Abstract
Retrospective evaluation of potassium hydroxide plus calcofluor white (KOH+CFW), Gram, Giemsa, and modified Ziehl-Neelsen (1% H2SO4, cold) stains for the detection of microsporidia in corneal scrapings from 30 patients showed KOH+CFW and acid-fast stains to be most efficient (29/30 [96.7%] and 28/30 [93.3%], respectively) in the diagnosis of microsporidial keratitis.
Microsporidia are intracellular protozoa of the phylum Microspora which parasitize both invertebrates and vertebrates (2). Two distinct clinical entities of this disease in the eye exist: corneal deep stromal infection in immunocompetent patients and superficial keratoconjunctivitis in patients with AIDS (14) and more recently in immunocompetent patients (3, 4). Definitive diagnosis has often depended upon transmission electron microscopy, which is time consuming and expensive, requires expertise, and is believed to be less sensitive than desired (15). The detection of specific antibodies in ocular specimens of AIDS patients is variable (16). Molecular methods are still in their infancy. A flexible diagnostic technique is the need of the hour in view of increasing reports of microsporidiosis in India (6, 11, 12). Though there have been a few reports of comparative evaluation of stains in detecting microsporidia in stools, bile, urine, and pulmonary specimens (1, 5, 9, 13), there are limited data evaluating various stains in the detection of microsporidia in ocular specimens and the feasibility of their routine use in laboratories. Therefore, we sought to evaluate the efficacy of various stains routinely used in our laboratory in the diagnosis of microsporidial keratitis.
Data were obtained by a retrospective review of records of patients diagnosed with microsporidial keratitis from 1 January 2002 to 30 September 2005 presenting to the Cornea Service at the L.V. Prasad Eye Institute, Hyderabad, India. All patients included in the study had undergone a complete ocular examination. Cases were confirmed to be microsporidiosis based on microbiological examination of the corneal scrapings. The microbiological work-up included the collection of corneal scrapings by an ophthalmologist using a sterile blade number 15 on a Bard Parker handle under a slit lamp biomicroscope. Multiple scrapings were taken from the lesions and smeared on presterilized slides for staining and various culture media for the growth of bacteria, fungi, or Acanthamoeba. We have described these procedures in detail in an earlier publication (7). The smears were stained with (i) potassium hydroxide plus calcofluor white (KOH+CFW), (ii) Gram, (iii) Giemsa, and (iv) modified Ziehl-Neelsen (1% H2SO4) (8). KOH+CFW staining was observed under a fluorescence microscope (BH2-RFC; Olympus), with cube U having filter combinations for the excitation spectrum region near 365 nm for a DAPI (4′,6′-diamidino-2-phenylindole) stain. The remaining stains were observed under a bright-field microscope. A paired comparison of the frequency of detection of spores by various stains was performed using the McNemar statistical test; a P value of ≤0.05 was considered significant.
Of the 30 patients diagnosed with microsporidial keratitis, the clinical diagnosis in 26 patients was superficial keratoconjunctivitis, and in 4 it was stromal keratitis. Direct examination of the corneal scrapings did not reveal any other bacteria or fungi in any of the cases except one (no. 4), which along with microsporidial spores also showed the presence of gram-positive cocci and bacilli in the Gram stain. Corneal scrapings from 6 of the 30 cases had grown bacteria in culture (Table 1), and none grew fungi. The culture media in the remaining cases were sterile after 7 days of incubation. Microsporidial spores were detected by KOH+CFW stain in 29/30 samples (96.7%; 95% confidence interval [CI], 90.23 to 100), by Gram stain in 27/30 samples (90%; 95% CI, 79.26 to 100), by Giemsa stain in 22/30 samples (73.3%; 95% CI, 57.51 to 89.15), and by modified Ziehl-Neelsen stain in 28/30 samples (93.3%; 95% CI, 84.41 to 100). The McNemar test showed no significant difference between the staining techniques used for visualization of microsporidial spores (KOH+CFW versus Gram stain, P = 0.625; KOH+CFW/Gram versus modified Ziehl-Neelsen stain, P = 1.0; Gram versus Giemsa stain, P = 0.125; and Giemsa versus modified Ziehl-Neelsen stain, P = 0.07) except for KOH+CFW versus Giemsa stain, with a P value of 0.016. The spores appeared elongated and oval to round and measured 1 to 3 μm in length and 1 to 1.5 μm in width in cases with keratoconjunctivitis. The spores appeared larger (3 to 5 μm by 2 to 2.5 μm) in the stromal cases. Figure 1 shows the appearance of these spores in the stains evaluated.
TABLE 1.
Direct smear examination and culture results of corneal scrapings from 30 patients with microsporidial keratitis
| Sample no. | Result for:a
|
Culture result(s) | |||
|---|---|---|---|---|---|
| Calcofluor white | Gram | Giemsa | Modified Ziehl-Neelsen | ||
| 1 | + | + | + | + | No growth |
| 2 | + | + | − | + | No growth |
| 3 | + | + | + | + | No growth |
| 4 | + | +* | −* | + | Staphylococcus epidermidis Corynebacterium spp. |
| 5 | + | + | + | + | No growth |
| 6 | + | + | + | + | No growth |
| 7 | + | + | + | − | Corynebacterium spp. |
| 8 | + | + | + | + | Corynebacterium spp. |
| 9 | + | − | − | + | No growth |
| 10 | + | + | + | + | No growth |
| 11 | − | + | − | +# | No growth |
| 12 | + | + | + | + | No growth |
| 13 | + | + | + | + | No growth |
| 14 | + | + | + | + | No growth |
| 15 | + | + | + | + | No growth |
| 16 | + | − | − | + | No growth |
| 17 | + | + | − | + | Proteus mirabilis |
| 18 | + | + | + | + | No growth |
| 19 | + | + | + | + | No growth |
| 20 | + | + | − | + | No growth |
| 21 | + | − | + | + | No growth |
| 22 | + | + | + | + | No growth |
| 23 | + | + | + | + | No growth |
| 24 | + | + | + | + | Pseudomonas aeruginosa |
| 25 | + | + | + | + | No growth |
| 26 | + | + | + | + | S. epidermidis |
| 27 | + | + | + | + | No growth |
| 28 | + | + | + | +# | No growth |
| 29 | + | + | − | − | No growth |
| 30 | + | + | + | + | No growth |
+, Microsporidial spores present; −, microsporidial spores absent; +*, microsporidial spores present along with gram-positive cocci and gram-positive bacilli; −*, microsporidial spores present along with cocci and bacilli; +#, non-acid-fast spores present.
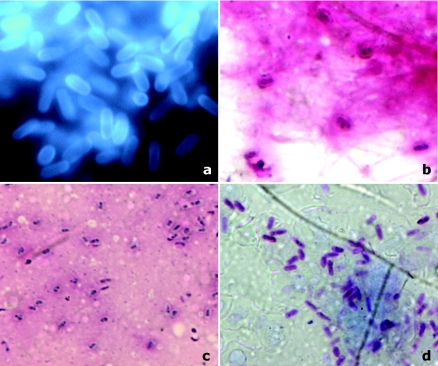
FIG. 1.
Microsporidal spores as observed under various stains on corneal scrapings. (a) KOH+CFW stain (magnification, ×1,000). Organisms were seen as bright turquoise to white oval bodies, often clustered in groups, against a relatively dark background. The spores displayed variable fluorescence intensities. Depending on the orientation of the microsporidia, the anterior end appeared concave. (b) Gram stain (magnification, ×1,000). Spores appeared ovoid and refractile and bright purple, resembling gram-positive organisms. The spores were scattered or highly clustered within the cytoplasm of occasional epithelial cells. Microsporidial spores show a dark staining belt girding them either diagonally or equatorially. (c) Giemsa stain (magnification, ×1,000). This stain is not taken up by the cell wall, and only the cytoplasm gets stained. The spores appear smaller than those in the other stains. There was also poor differentiation from other bacteria and debris. The darkly stained belt could be identified in 18/30 cases, aiding preliminary diagnosis. (d) Modified Ziehl-Neelsen stain (magnification, ×1,000). Except for two, all cases of microsporidial spores were acid fast (1% H2SO4). The acid-fast spores appeared bright red on a blue background, and a posterior vacuole and central diagonal strip within the spores were often visible. Bacteria and other cell debris appeared blue, owing to methylene blue counterstain.
We have previously reported that simple smear examination methods may be adequate to diagnose a case of ocular microsporidiosis (12). According to our institutional protocol, a combination of four staining techniques, i.e., Gram, Giemsa, KOH+CFW, and modified Ziehl-Neelsen stains, is used in the diagnosis of nonviral keratitis. We evaluated these staining techniques for their feasibility in the diagnosis of ocular microsporidosis. In this study, the KOH+CFW stain along with the modified Ziehl-Neelsen stain most frequently detected microsporidia, followed by the Gram stain, while Giemsa staining had the least detection efficacy among the four. The study is limited as it is retrospective and unmasked; however, a statistical comparison of frequency of detection by the stains revealed a significant difference between the KOH+CFW and Giemsa stains by McNemar's test. According to our protocol, the first corneal scraping taken from the patients with suspected microbial keratitis is always used for KOH+CFW staining. Although this may account for greater detection of organisms in this stain, we would like to emphasize that KOH+CFW staining could identify these organisms relatively easily, distinguishing them clearly from other cell debris, and that the appearance of the spores was esthetically appealing. The frequency of detection of microsporidia by the Gram and Giemsa stains was reasonably high. However, the morphology of the spores was not as clear as that in the KOH+CFW stain. Yeast cells are closest in morphology to microsporidial spores. However, the uniformly ovoid spores of microsporidia are quite characteristic, and the absence of budding and pseudohyphae (not always present in yeast) may help identify them. In addition, yeast would grow in culture. Since the smears stained with Gram and Giemsa were always seen after KOH+CFW staining, a certain amount of bias may have heightened the percentage of detection in these stains. From this unmasked observational series, despite the limitations, we are tempted to recommend that calcofluor white is the stain of choice for the diagnosis of microsporidial keratitis when a fluorescence microscope is available. In the absence of a fluorescence microscope, acid-fast stain seems to be the best alternative. For clinical samples with extremely scanty microsporidial spores, the use of two or more methods may yield more convincing results.
Our results are in agreement with a previous study in which CFW and modified Ziehl-Neelsen stains were reported to be most efficient in diagnosing microsporidiosis (1). In another study comparing the effectiveness of CFW, Giemsa, and modified trichrome stains in detecting microsporidia in fire ants (10), the Giemsa stain was found to be least sensitive and CFW the most sensitive. The frequency of detection of spores was lowest in the Giemsa stain in our study as well. We are still in the process of understanding this rare entity, and our ongoing prospective study using a larger sample size will allow us to determine the sensitivity and specificity of each of these stains for the detection of microsporidia and to define their complete clinical utility. The stains used in this study are commonly used in the diagnosis of microbial keratitis in several laboratories, and our results suggest that these stains are adequate for the diagnosis of microsporidiosis and are of great clinical utility.
Acknowledgments
Financial support was provided by the Department of Biotechnology, DBT (BT/PR4951/MED/14/573/2004), Government of India.
REFERENCES
- 1.Awadalla, H. N., I. F. el Naga, M. M. el-Temsahi, and A. Y. Negm. 1998. Detection of microsporidia by different staining techniques. J. Egypt. Soc. Parasitol. 28:729-738. [PubMed] [Google Scholar]
- 2.Canning, E. U., and W. S. Hollister. 1987. Microsporidia of mammals—widespread pathogens or opportunistic curiosities? Parasitol. Today 9:267-273. [DOI] [PubMed] [Google Scholar]
- 3.Chan, C. M., J. T. Theng, L. Li, and D. T. Tan. 2003. Microsporidial keratoconjunctivitis in healthy individuals: a case series. Ophthalmology 110:1420-1425. [DOI] [PubMed] [Google Scholar]
- 4.Davis, R., R. Font, M. Keisler, and J. Shadduck. 1990. Corneal microsporidiosis: a case report including ultrastructural observations. Ophthalmology 97:953-957. [PubMed] [Google Scholar]
- 5.Joste, N. E., P. E. Sax, and W. S. Pieciak. 1999. Cytologic detection of microsporidia spores in bile. A comparison of stains. Acta Cytol. 43:98-103. [DOI] [PubMed] [Google Scholar]
- 6.Kumar, S. S., S. Ananthan, and P. Saravanan. 2002. Role of coccidian parasites in causation of diarrhoea in HIV infected patients in Chennai. Indian J. Med. Res. 116:85-89. [PubMed] [Google Scholar]
- 7.Kunimoto, D. Y., S. Sharma, P. Garg, U. Gopinathan, D. Miller, and G. N. Rao. 2000. Corneal ulceration in the elderly in Hyderabad, south India. Br. J. Ophthalmol. 84:54-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luna, L. G. 1968. Manual of histologic staining methods of the Armed Forces Institute of Pathology, 3rd ed. McGraw-Hill Book Co., New York, N.Y.
- 9.Matos, O., M. L. Lobo, and F. Antunes. 2001. Methodology of the diagnosis of microsporidiosis in urine and pulmonary specimens from AIDS patients. J. Eukaryot. Microbiol. 48(Suppl.):69S-70S. [DOI] [PubMed] [Google Scholar]
- 10.Milks, M. L., Y. Y. Sokolova, I. A. Isakova, J. R. Fuxa, F. Mitchell, K. F. Snowden, and S. B. Vinson. 2004. Comparative effectiveness of light-microscopic techniques and PCR in detecting Thelohania solenopsae (Microsporidia) infections in red imported fire ants (Solenopsis invicta). J. Eukaryot. Microbiol. 51:187-191. [DOI] [PubMed] [Google Scholar]
- 11.Shenoy, S., S. Baliga, T. Kurnvilla, H. V. Prashanth, and R. M. Dominic. 2003. Opportunistic intestinal parasitic infections in human immunodeficiency virus infected patients in Mangalore, South India. Trop. Dr. 33:250. [DOI] [PubMed] [Google Scholar]
- 12.Sridhar, M. S., and S. Sharma. 2003. Microsporidial keratoconjunctivitis in a HIV-seronegative patient treated with debridement and oral itraconazole. Am. J. Ophthalmol. 136:745-746. [DOI] [PubMed] [Google Scholar]
- 13.Vavra, J., and J. Chalupsky. 1982. Fluorescence staining of microsporidian species with the brightener calcofluor white M2R. J. Protozool. 29(Suppl.):503.
- 14.Weber, R., R. T. Bryan, D. A. Schwartz, and R. L. Owen. 1994. Human microsporidial infections. Clin. Microbiol. Rev. 7:426-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber, R., D. A. Schwartz, and P. Deplazes. 1999. Laboratory diagnosis of microsporidiosis, p. 315-362. In M. Wittner and L. M. Weiss (ed.), The microsporidia and microsporidiosis. American Society for Microbiology, Washington, D.C.
- 16.Weber, R., R. Bryan, R. Owen, C. Wilcox, L. Gorelkin, and G. Visvesvara. 1992. Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates. N. Engl. J. Med. 326:161-166. [DOI] [PubMed] [Google Scholar]