Abstract
The functional organization of somatosensory and motor cortex was investigated in an individual with a high cervical spinal cord injury, a 5-year absence of nearly all sensory/motor function at and below the shoulders, and rare recovery of some function in years 6–8 after intense and sustained rehabilitation therapies. We used functional magnetic resonance imaging to study brain activity to vibratory stimulation and voluntary movements of body parts above and below the lesion. No response to vibratory stimulation of the hand was observed in the primary somatosensory cortex (SI) hand area, which was conversely recruited during tongue movements that normally evoke responses only in the more lateral face area. This result suggests SI reorganization analogous to previously reported neuroplasticity changes after peripheral lesions in animals and humans. In striking contradistinction, vibratory stimulation of the foot evoked topographically appropriate responses in SI and second somatosensory cortex (SII). Motor cortex responses, tied to a visuomotor tracking task, displayed a near-typical topography, although they were more widespread in premotor regions. These findings suggest possible preservation of motor and some somatosensory cortical representations in the absence of overt movements or conscious sensations for several years after spinal cord injury and have implications for future rehabilitation and neural-repair therapies.
Severe sensory deprivation due to amputation or peripheral nerve damage profoundly alters responsiveness and topographical organization of primary somatosensory cortex (SI) (see reviews in refs. 1–3). Less well known are the alterations in cortical responses after spinal cord injury (SCI). Individuals with SCI, as opposed to those with amputations, retain a normal body, which may influence cortical reorganization significantly, especially in individuals with partial SCI who have surviving fibers and potentially some functional connections across the level of damage. It will become an important practical issue to assess cortical responsiveness immediately after damage and in the course of recovery if ongoing efforts for restoring function by transplantation or other means are successful (4). Functional MRI (fMRI) with blood oxygenation level-dependent (BOLD) contrast provides a noninvasive method to assess neuronal activity by monitoring task-related changes in the local tissue concentration of deoxyhemoglobin (5).
In this fMRI study we mapped cortical somatosensory-motor areas in a subject with a high cervical traumatic injury at the level of the second cervical vertebra (C2). This subject had complete loss of motor and sensory function below the C3 level except for spotty sensation in the left hemibody for 5 years after injury and then progressively regained some capabilities in years 6–8. This late partial recovery was unexpected and followed a regimen of physical therapy that was more vigorous, frequent, and prolonged than is usual in most SCI patients (6). This study sought to determine the topographical normality of his somatosensory and motor cortical responses above and below the level of damage and investigate possible cortical reorganization subsequent to a late recovery from SCI.
Methods
Subjects.
A prior report (6) describes in detail the clinical history of the 50-year-old right-handed male who sustained a displaced C2 type II odontoid fracture from an equestrian accident in 1995 at age 42. No other permanent injuries, particularly a head injury, complicated the SCI. By clinical assessment, motor or somatosensory functions were absent below the lesion level for 5 years except for spotty sensation in the left hemitorso. He is ventilator dependent with hypophonic vocalization due to impaired function of the chest diaphragm and muscles of vocalization.
The control subject was a 23-year-old male who has a normal neurological and psychiatric history.
Visuomotor Tracking.
Subjects were required to synchronize movements to a video image of a yellow-green tennis ball against a black background. Subjects viewed the image on a back projection screen, which was seen in a mirror mounted on the head coil. The ball (≈4° in diameter) jumped regularly left/right of a fixation point (≈±4° jumps, 0.83-Hz rate) to guide tongue left/right movements (tongue extruded and moved against lips), jumped above/below the fixation point to guide left index-finger movement (at metacarpal–phalangeal joint) and remained stationary for rest periods. Visual monitoring indicated that both subjects consistently followed the ball motion. Movement range and vigor were less in the SCI subject. The left index finger was tested because the SCI subject sustained better following with this finger movement. Subjects could not see their finger during fMRI. For testing consistency all tactile stimulation was also applied to the left extremities.
Vibrotactile Stimulation.
A massage vibrator delivered suprathreshold tactile stimulation. The device, previously used in positron-emission tomography studies (7), was made magnetic resonance-compatible by replacing the electric motor with a pneumatic drive that was connected to a remote air compressor. The vibrator delivered ≈2-mm displacement vibrations centered on a base frequency of ≈100 Hz. The vibrator head was manually held against the left fingers and palm or sole of the left foot throughout stimulation and rest periods. Precise skin displacements were unknown, although stimulus magnitudes probably activated most skin mechanoreceptors, adjoining deeper tissues, and proprioceptors throughout distal parts of the stimulated limb.
MRI Acquisitions.
During MRI the SCI subject was ventilated, and physiologic parameters were monitored continuously (Magnitude/Millenium anesthesia monitoring, In Vivo Research, Orlando, FL) in a custom magnetic resonance-compatible setup (Shielding Resources Group, Tulsa, OK) as in ref. 8. All MRI used a 1.5-Tesla Magnetom Vision scanner and circularly polarized head coil (Siemens, Erlangen, Germany). Structural 3D T1-weighted magnetization-prepared/rapid gradient echo MRI was acquired. fMRI used a custom T2*-weighted asymmetric spin-echo echo-planar sequence sensitive to BOLD contrast (repetition time = 2,360 ms, T2* evolution time = 50 ms, α = 90°). During each fMRI run, 128 sets of 20 contiguous, 6-mm-thick slices were acquired parallel to the anterior commissure–posterior commissure plane (3.75 × 3.75 mm in-plane voxel size), allowing complete brain coverage. This protocol yielded images of the SCI subject without significant signal loss or distortions in the brain despite surgical metal in the neck.
Sensory and motor fMRI were acquired in different imaging sessions by using 8–10 fMRI runs per session and 128 frames (346.75 s) per run. For sensory fMRI, four runs were obtained for each stimulated limb, and each run contained eight baseline frames followed by 15 trials with three frames of stimulation alternating with five frames of no stimulation. For motor fMRI, four to five runs were obtained for each task (tongue and finger), each run having 12 trials of five task frames alternating with five rest frames.
MRI Data Analysis.
The cord was segmented from structural MRI for area measurement and 3D-rendered display by using ANALYZE AVW 4.0 (Mayo Foundation, Rochester, MN) and a Sun Fire V880 computer (Sun Microsystems, Santa Clara, CA). fMRI data were analyzed as described in refs. 9 and 10. General linear models (11) estimated the BOLD responses in each subject and each task (e.g., tongue movement) without assuming a hemodynamic response shape (12). BOLD time courses were estimated in each voxel, over eight frames (21.67 s) for somatosensory tasks and 10 frames (27.09 s) for motor tasks. fMRI data were smoothed with a 3D 2-voxel Gaussian kernel and transformed to the Talairach atlas (13) before statistical analysis. Statistical maps were based on cross-correlation between estimated BOLD time course and a reference hemodynamic response function that was obtained by convolving a delayed gamma function with a rectangular function representing task and control periods. The derived t statistics per voxel were converted to normally distributed z scores and corrected for multiple comparisons across the entire brain by using distributions obtained from Monte Carlo simulations (based on methods described in ref. 14). These images were inspected by using a threshold of P = 0.05 for a z score value of 4.5 over at least three face-contiguous voxels. The statistical maps were projected onto a standard brain atlas in both 3D view of the lateral hemispheric surface and 2D view of flattened cortex (ref. 15; see http://stp.wustl.edu/resources/caretnew.html).
Results
Structural MRI of the Cord.
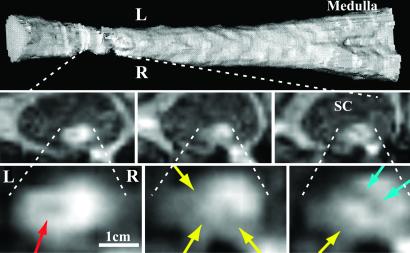
T1-weighted MRI in the SCI subject showed >75% loss in cross-sectional tissue area throughout the C2 region (Fig. 1). Additionally, there were multiple focal areas of tissue damage (myelomalacia) in different parts of the remaining spinal cord (Fig. 1). The continuity of white-matter pathways across the lesion cannot be determined from these images alone, particularly given the small cord size and the multiple areas of damage within and across levels.
Fig 1.
T1-weighted MRI of cervical SCI in SCI subject. (Top) Longitudinal 3D-rendered image (view from behind) of lower-brainstem and cervical spinal cord segments from the tip of the C2 odontoid process through the bottom of the C2 vertebral body. (Middle) Selected low-magnification images through the zone of injury show the small size of the cord relative to the spinal canal (SC). Images are transverse to the cord's long axis and at levels 40, 46, and 51 mm below the cerebellar tonsils (Left, Center, and Right, respectively). (Bottom) Higher-magnification view of the same three transverse images show focal regions of low T1-weighted signal that are consistent with chronic tissue damage (myelomalacia) or scarring. The location and shape of these sites vary and include a central oval (red arrow), a cleft (blue arrow), and several peripheral lesions (yellow arrows). L, left; R, right.
Clinical History of SCI Patient.
Initial recovery of motor and somatosensory function began in year 2000 after several years of standard physical therapy including passive range of motion and supported standing. The recovery accelerated through 2002 after the subject was enrolled in a more intense regime of physical therapy. Physical therapy included thrice-weekly, hour-long sessions of functional electrical stimulation of leg muscles that was computer-synchronized to drive an exercise bicycle (6). Frequent standard clinical examinations in the last 2 years documented self-initiated small movements with the left index finger, right wrist, and more recently, lower extremities. In parallel, the SCI subject reported feeling strong sensation upon tactile stimulation and passive movement of the upper and lower extremities. Sensations from the lower extremities were stronger. He had good accuracy in roughly localizing a tactile stimulus on the hand or foot including dorsal and volar surfaces. He also could identify a stimulated finger or toe (fingers better than toes).
Motor Tracking Tasks.
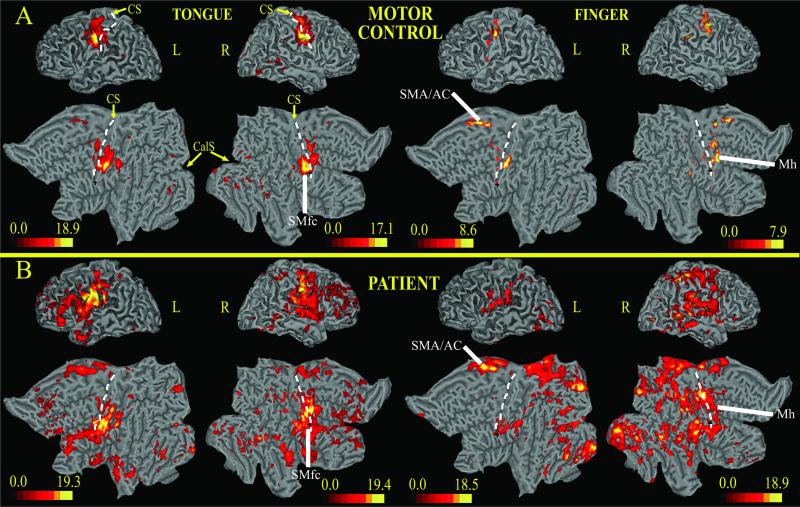
Fig. 2 shows BOLD responses to the visually guided motor tasks on 3D and flattened representations of a standard brain. Tongue movements in the control subject activated a ventral segment of precentral gyrus, central sulcus, and postcentral gyrus bilaterally (Fig. 2A, SMfc). These regions likely correspond to the primary sensory and motor cortex face area. Recruitment of SI in a visuomotor tracking task probably reflects tactile and proprioceptive sensory feedback signals associated with normal movements. Significant activity also occurred in medial frontal cortex along the cingulate sulcus (supplementary motor area/anterior cingulate) and in visual occipital cortex. Left index-finger movements produced the strongest activation in a middle segment of the contralateral (right) precentral gyrus/sulcus (Fig. 2A, Mh). This response was ≈2 cm superior to the foci active during tongue movements. Smaller responses occurred in the right parietal operculum and bilaterally within the central sulcus and postcentral gyrus (SI, area 3b); these likely related to sensory feedback. Finally, bilateral responses were observed in supplementary motor area/anterior cingulate similar to those observed for tongue movements.
Fig 2.
3D and 2D flattened views of atlas brain (15) with projected BOLD responses for visuomotor tracking task; the color scale indicates z scores. (A) Control subject. (B) Subject with SCI. CS, central sulcus (dotted line); CalS, calcarine sulcus; Mh, motor cortex, hand area; SMfc, primary somatosensory-motor cortex, face area; SMA/AC, supplementary motor area/anterior cingulate; L, left; R, right.
In the subject with SCI, BOLD responses during both motor tasks were stronger and more widespread than those observed in the control subject (Fig. 2 B vs. A). Tongue movements activated the face area of SI/M1 in ventral precentral gyrus, central sulcus, and postcentral gyrus (primary somatosensory-motor cortex, SMfc). Activity spread dorsally into the hand area and more extensively into adjacent regions such as the second somatosensory cortex (SII) (16) and frontal operculum. There was also strong activation of dorsolateral prefrontal cortex and supplementary motor area/anterior cingulate. Left index-finger movements intensely activated the contralateral M1 hand area (Mh), frontal operculum, SII, cingulate cortex, and lateral and medial parietal cortex (Fig. 2B). There was no spread into the SI/M1 face area. Recruitment of visual cortex, both ventrally and dorsally, was much stronger in the SCI than control subject and during finger than tongue movements in the SCI subject (Fig. 2B).
In one experiment, fMRI was acquired while the SCI subject performed left finger movements without visual feedback for 20-s intervals. Voluntary movements were internally planned and executed after a brief verbal “GO” signal. Performance was less accurate and less sustained, and the subject reported more effort, compared with visuomotor tracking. BOLD responses (movement vs. rest) were weaker and less localized in M1, and there was no recruitment of premotor or higher-order regions (not shown).
Vibrotactile fMRI.
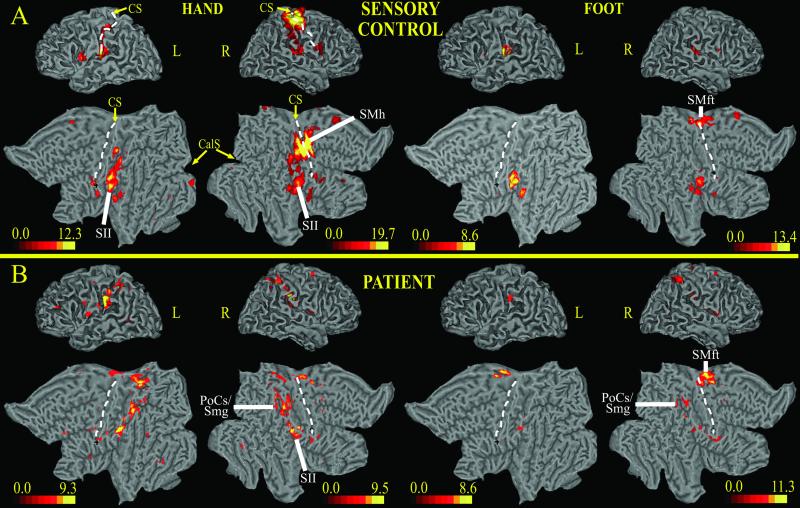
Fig. 3 shows BOLD responses to vibratory stimulation of the left hand or left foot. In the control subject (Fig. 3A), left hand and fingers vibration activated the contralateral (right) SI/M1 cortex. Responses occupied the dorsal segment of the postcentral gyrus extending into the central sulcus and the precentral gyrus, thus identifying the normal location of the hand area (Fig. 3A, SMh). There was also bilateral activation of SII. Left foot vibration evoked activity in the right medial frontal gyrus ≈1.2 cm dorsal to the hand area (Fig. 3A Right). This response likely involved the SI/M1 foot area (SMft). SII was activated bilaterally.
Fig 3.
3D and 2D flattened views of atlas brain (15) on which BOLD responses for sensory vibrotactile stimuli have been projected; the color scale indicates z scores. (A) Control subject. (B) Subject with SCI. CS, central sulcus (dotted line); CalS, calcarine sulcus; PoCs/Smg, postcentral sulcus/supramarginal gyrus; SMh, primary somatosensory-motor cortex, hand area; SMft, primary somatosensory-motor cortex, foot area; L, left; R, right.
The subject with SCI reported a better sensation of vibration in the left foot than in the left hand. Left hand vibration failed to activate expected hand areas of SI/M1 in the right postcentral gyrus, central sulcus, and precentral gyrus but produced bilateral responses in SII (Fig. 3B Left). Several other regions not recruited in the control subject responded in the SCI subject. These regions included, in order of strength, the contralateral (right) postcentral sulcus, and bilaterally, the posterior postcentral gyrus, and supramarginal gyrus (PoCs/Smg); the ipsilateral (left) postcentral gyrus; and contralateral medial frontal gyrus near the SI/M1 foot area. The contralateral regions correspond to likely higher-order somatosensory areas.
Left foot stimulation in the SCI subject (Fig. 3B Right) elicited a normal contralateral response in the right medial frontal gyrus, central sulcus, and postcentral gyrus that corresponds to the normal SI/M1 foot area (SMft, compare Fig. 3 A with B). An ipsilateral SI response also occurred in the left hemisphere. SII responses again were located along the parietal operculum but were weaker than during hand stimulation. There was some recruitment of higher-order parietal regions in right supramarginal gyrus during foot stimulation.
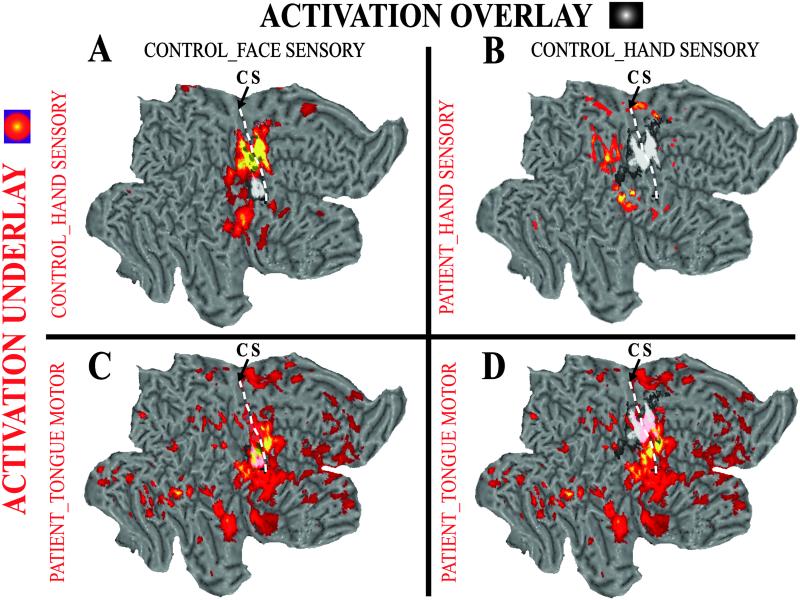
We overlaid the fMRI from the SCI and control subjects to compare somatosensory-motor cortex areas. Fig. 4A contrasts activation maps for left hand vibration and tracking tongue movements in the control subject. Fig. 4B shows the absence of a normal hand area in the SCI subject. Fig. 4C shows that the face area is similarly active in both subjects. However, the active region expands dorsally in the SCI subject where it encroaches onto the normal hand area (Fig. 4D).
Fig 4.
Comparison of the functional topography in SI between the SCI and control subjects. Overlay responses are shown in a white/black color scale; underlay responses are shown in a red–yellow color scale. (A) Normal topography of somatosensory-motor cortex in control subject: response to vibrotactile hand stimulation (yellow–red) is more dorsal than sensory face response to tongue movements (white/black). (B) Hand sensory response in control subject (white/black) overlaid on unresponsive hand area in the subject with SCI. (C) Sensory face response to tongue movement in control (white/black) matches face-area activation for tongue movements in the subject with SCI. (D) Hand sensory response in control subject matches location of “abnormal” hand response for tongue movements in the subject with SCI. CS, central sulcus.
Discussion
fMRI in an SCI subject with late partial recovery of sensory-motor function revealed BOLD responses in the SI foot area during vibratory foot stimulation, responses in higher-order somatosensory areas during stimulation of the hand, and responses in motor cortex areas during finger movements. In contrast, no response was observed in the S1 hand area upon vibratory hand stimulation. This region instead was recruited during sensory-motor stimulation of the tongue and lips.
The brain responses to vibratory stimuli occurred despite >75% loss in spinal cord area and multiple regions of chronic damage in the remaining cord. These responses indicate the existence of functional neural connections that traverse the site of cord injury. The subject's late clinical recovery is surprising given the 5-year history of no voluntary movements and nearly absent sensations from the extremities. Potentially contributing to partial recovery was a coincident period of extraordinary physical therapy. Because we have no longitudinal fMRI data, we can only speculate on the relationship between brain activations and either clinical recovery and/or the potential effect of physical therapy. Although some brain responses were consistent with known neuroplasticity effects demonstrated mostly in animal studies, others were unexpected and suggest preservation of normal topography and recruitment of compensatory areas.
The S1 hand area responded to sensory stimulation of the face but not the hand, which possibly reflects neural plasticity based on competitive interaction from a normally innervated face (1, 17–21). This response of the (deafferented) hand area to (invading) face inputs might correlate with an enhanced resolution of tactile sensation in the face, as demonstrated on a much smaller spatial scale within the digit representation of monkey area 3b (1). Unfortunately, we do not have any objective or anecdotal evidence supporting this perceptual substitution.
Another potential behavioral correlate of cortical remapping is the generation of phantom sensations from the stimulation of nondeafferented body parts (21–24). For instance, patients with complete thoracic SCI and no residual sensory function below the lesion experienced phantom sensations in the deafferented chest upon stimulation of the arm, which correlated with a coactivation of both SI chest and arm representations (24). However, the subject herein, who had an incomplete SCI and some sensation in the deafferented hand, never reported any phantom perception from stimulation of the face despite the coactivation of the SI hand area from lower-face stimulation. Residual sensory hand function was likely mediated by higher-order somatosensory areas that were activated by vibratory stimulation of the hand (see below). It is therefore possible that the preservation of some sensory input from the hand was sufficient to maintain a normal percept and prevent the generation of phantom sensations. Thus, the expanded cortical response from stimulation of the lower face may not have a clear perceptual correlate.
Because there are few cortical connections between SI face and hand areas (25) and given the long delay before sensations reappeared in the SCI subject, this reorganization likely involved large-scale thalamic (26) or brainstem (2) events. The reorganization possibly involved subcortical transneuronal changes, which are progressive and delayed.
Cortical activity is likely to reflect this mechanism by initial silence followed by late recovery of responses (26). Similar to prior results in monkeys with long-term dorsal rhizotomies, expansion in the SCI subject involved SI hand area responses to stimulation in the lower face, which borders the thalamic hand area (26).
A conjunction of mechanisms are likely responsible for the SI foot-area responses and the reported better sensations from the foot. Absent was competition from intact representations because all cortical and subcortical areas adjacent to the foot were severely deprived. This likely left the SI foot area accessible to any input that was conveyed through surviving fibers at the level of the injury (Fig. 1). Clinical changes accelerated after more intense rehabilitation involving functional electrical stimulation of the lower extremities (6). Sustained and synchronized stimulation is a known mechanism for behaviorally induced neuroplasticity (1). Thus, coordinated spinal activity from training possibly was sufficient to propagate through retained connections to influence the cortex.
The SCI subject was able to localize tactile stimulation on the hand, despite little evidence of responses in the SI hand area to intense tactile vibrations. Vibratory stimulation of the hand recruited SII and additional postcentral and posterior parietal somatosensory regions that normally do not respond during passive stimulation but become active during attention-requiring tactile discrimination tasks (27, 28). Thus, these regions possibly served his retained sensations, which suggests potential substitutions in recovery from severe spinal injury. In addition, we observed recruitment of more posterior multimodal parietal regions (27, 29), which may indicate the engagement of attention mechanisms by the SCI subject to detect weak somatosensory signals. These regions might be active when attempting to resolve small signals in primary or secondary somatosensory areas.
In contrast to the changes found in somatosensory maps, the primary motor areas were more nearly normal despite years without movements. Finger movement activated a confined middle segment of M1, whereas tongue movements, although activating hand SI areas, did not invade adjacent M1 regions. Possibly a more typical motor cortex network persisted because of attempted or imagined movements (motor imagery) during the 8 years after SCI, which can evoke patterned motor activity (even during sleep) without frank movements. Several experiments show similar cortical activity during mental rehearsal vs. execution of motor sequences (30, 31). Motor imagery enhances performance in sports (32). Sleep also improves motor performance (33). Thus, motor imagery perhaps recruits motor networks and might have maintained more typical topography of M1 cortex despite SCI.
The secondary motor areas (premotor and cingulate cortex) were activated more in the SCI vs. control subject. Additionally, many higher-order regions (e.g., posterior parietal, temporal, and dorsolateral prefrontal cortex) were recruited in the SCI subject, more for finger than tongue movements. Although tongue movements use neurons above the lesion, it is not surprising that an abnormally widespread network of activity was evoked, because these movements require coordination of several muscle groups (e.g., diaphragm or accessory respiratory muscles) that have C2–C5 myotomes at or below the lesion.
There are several likely and potentially intertwined explanations for the stronger M1/secondary motor responses and widespread engagement of higher-order cortical regions in the SCI subject. First, this activity might reflect arousal or attentional load related to greater effort needed to move a partially paralyzed body part. Second, the absence of normal proprioceptive feedback might cause disorganized or dysfunctional activity. Third, compensatory strategies (e.g., use of visuomotor associations) might rely on more widespread recruitment of cortical responses. Coupling of visual and motor information aided motor performance and led to stronger, more widespread activity compared with self-timed movements. The differences in visually guided vs. nonguided movements cannot be explained solely by a greater effort or lack of proprioceptive feedback, because these effects were similar in the two tasks. Thus, it is likely that the regular oscillation of the tennis ball image provided necessary timing and directional information to plan and execute more sustained and regular movements.
In studies of SCI without recovery, Sabbah et al. found normal motor cortical responses during attempted/imagined toe movements in 9/9 subjects with thoraco-lumbar paraplegia but weaker sensory cortical responses to passive unconscious mobilization in 3/9 subjects (34). Ionnides et al. found normal SII activation in 3/3 paraplegics but only weak SI foot activation in one subject (35). The data herein extend those studies by assessing preservation and rearrangement of cortical topography (face vs. hand vs. foot) in the rare case of partial recovery years after SCI.
Finding any normal topography in somatosensory and motor areas of a tetraplegic is surprising given a long history of no sensations or movements from below the injury. These fMRI findings were coincident with extraordinary and intensive rehabilitation therapies in a patient with severe but not total SCI. The mere presence of these cortical responses years after deafferentation is good news for strategies aimed at restoring spinal cord connections (e.g., stem cell transplants) because they suggest that restoration of any neural links across the lesion appear capable of reestablishing motor and sensory functions. Finally, fMRI provides a valuable objective tool for assessing neural integrity across cord lesions, the functional effects of SCI on the brain, and, potentially, the efficacy of novel therapies.
Acknowledgments
We thank the SCI subject and his assistants for their interest, enthusiasm, and cooperation. We acknowledge J. Diamond, V. Raja, A. Tansy, M. Cowan, C. Stanley, and Dr. Avi Snyder for data analysis; Drs. J. Lu and E. Diebert for monitoring patient health during MRI sessions; Drs. J. Shimony and J. Wippold for radiology exams; Mr. G. Foster for technical assistance; and Dr. L. Schultz for overall coordination and scheduling. This research was supported by National Institutes of Health Grants R01 NS31005 and P50 NS06833 and by the J. S. McDonnell Foundation.
Abbreviations
SI, primary somatosensory cortex
SCI, spinal cord injury
fMRI, functional MRI
BOLD, blood oxygenation level-dependent
Cn, cervical vertebra n
M1, primary motor cortex
SII, second somatosensory cortex
References
- 1.Buonomano D. V. & Merzenich, M. M. (1998) Annu. Rev. Neurosci. 21, 149-186. [DOI] [PubMed] [Google Scholar]
- 2.Jones E. G. (2000) Annu. Rev. Neurosci. 23, 1-37. [DOI] [PubMed] [Google Scholar]
- 3.Ramachandran V. S. & Hirstein, W. (1998) Brain 121, 1603-1630. [DOI] [PubMed] [Google Scholar]
- 4.McDonald J. W. (1999) Sci. Am. 281, (281), 64-73. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa S., Menon, R. S., Tank, D. W., Kim, S. G., Merkle, H., Ellermann, J. M. & Ugurbil, K. (1993) Biophys. J. 64, 803-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald J. W., Becker, D., Sadowsky, C. L., Jane, J. A., Conturo, T. E. & Schultz, L. M. (2002) J. Neurosurg. Spine 97, 252-265. [DOI] [PubMed] [Google Scholar]
- 7.Burton H., Videen, T. O. & Raichle, M. E. (1993) Somatosens. Mot. Res. 10, 297-308. [DOI] [PubMed] [Google Scholar]
- 8.McDonald J. W., Becker, D., Sadowsky, C. L., Jane, J. A., Conturo, T. E. & Schultz, L. M. (2002) J. Neurosurg. Spine 97, 405-406. [DOI] [PubMed] [Google Scholar]
- 9.Burton H., Snyder, A. Z., Conturo, T. E., Akbudak, E., Ollinger, J. M. & Raichle, M. E. (2002) J. Neurophysiol. 87, 589-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corbetta M., Akbudak, E., Conturo, T. E., Snyder, A. Z., Ollinger, J. M., Drury, H. A., Linenweber, M. R., Petersen, S. E., Raichle, M. E., Van Essen, D. C. & Shulman, G. L. (1998) Neuron 21, 761-773. [DOI] [PubMed] [Google Scholar]
- 11.Friston K., Holmes, A., Worsley, K., Poline, J., Frith, C. & Frackowiak, R. (1995) Hum. Brain Mapp. 2, 189-210. [DOI] [PubMed] [Google Scholar]
- 12.Ollinger J. M., Corbetta, M. & Shulman, G. L. (2001) Neuroimage 13, 218-229. [DOI] [PubMed] [Google Scholar]
- 13.Talairach J. & Tournoux, P., (1988) Coplanar Stereotaxic Atlas of the Human Brain (Thieme, New York).
- 14.Forman S. D., Cohen, J. D., Fitzgerald, M., Eddy, W. F., Mintun, M. A. & Noll, D. C. (1995) Magn. Reson. Med. 33, 636-647. [DOI] [PubMed] [Google Scholar]
- 15.Van Essen D. C., Drury, H. A., Joshi, S. & Miller, M. I. (1998) Proc. Natl. Acad. Sci. USA 95, 788-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burton H., MacLeod, A. M., Videen, T. O. & Raichle, M. E. (1997) Cereb. Cortex 7, 3-17. [DOI] [PubMed] [Google Scholar]
- 17.Merzenich M. M., Kaas, J. H., Wall, J., Nelson, R. J., Sur, M. & Felleman, D. (1983) Neuroscience 8, 33-55. [DOI] [PubMed] [Google Scholar]
- 18.Merzenich M. M., Kaas, J. H., Wall, J. T., Sur, M., Nelson, R. J. & Felleman, D. J. (1983) Neuroscience 10, 639-665. [DOI] [PubMed] [Google Scholar]
- 19.Merzenich M. M., Nelson, R. J., Stryker, M. P., Cynader, M. S., Schoppmann, A. & Zook, J. M. (1984) J. Comp. Neurol. 224, 591-605. [DOI] [PubMed] [Google Scholar]
- 20.Pons T. P., Garraghty, P. E., Ommaya, A. K., Kaas, J. H., Taub, E. & Mishkin, M. (1991) Science 252, 1857-1860. [DOI] [PubMed] [Google Scholar]
- 21.Ramachandran V. S. (1993) Proc. Natl. Acad. Sci. USA 90, 10413-10420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aglioti S., Cortese, F. & Franchini, C. (1994) NeuroReport 5, 473-476. [DOI] [PubMed] [Google Scholar]
- 23.Aglioti S., Bonazzi, A. & Cortese, F. (1994) Proc. R. Soc. London Ser. B 255, 273-278. [DOI] [PubMed] [Google Scholar]
- 24.Moore C. I., Stern, C. E., Dunbar, C., Kostyk, S. K., Gehi, A. & Corkin, S. (2000) Proc. Natl. Acad. Sci. USA 97, 14703-14708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burton H. & Fabri, M. (1995) J. Comp. Neurol. 355, 508-538. [DOI] [PubMed] [Google Scholar]
- 26.Jones E. G. & Pons, T. P. (1998) Science 282, 1121-1125. [DOI] [PubMed] [Google Scholar]
- 27.Burton H., Abend, N. S., MacLeod, A. M., Sinclair, R. J., Snyder, A. Z. & Raichle, M. E. (1999) Cereb. Cortex 9, 662-674. [DOI] [PubMed] [Google Scholar]
- 28.Burton H. & Sinclair, R. J. (2000) J. Clin. Neurophysiol. 17, 575-591. [DOI] [PubMed] [Google Scholar]
- 29.Corbetta M. & Shulman, G. L. (2002) Nat. Rev. Neurosci. 3, 201-215. [DOI] [PubMed] [Google Scholar]
- 30.Porro C. A., Francescato, M. P., Cettolo, V., Diamond, M. E., Baraldi, P., Zuiani, C., Bazzocchi, M. & di Prampero, P. E. (1996) J. Neurosci. 16, 7688-7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richter W., Somorjai, R., Summers, R., Jarmasz, M., Menon, R. S., Gati, J. S., Georgopoulos, A. P., Tegeler, C., Ugurbil, K. & Kim, S. G. (2000) J. Cognit. Neurosci. 12, 310-320. [DOI] [PubMed] [Google Scholar]
- 32.Roure R., Collet, C., Deschaumes-Molinaro, C., Delhomme, G., Dittmar, A. & Vernet-Maury, E. (1999) Physiol. Behav. 66, 63-72. [DOI] [PubMed] [Google Scholar]
- 33.Fischer S., Hallschmid, M., Elsner, A. L. & Born, J. (2002) Proc. Natl. Acad. Sci. USA 99, 11987-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabbah P., de, S. S., Leveque, C., Gay, S., Pfefer, F., Nioche, C., Sarrazin, J. L., Barouti, H., Tadie, M. & Cordoliani, Y. S. (2002) J. Neurotrauma 19, 53-60. [DOI] [PubMed] [Google Scholar]
- 35.Ioannides A. A., Liu, L., Khurshudyan, A., Bodley, R., Poghosyan, V., Shibata, T., Dammers, J. & Jamous, A. (2002) Neuroimage 16, 115-129. [DOI] [PubMed] [Google Scholar]