box. (iv) Duplicate swabs were transported at ambient temperature and humidity (ambient versus ambient). To assess the sampling differences from uneven distribution of BHS in the throat and on the swabs, duplicate throat swabs were taken, separated, handled, and transported in an identical manner under ambient conditions.
For logistical reasons, paired comparisons were done in sequential batches over time; first, plated compared with ambient, then plated compared with desiccant, then desiccant compared with ambient, and last, ambient compared with ambient. Swabs from communities 1 and 3 were subjected to all four comparisons over the course of the study, whereas swabs from community 2 were only handled by the plated and ambient methods.
Laboratory methods.
Upon arrival in the central laboratory, swabs transported in the presence of desiccant or under ambient conditions were inoculated onto HBA and CNA. All culture plates were then incubated at 37°C in 5% CO2. The plates were examined after 24 h and again at 48 h, and colonies of BHS were subcultured onto HBA plates for subsequent identification using a Streptococcal Grouping Kit (Oxoid Diagnostic Reagents, England, United Kingdom). Isolates identified as GCS or GGS (Streptococcus dysgalactiae subsp. equisimilis) and GAS were suspended in tryptone soy broth and 15% glycerol prior to storage at −80°C. The emm pattern types of GAS isolates were determined by PCR-based mapping according to the method of Bessen and coworkers (4).
Data analysis and comparison of methods.
Recovery rates and median point prevalence (throat carriage) were calculated for the BHS by Lancefield group and by community. For intraprocess comparisons (e.g., plated versus ambient), the yields of the different methods were compared using paired-swab data for total BHS and for streptococcal Lancefield groupings A, C, and G. The relative sensitivity of each method was calculated, including 95% confidence intervals. In addition, for interprocess comparisons (e.g., all plated versus all ambient), total yields were calculated for each of the methods. P values were calculated using chi-square analysis.
RESULTS
The study was conducted from August 2003 to June 2005 (inclusive). Households in community 1 (population, approximately 2,500) were studied for the whole period. There were difficulties with logistics, ongoing household consent, and employment of research staff in community 2 (population, 800), and the study was transferred from community 2 to community 3 (population, 1,800) in July 2004.
Over the study period, 4,842 throat swabs were taken; 4,122 of these were paired swabs. For all the swabs, beta-hemolytic streptococci were recovered on 558 occasions, with more than one BHS Lancefield group isolated on 29 occasions. There were 216 isolates of GAS, 80 isolates of GCS, and 262 isolates of GGS, as shown in Table 1. Only nine people had sore throats on direct questioning, and GAS was recovered on two of those occasions, both from adults. Across all communities, the median point prevalences (throat carriage) were 9.3% for BHS, 4.2% for GAS, 1.5% for GCS, and 3.5% for GGS. For children aged <15 years, the median point prevalences were 10.1% for BHS and 4.9% for GAS. Recovery rates for GCS were significantly higher in community 1 than the other communities, and overall recovery rates were much lower in community 2, reflecting difficulties with on-site collection and processing. In each community, recovery rates from children aged <15 years were higher than for adults.
TABLE 1.
Recovery rates of BHS from the throat by all methods
| Community no. | Group | Total no. of throat swabs | No. (%) of:
|
|||
|---|---|---|---|---|---|---|
| Total BHS | GAS | GCS | GGS | |||
| 1 | Total | 3,016 | 387 (12.8) | 134 (4.4) | 75 (2.5) | 178 (5.9) |
| <15 yr | 1,701a | 274 (16.1) | 88 (5.2) | 55 (3.2) | 131 (7.7) | |
| 2 | Total | 295 | 12 (4.1) | 6 (2.0) | 6 (2.0) | |
| <15 yr | 135a | 10 (7.4) | 5 (3.7) | 5 (3.7) | ||
| 3 | Total | 1,531 | 159 (10.4) | 76 (5.0) | 5 (0.3) | 78 (5.1) |
| <15 yr | 858a | 113 (13.2) | 58 (6.8) | 4 (0.5) | 51 (5.9) | |
| Total | Total | 4,842 | 558 (11.5) | 216 (4.5) | 80 (1.7) | 262 (5.4) |
| <15 yr | 2,694a | 397 (14.7) | 151 (5.6) | 59 (2.2) | 187 (6.9) | |
No date of birth was available for 13 people, representing 15 swabs.
Comparison of transportation methods.
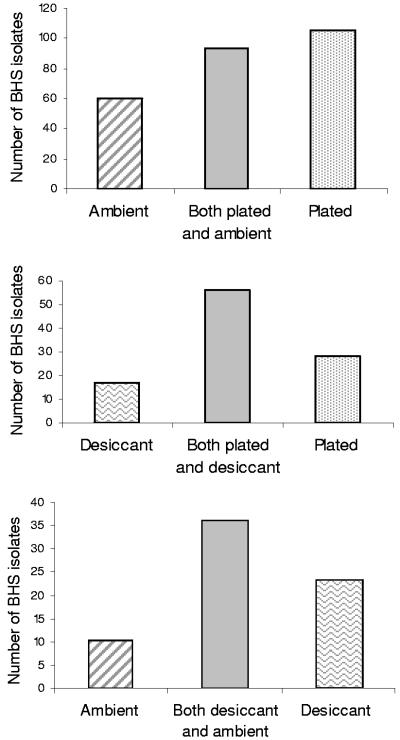
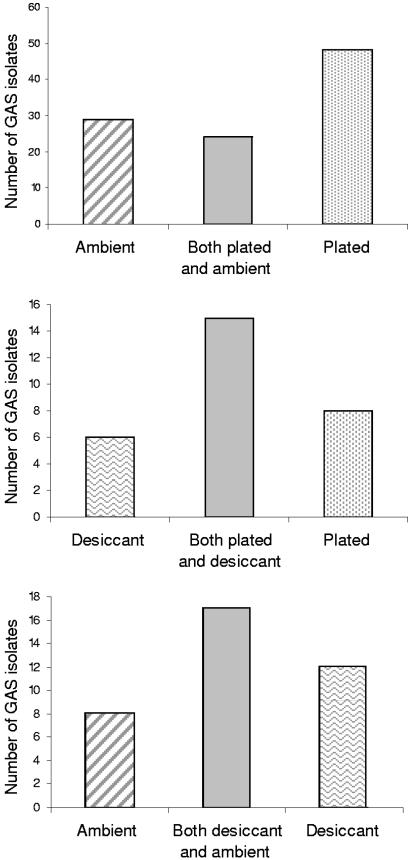
Recovery rates of BHS from duplicate swabs are shown in Fig. 1. On-site inoculation of culture plates, followed by cold-box transport, provided a better yield of BHS than transport in the presence of desiccant (P = 0.04) and was also superior to transport under ambient conditions (P = <0.001). Transport in the presence of desiccant was also significantly better than transport under ambient conditions alone (P = 0.009). Paired-swab results for GAS (Fig. 2) indicated that on-site inoculation of culture plates was superior to transport under ambient conditions (P = 0.006), but the other comparison data did not achieve statistical significance. The same relationship held with isolates of GCS and GGS (not shown).
FIG. 1.
Recovery of BHS using paired throat swabs: swabs inoculated onto culture media in the community clinic (plated) were compared with swabs transported with desiccant (desiccant) and swabs transported under ambient conditions (ambient) (see Materials and Methods). (Top) Number of isolates, 258 of 2,571 paired swabs. Ambient sensitivity, 59.3% (95% confidence interval [CI], 53.2% to 65.1%), and plated sensitivity, 76.7% (95% CI, 71.2% to 81.5%); P < 0.001. (Middle) Number of isolates, 101 of 521 paired swabs. Desiccant sensitivity, 72.3% (95% CI, 62.5% to 80.7%), and plated sensitivity, 83.2% (95% CI, 74.4% to 89.9%); P = 0.04. (Bottom) Number of isolates, 69 of 515 paired swabs. Ambient sensitivity, 66.7% (95% CI, 54.3% to 77.6%), and desiccant sensitivity, 85.5% (95% CI, 75.0% to 92.8%); P = 0.009.
FIG. 2.
Recovery of GAS using paired throat swabs. (Top) Number of GAS isolates, 101. Ambient sensitivity, 52.5% (95% confidence interval [CI], 42.3% to 62.5%), and plated sensitivity, 71.3% (95% CI, 61.4% to 79.6%); P = 0.006. (Middle) Number of GAS isolates, 29. Desiccant sensitivity, 72.4% (95% CI, 52.8% to 87.3%), and plated sensitivity, 79.3% (95% CI, 60.3% to 92.0%); P = 0.54. (Bottom) Number of GAS isolates, 37. Ambient sensitivity, 67.6% (95% CI, 50.2% to 83.0%), and desiccant sensitivity, 78.4% (95% CI, 61.8% to 90.2%); P = 0.29.
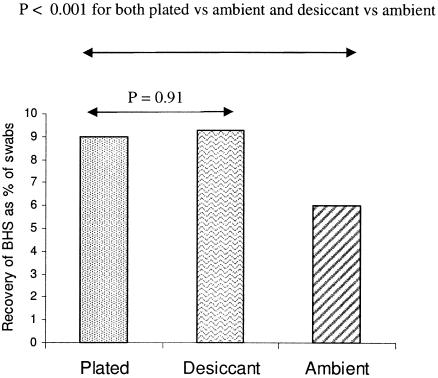
Pooled data from all swabs are shown in Fig. 3. Comparisons using specimen transport in the presence of desiccant were done in the second year of the study. In May 2005, there was an outbreak of GAS and GGS carriage and infection in communities 1 and 3, although they are more than 600 kilometers apart. This substantially skewed the BHS recovery results. Thus, data from May 2005 were excluded when the pooled recovery rates were calculated. By this method, recovery rates from cold-box transport in the presence of desiccant were equivalent to those from on-site inoculation of culture plates (P = 0.91); however, the differences between plated and ambient, and desiccant and ambient, were highly significant (P < 0.001).
FIG. 3.
Pooled data (not paired) for all swabs taken showing the recovery rates of BHS using three different transportation methods.
Sampling variation.
Sampling variations were assessed by documenting recovery rates from 515 paired swabs transported under ambient conditions where yields were expected to be lower and sampling variations were expected to have a greater impact. The overall yield of BHS from these swabs was low (6.2%) compared to the recovery rate by the other methods, excluding ambient (11.9%). There were 32 isolates of BHS; 23 were recovered from only one swab in each pair and 9 from both swabs. This indicates a substantial sampling variability from using two swabs together combined with poorer survival rates of BHS on swabs handled under ambient conditions.
Correlation with emm pattern type.
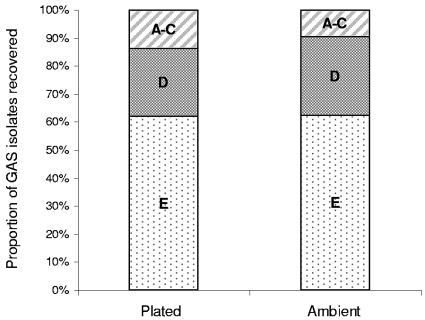
When the emm pattern types of the first 155 GAS isolates were examined, 28 isolates were found to have pattern types A to C (18%), 33 pattern type D (22%), and 73 pattern type E (47%). Twenty-one isolates (14%) could not be pattern typed using the method described. There appeared to be slightly improved recovery rates of types A to C with the community plating and cold-box transport compared to transport under ambient conditions, but they were not statistically significant (Fig. 4).
FIG. 4.
Comparison of emm pattern types of GAS isolates recovered; on-site inoculation of cultures plates (plated) and transport under ambient conditions (ambient). Number of GAS isolates, 101. There was no significant difference in the proportions of emm pattern types between methods.
DISCUSSION
Nonsuppurative complications of beta-hemolytic streptococcal infection have profound consequences; acute rheumatic fever is the world's leading cause of acquired heart disease in children, and acute poststreptococcal glomerulonephritis is a major cause of renal morbidity in some regions (6, 36). The greatest burden of disease falls on people in developing countries and minority indigenous communities in industrialized countries. The reported throat carriage rates of GAS vary widely from population to population, ranging from less than 3% to greater than 25% (13, 15, 25, 27, 28). Previous reports of carriage rates in remote Australian Aboriginal communities have suggested that they are low, usually less than 5% (5). Using the “gold standard,” on-site inoculation of culture plates, the median point prevalence for throat carriage of GAS in children <15 years old was 4.9%. This is much lower than throat carriage rates of GAS in urban non-Aboriginal Australian children in Melbourne, where the spring, summer, and winter carriage rates were 13.0%, 8.0%, and 16.0%, respectively (11).
Culturing throat swabs in remote Aboriginal communities is logistically difficult, as is the case in many settings around the world with a high incidence of streptococcal disease. Recovery of BHS from the throat depends on how well the swab is collected (19), because GAS are not evenly distributed in the pharynx (32). The highest counts come from the tonsils or tonsillar fossae (20). Ideally, one swab should be swept across the back of the pharynx, including both tonsils or tonsillar fossae plus the posterior pharynx. The tongue and buccal mucosa should be avoided (7). When duplicate swabs were used, we found that there was significant sampling variation; this may, in part, account for the reduced sensitivity of the “gold standard.” Results from the duplicate swab comparisons indicated that direct inoculation of culture plates close to the time of collection (within 2 h) was superior to transportation of specimens in a cold box in the presence of silica gel desiccant, although the pooled data suggested that they were equivalent. The yield from either of these methods was clearly better than that for transportation under ambient conditions.
Keeping the process simple is an important consideration when working under difficult circumstances. Successful transportation of throat swabs in the presence of desiccant was described in the 1960s (16, 29). Redys and coworkers found that recovery rates at low temperatures (4°C) and low humidity were better than at high temperatures (>25°C) and high humidity. Several years later, a study by Taplin and Lansdell showed that transport of specimens in the presence of desiccant improved the yield of BHS in tropical settings; however, the numbers were too small for statistical analysis (35). Transportation of specimens sealed with a desiccant is technically straightforward and inexpensive; ziplock plastic bags are now readily available in most countries, and sachets of silica gel desiccant can be regenerated by heating them for an hour in a dry oven. Weight is also important; in many remote settings, the cost of transporting culture plates by air is three to five times the cost of transporting swabs sealed with desiccant. There may also be practical difficulties in providing refrigerated fresh culture media in the field (35). Thus, the high yield from on-site inoculation of cultures plates can be balanced against the lower cost and reduced complexity of the alternative.
The roles of enrichment media and transport media are less clear. Facklam reviewed the role of Pike's enrichment medium containing crystal violet and sodium azide (and several modifications) before plating to increase recovery rates (14), but controlled studies are lacking. Smith et al. found that primary plating was 30% better than subculture from Todd-Hewitt broth, but it was not compared to a delayed plating method (34). Roddey et al. found that direct plating on a selective and a nonselective medium, delayed plate inoculation (2 to 6 h), and inoculation of Todd-Hewitt broth were equally effective (30). An enrichment medium for throat swabs that contained calf serum has also shown better yields of GAS than primary plating (9).
In a review of the microbiology of upper respiratory infections, Carroll and Reimer (7) recommended the use of Stuart's or Amies transport medium if the expected transport delay is greater than 24 h, although they did not quote supportive data. Indeed, higher counts of BHS have been reported from Amies transport medium than from dry swabs at 24 h (2), but this was a laboratory rather than a field study. Yrios et al. (37) found that GAS counts were maintained over 48 h in modified Stuart's transport medium, whereas other workers have reported less than acceptable recovery rates from both Stuart's and Amies transport media (35). A Scottish study of Amies and Stuart's transport media and Pike's enrichment medium versus direct transfer of dry swabs to the laboratory showed that they provided no significant benefit (10). Stuart's transport medium has been used, apparently successfully, for more than 20 years in Central Australia, but no formal evaluation in this setting has been published (F. Morey, unpublished data). A serum glucose agar transport system has also been suggested but never widely utilized (26). Other published methods have included transport of isolates on filter paper and the use of swab transport medium kits (21, 34). They have no proven advantage over simpler methods (18, 33). Enrichment and transport media for recovery of GAS and other BHS are no longer routinely recommended in the eighth edition of the Manual of Clinical Microbiology (24).
In this study, emm pattern types of GAS strains did not correlate with recovery rates using different methods. The hypothesis that “throat” strains, types A to C, would be better suited to moist culture media and types D and E would survive better under dry conditions was not supported by the evidence. As with a previous study of emm patterns of GAS isolates from a remote Aboriginal community with a high incidence of acute rheumatic fever, supposed “skin” strains of GAS (emm pattern types D and E) predominated in the throat (4).
In summary, direct inoculation of throat swabs onto culture plates remains the “gold standard” for recovering BHS in the field. In tropical and remote settings, such as those in “outback” Australia, cold-box transport of specimens sealed with desiccant and subsequent inoculation of culture plates in the laboratory is a practical alternative. Results from this throat carriage study may not apply to studies of acute pharyngitis, where organism loads are likely to be higher and alternative methods may be more acceptable. Further studies of methods that do not require cold transport could help to make the reliable recovery of BHS from throat swabs simpler and less expensive in regions of the world where the burden of streptococcal disease is high.
Acknowledgments
We thank the study households, community clinic staff, the Aboriginal research officers, the community councils, Pallave Dasari, Stephen Halpin, Murin Air (NT), and Western Pathology, which provided transport of specimens.
The study was supported by grants from the National Heart Foundation of Australia (PB 02 M 0996) and the National Health and Medical Research Council (ID 251690).
REFERENCES
- 1.Anhalt, J. P., B. J. Heiter, D. W. Naumovitz, and P. P. Bourbeau. 1992. Comparison of three methods for detection of group A streptococci in throat swabs. J. Clin. Microbiol. 30:2135-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry, A. L., G. D. Fay, and R. L. Sauer. 1972. Efficiency of a transport medium for the recovery of aerobic and anaerobic bacteria from applicator swabs. Appl. Microbiol. 24:31-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bessen, D., C. Sotir, T. Readdy, and S. Hollingshead. 1996. Genetic correlates of throat and skin isolates of group A streptococci. J. Infect. Dis. 173:896-900. [DOI] [PubMed] [Google Scholar]
- 4.Bessen, D. E., J. R. Carapetis, B. Beall, R. Katz, M. Hibble, B. J. Currie, T. Collingridge, M. W. Izzo, D. A. Scaramuzzino, and K. S. Sriprakash. 2000. Contrasting molecular epidemiology of group A streptococci causing tropical and nontropical infections of the skin and throat. J. Infect. Dis. 182:1109-1116. [DOI] [PubMed] [Google Scholar]
- 5.Carapetis, J., C. Connors, D. Yarmirr, V. Krause, and B. Currie. 1997. Success of a scabies control program in an Australian Aboriginal community. Pediatr. Infect. Dis. J. 16:494-499. [DOI] [PubMed] [Google Scholar]
- 6.Carapetis, J. R., M. McDonald, and N. J. Wilson. 2005. Acute rheumatic fever. Lancet 366:155-168. [DOI] [PubMed] [Google Scholar]
- 7.Carroll, K., and L. Reimer. 1996. Microbiology and laboratory diagnosis of upper respiratory tract infections. Clin. Infect. Dis. 23:442-448. [DOI] [PubMed] [Google Scholar]
- 8.Chapin, K. C., P. Blake, and C. D. Wilson. 2002. Performance characteristics and utilization of rapid antigen test, DNA probe, and culture for detection of group a streptococci in an acute care clinic. J. Clin. Microbiol. 40:4207-4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colling, A., I. Kerr, W. R. Maxted, and J. P. Widdowson. 1980. Streptococcal infection in a Junior Detention Centre: a five-year study. J. Hyg. 85:331-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cumming, C. G., P. W. Ross, and H. Lough. 1981. Optimal methods for the isolation of groups A, B, C and G streptococci. J. Laryngol. Otol. 95:377-384. [DOI] [PubMed] [Google Scholar]
- 11.Danchin, M. H., S. Rogers, G. Selvaraj, L. Kelpie, P. Rankin, R. Vorich, M. Howson, J. B. Carlin, N. Curtis, T. M. Nolan, and J. R. Carapetis. 2004. The burden of group A streptococcal pharyngitis in Melbourne families. Indian J. Med. Res. 119(Suppl.):144-147. [PubMed] [Google Scholar]
- 12.Del Mar, C. 1992. Managing sore throat: a literature review. I. Making the diagnosis. Med. J. Aust. 156:572-575. [PubMed] [Google Scholar]
- 13.Dierksen, K. P., M. Inglis, and J. R. Tagg. 2000. High pharyngeal carriage rates of Streptococcus pyogenes in Dunedin school children with a low incidence of rheumatic fever. NZ Med. J. 113:496-499. [PubMed] [Google Scholar]
- 14.Facklam, R. R. 1976. A review of the microbiological techniques for the isolation and identification of streptococci. Crit. Rev. Clin. Lab. Sci. 6:287-317. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann, S. 1985. The throat carrier rate of group A and other beta hemolytic streptococci among patients in general practice. Acta Pathol. Microbiol. Immunol. Scand. 93:347-351. [DOI] [PubMed] [Google Scholar]
- 16.Hosty, T., M. Johnson, M. Freear, R. Gaddy, and F. Hunter. 1964. Evaluation of the efficiency of four different types of swabs in the recovery of group A streptococci. Health Lab. Sci. 1:163-169. [Google Scholar]
- 17.Kaplan, E. L., F. H. Top, Jr., B. A. Dudding, and L. W. Wannamaker. 1971. Diagnosis of streptococcal pharyngitis: differentiation of active infection from the carrier state in the symptomatic child. J. Infect. Dis. 123:490-501. [DOI] [PubMed] [Google Scholar]
- 18.Kellogg, J. 1990. Suitability of throat culture procedures for detection of group A streptococci and as reference standards for evaluation of streptococcal antigen detection kits. J. Clin. Microbiol. 28:165-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kellogg, J., and J. Manzella. 1986. Detection of group A streptococci in the laboratory or physician's office. JAMA 255:2638-2642. [PubMed] [Google Scholar]
- 20.Kim, S. J. 1993. Optimal site of throat swab for the isolation of beta-hemolytic streptococci. J. Korean Med. Sci. 8:453-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koshi, G., K. N. Brahmadathan, C. P. Thangavelu, and R. Pandian. 1979. Evaluation of different methods for the transport of swabs for streptococci. Indian J. Med. Res. 69:26-31. [PubMed] [Google Scholar]
- 22.Majeed, H., L. Al-Doussary, M. Moussa, A. Yusuf, and A. Suliman. 1993. Office diagnosis and management of group A streptococcal pharyngitis employing the rapid antigen detecting test. A 1-year prospective study of reliability and cost in primary care centres. Ann. Trop. Pediatr. 13:65-72. [DOI] [PubMed] [Google Scholar]
- 23.Martin, D., J. Stanhope, and L. Finch. 1977. Delayed culture of group A streptococci: an evaluation of variables in methods of examining throat swabs. J. Med. Microbiol. 10:249-253. [DOI] [PubMed] [Google Scholar]
- 24.Miller, J. M., H. T. Holmes, and K. Krisher. 2003. General principles of specimen collection and handling, p. 55-66. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, D.C.
- 25.Nicolle, L. E., B. Law, B. Postl, B. Urias, N. Ling, and M. Fast. 1988. Group A streptococcal pharyngeal carriage and impetigo in two northern native communities. Arctic Med. Res. 47:669-671. [PubMed] [Google Scholar]
- 26.Perks, E. M., and R. T. Mayon-White. 1984. Serum glucose agar, a transport medium for Streptococcus pyogenes. J. Clin. Pathol. 37:226-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pichichero, M. E., S. M. Marsocci, M. L. Murphy, W. Hoeger, J. L. Green, and A. Sorrento. 1999. Incidence of streptococcal carriers in private pediatric practice. Arch. Pediatr. Adolescent Med. 153:624-628. [DOI] [PubMed] [Google Scholar]
- 28.Rajkumar, S., and R. Krishnamurthy. 2001. Isolation of group A beta-hemolytic streptococci in the tonsillopharynx of school children in Madras City and correlation with their clinical features. Jpn. J. Infect. Dis. 54:137-139. [PubMed] [Google Scholar]
- 29.Redys, J. J., E. W. Hibbard, and E. K. Borman. 1968. Improved dry-swab transportation for streptococcal specimens. Public Health Rep. 83:143-149. [PMC free article] [PubMed] [Google Scholar]
- 30.Roddey, O. F., Jr., H. W. Clegg, E. S. Martin, R. L. Swetenburg, and E. W. Koonce. 1995. Comparison of throat culture methods for the recovery of group A streptococci in a pediatric office setting. JAMA 274:1863-1865. [PubMed] [Google Scholar]
- 31.Roddey, O. F., C. U. Mauney, H. W. Clegg, E. S. Martin, and R. L. Swetenburg. 1989. Comparison of immediate and delayed culture methods for isolation of group A streptococci. Pediatr. Infect. Dis. J. 8:710-712. [DOI] [PubMed] [Google Scholar]
- 32.Ross, P. 1977. The isolation of Streptococcus pyogenes from throat swabs. J. Med. Microbiol. 10:69-76. [DOI] [PubMed] [Google Scholar]
- 33.Ross, P., C. Cumming, and H. Lough. 1982. Swabs and swab-transport media kits in the isolation of upper respiratory bacteria. J. Clin. Pathol. 35:223-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith, R. E., N. M. Pease, C. W. Reiquam, and E. C. Beatty, Jr. 1965. A comparison of multiple techniques in the recovery of group A streptococci from throat cultures of children. A study of 13,476 cases. Am. J. Clin. Pathol. 44:689-694. [DOI] [PubMed] [Google Scholar]
- 35.Taplin, D., and L. Lansdell. 1973. Value of desiccated swabs for streptococcal epidemiology in the field. Appl. Microbiol. 25:135-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White, A. V., W. E. Hoy, and D. A. McCredie. 2001. Childhood post-streptococcal glomerulonephritis as a risk factor for chronic renal disease in later life. Med. J. Aust. 174:492-496. [DOI] [PubMed] [Google Scholar]
- 37.Yrios, J. W., E. Balish, A. Helstad, C. Field, and S. Inhorn. 1975. Survival of anaerobic and aerobic bacteria on cotton swabs in three transport systems. J. Clin. Microbiol. 1:196-200. [DOI] [PMC free article] [PubMed] [Google Scholar]