Abstract
In vitro expanded neural stem/progenitor cells can undergo region-specific differentiation after transplantation to the developing or adult brain, and display morphologies and markers characteristic of mature neurons. Here we have used patch-clamp techniques to explore whether grafted stem cells also can develop physiological properties of mature neurons and become functionally integrated within host neural circuitry. The immortalized neural progenitor cell line, RN33B, prelabeled with GFP by using a lentiviral vector, was transplanted into the cortex or hippocampus of neonatal rats. We found that the grafted GFP-positive cells differentiated into cells with morphological features of cortical or hippocampal pyramidal neurons, and that many of them had established appropriate cortico-thalamic and contralateral hippocampal connections, respectively, as revealed by retrograde tracing. Whole-cell patch-clamp recordings from grafted cells with morphological characteristics of pyramidal neurons showed that they were able to generate action potentials, and received functional excitatory and inhibitory synaptic inputs from neighboring cells. These data provide evidence that grafted neural progenitors can differentiate into morphologically mature pyramidal projection neurons, establish appropriate long-distance axonal projections, exhibit normal electrophysiological properties, and become functionally integrated into host cortical circuitry.
Keywords: progenitor cells, GFP, electrophysiology, whole-cell recording, retrograde tracing
Multipotent neural stem or progenitor cell lines can differentiate to neuronal and glial phenotypes, both in vitro and after transplantation into the developing or adult brain (1–3). Immature progenitors possess the capability to migrate within the host brain parenchyma, and can, at least in some cases, adopt morphological features and express markers of mature neurons. It remains unclear, however, whether grafted neural stem/progenitor cell lines also can develop the physiological properties of mature neurons and become functionally integrated into host neural circuitry.
In the present study, we have used whole-cell patch-clamp recording to analyze the electrophysiological properties and functional integration of intracerebrally grafted cells derived from a conditionally immortalized neural progenitor cell line, RN33B, generated from embryonic rat brainstem by retroviral transduction of the temperature-sensitive simian virus 40 large-T-antigen (4, 5). The RN33B cell line has a remarkable neurogenic capacity both in neonatal and adult recipients, and can differentiate into neuron-like cells with morphologies of pyramidal cells and interneurons after transplantation into cortex or hippocampus, and into medium-sized densely spiny neurons after transplantation into the striatum (5–9). The RN33B cells were first transduced with the GFP gene by using a lentiviral vector, and then implanted into the cortex and hippocampus. The GFP marker allows visualization of the grafted cells in their entirety, including fine details of their axons and dendrites (9), and identification of grafted cells by their native fluorescence for electrophysiological recordings in brain slices. To increase the success of whole-cell recordings, neonatal rats were used because of higher numbers of GFP-labeled neurons detected as compared with adults (12). Axonal connections of the grafted cells were studied by using the retrograde tracer Fluorogold (FG).
Our data indicate that transplanted GFP-positive (GFP+) RN33B cells with morphological features of pyramidal neurons extend long-distance axonal projections to appropriate brain regions, develop a physiologically mature neuronal phenotype, and become functionally integrated into host cortical circuitry.
Materials and Methods
Cell Culture and Lentiviral Transduction.
RN33B cells were expanded as described (4, 5). A recombinant lentiviral vector carrying the reporter gene for GFP was produced (refs. 10 and 11; see refs. 9 and 12 for details), and added to the culture medium at a multiplicity of infection of 5 (based on the transducing units/ml on 293T cells) for 48 h, after which the medium was changed. After transduction, ≈85% of the RN33B-cells expressed GFP.
Transplantation.
Postnatal day 1–2 (P1–P2) Sprague–Dawley rats (B&K Universal, Stockholm) were used as recipients. Surgery was performed under deep hypothermia and with the animals mounted in a miniaturized stereotaxic frame (for details see ref. 13). All animal-related procedures were conducted in accordance with local ethical guidelines and approved animal care protocol. The cell suspensions (100,000 cells per μl in Hanks' balanced salt solution) were injected with a glass capillary (70 μm i.d.), attached to a 2-μl Hamilton syringe, either into hippocampus (100,000 cells) at anterior (A) = −1.2 and −1.5, lateral (L) = −1.5, and ventral (V) = −1.5 and −1.8; or cortex (100,000 cells) at A = 0.5, L = 2.2, and V = −2.5 and −3.0. The latter ventral coordinates fell within the striatum, but the cells investigated here were all located in the cortex.
For retrograde tracing of cortico-thalamic and contralateral hippocampal projections, respectively, eight animals received RN33B cell transplants either unilaterally (n = 6) or bilaterally (n = 2) into cortex, and unilaterally into the hippocampus (n = 5). Twelve rats were grafted bilaterally into the cortex for electrophysiological recordings.
FG Injections.
At 15 weeks after transplantation, FG (2% solution in sterile saline; Molecular Probes) (14) was iontophoretically injected from a glass micropipette (20 μm i.d.) with a 5-μA positive current, pulsed for 7 of every 14 s over 20 min, by using a constant current generator Midgard (EDCO Scientific, Chapel Hill, NC). For tracing of cortico-thalamic projections, FG was injected into ipsilateral thalamus, at two sites centered on the ventro-lateral and ventro-basal thalamic nuclei (A = −2.0, L = 2.3, V = −5.4 and A = −3.0, L = 2.3, V = −5.4, respectively, with tooth bar (TB) at −3.0), i.e., in the area of the thalamus where we have previously observed dense GFP+ terminal projections from intracortical grafts of RN33B cells (9). Commissural projections were traced by FG injections into the contralateral CA3, by using two injections at A = −3.0 to −1.5, L = 3.0, V = −3.2; and A = −4.0 to −1.5, L = 3.0, V = −3.2, with TB set at −2.3.
Electrophysiology.
Four to seven weeks after transplantation, rats were anaesthetized with halothane (Fluothane, Astra Zeneca, London), and their brains were taken out and placed into gassed (95% O2/5% CO2) ice-cold artificial cerebrospinal fluid (aCSF) containing 119 mM NaCl, 2.5 mM KCl, 1.3 mM MgSO4, 2.5 mM CaCl2, 26.2 mM NaHCO3, 1 mM NaH2PO4, and 11 mM glucose. We focused on the cortex because, compared with the hippocampus, it contained higher number of GFP-expressing neurons, increasing our chances for successful whole-cell recordings. Coronal cortical slices (300 μm thick) were cut on a Vibratome (Ted Pella, Inc., Redding, CA), placed into a submerged chamber with gassed aCSF, warmed up to 35°C for 30 min, and stored at room temperature for 1–4 h. Slices were then taken into the submerged recording chamber, which was permanently perfused with gassed aCSF at room temperature. Transplanted GFP+ cells were identified by using UV light, whereas IR light in combination with differential interference contrast microscopy technique were used for visual approach and patch-clamping. Whole-cell recordings were made with glass pipettes filled with one of three different solutions: for current-clamp recordings, 140 mM KCl/10 mM NaCl/10 mM Hepes/4 mM MgATP/0.4 mM GTP/0.2 mM EGTA; for voltage-clamp and excitatory postsynaptic current (EPSC) recordings, 117.5 mM Cs gluconate/17.5 mM CsCl/10 mM Hepes/8 mM NaCl/0.2 mM EGTA/2 mM MgATP/0.3 mM GTP/5 mM QX-314 Br; for voltage-clamp and inhibitory postsynaptic current (IPSC) recordings, 135 mM CsCl/10 mM Hepes/0.2 mM EGTA/8 mM NaCl/2 mM MgATP/0.3 mM GTP/5 mM QX-314 Br, pH 7.2; osmolarity 295 mOsm, pipette resistance 4–6 MΩ. Before each experiment, biocytin (final concentration of 0.5%) was added to the pipette solution. This biocytin diffused into the cells during whole-cell recordings and labeled them. After each electrophysiological experiment, slices were stained either with AMCA-avidin (7-amino-4-methylcoumarin-3-acetic acid; Vector Laboratories) or Cy3-avidin (Jackson ImmunoResearch) conjugates (1:200) to visualize biocytin, and double-labeling with GFP in the recorded RN33B cells was confirmed. In total, 12 double-labeled transplanted RN33B cells and 20 control (host) cells were recorded in 12 animals. The average age of the animals from which the transplanted RN33B and corresponding control cells were recorded was 5.2 ± 0.3 and 4.6 ± 0.5 weeks, respectively.
The EPSCs, IPSCs, and excitatory postsynaptic potentials (EPSPs) were filtered at 2.9 kHz and sampled at 10 kHz with a patch-clamp amplifier (HEKA Electronics, Lambrecht, Germany). Data were stored on a G4 Macintosh computer for off-line analysis. The non-N-methyl-d-aspartate (NMDA) receptor-mediated EPSCs and γ-aminobutyric acid type A (GABAA) receptor-mediated IPSCs were recorded at holding potential of −70 mV, whereas NMDA receptor-mediated EPSCs were recorded at +40 mV. 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline (NBQX; 5 μM), d(−)-2-amino-5-phosphonopentanoic acid (d-AP5; 50 μM), and picrotoxin (PTX; 100 μM) were added to the perfusion medium to block non-NMDA, NMDA, and GABAA receptor-mediated currents, respectively. EPSP/EPSCs and IPSCs were induced by electrical stimulation of the cortical areas close to the recorded cells by using bipolar stainless steel electrodes. To induce action potentials in current-clamp mode, depolarization current pulses of 1 s duration and of increasing magnitude were delivered to the recorded cells through patch pipettes. PTX or NBQX plus d-AP5 were always present in the perfusion medium when EPSP/EPSCs or IPSCs were recorded, respectively.
Immunohistochemistry.
At 1 week after the FG injections (i.e., 16 weeks after transplantation), animals were killed. Rats were anaesthetized with an overdose of pentobarbital (70 mg/kg, Apoteksbolaget, Umeå, Sweden) and transcardially perfused (for details see ref. 9). Briefly, coronal or sagittal sections were cut on a freezing microtome at a thickness of 40 μm. The primary antibodies used were GFP, chicken polyclonal (1:5,000, Chemicon) and FG, rabbit polyclonal (1:2,000, Chemicon). For 3,3-diaminobenzidine (DAB)-reacted specimens, sections were pretreated with 3% H2O2 and 10% methanol, and then incubated for 24 h with the primary antiserum. For fluorescent double labeling, sections were first incubated with the two primary antibodies together and then for 2 h with the Cy2 and Cy3 conjugated secondary antibodies (1:400, Jackson ImmunoResearch).
Microscopy and Cell Counting.
The number of GFP+ cells double-labeled with FG in the cortex (n = 4 rats) and hippocampus (n = 4 rats) was counted in every sixth section. Colocalization analysis was performed by using the openlab software (ImproVision, Coventry, U.K.) for scanning the sections in the z plane. An average of 9 and 6 sections were counted for the cortical and hippocampal grafts, respectively, in each animal. The GFP+ cells with morphological features of pyramidal projection neurons (angular cell bodies and apical and basal branching dendrites) were counted in the deep cortical layers, where the majority of FG-labeled host cortico-thalamic neurons were located. In the hippocampus, we counted grafted GFP+ cells located in the FG-labeled pyramidal layer of the CA3 region and displaying pyramidal-like cellular profile with branching apical and basal dendrites.
Results
Neuronal Differentiation.
In the dorsal fronto-parietal cortex, GFP+ cells were found scattered in an area extending 2–3 mm in rostro-caudal and medio-lateral directions, primarily in the deep cortical layers (IV–VI) (Figs. 1A and 2A). A large fraction of the differentiated cells exhibited morphologies of cortical pyramidal neurons, with large angular somas and rich trees of branching and spine-bearing apical and basal dendrites. The cells had the normal vertical alignment, i.e., perpendicular to the cortical layers, with the apical dendrite extending toward the pial surface (Fig. 1A). Other GFP+ grafted cells, albeit lower in number, had differentiated into smaller, multipolar cells with interneuron-like morphologies, as well as into cells with characteristic glial morphologies, including astrocyte-like cells in the cortical gray matter (Fig. 1A), and oligodendrocyte-like cells in and near the corpus callosum.
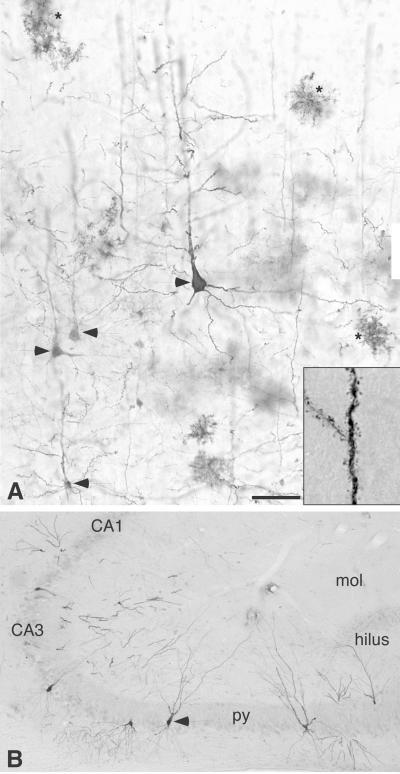
Fig 1.
Morphologies of GFP+ grafted cells in the cortex and hippocampus at 16 weeks after transplantation. (A) In the cortex, a large fraction of the GFP+ cells differentiated into cells displaying morphologies of cortical pyramidal neurons (arrowheads), with large angular somas and rich trees of branching and spine-bearing apical and basal dendrites (see Inset for high magnification). The cells had the normal vertical alignment, perpendicular to the cortical layers, with the apical dendrite extending toward the pial surface. In addition, some of the GFP+ cells in the hippocampus had differentiated into typical astrocyte-like cell profiles (asterisks). (B) A significant number of the GFP+ cells had integrated into the pyramidal layer of CA3 and exhibited morphology and position closely resembling the intrinsic hippocampal pyramidal neurons (arrowhead), with bipolar branching dendrites and a normal orientation perpendicular to the cell layer. (Scale bar = 65 μm in A and 180 μm in B.) mol, molecular layer; py, pyramidal layer.
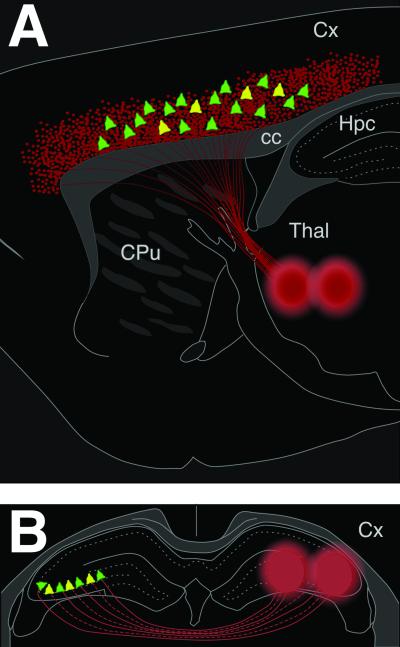
Fig 2.
Schematic illustration of the distribution of GFP+ grafted cells (green and yellow pyramids), the injection sites of the retrograde tracer FG (large red circles), and the distribution of FG-labeled host cells (red dots). (A) In the fronto-parietal cortex, the GFP+ cells labeled by FG from the thalamus were found in the same layers as the FG-labeled host cells, intermingled with the intrinsic FG+ cortico-thalamic neurons. About one-fifth of the GFP+ pyramidal-like cells (yellow pyramids) were found to contain the FG label. (B) In the hippocampus, FG was injected contralateral to the grafts in the medial and lateral CA3 region (large red circles). About one-third of the GFP+ cells possessing pyramidal neuron morphologies were GFP/FG-double-labeled (yellow pyramids). cc, corpus callosum; Cx, cortex; CpU, cuadate putamen; Hpc, hippocampus; Thal, thalamus.
In the hippocampal formation, GFP+ cells with neuronal morphologies were distributed throughout the CA3 region and the hilus of the dentate gyrus (Fig. 1B). Many of these cells were located in the CA3 pyramidal layer with a morphology and position closely resembling intrinsic hippocampal pyramidal neurons, with bipolar branching dendrites and a normal orientation perpendicular to the cell layer (Fig. 1B). The GFP+ cells located within the hilus exhibited morphologies of both pyramidal cells and interneurons. Occasionally, GFP+ cells with morphological features of granule cells were found within the dentate granule cell layer (data not shown). Similar to the cortex, some GFP+ grafted cells in the hippocampus had differentiated into astrocyte-like cells. Overall, <30% of the transplanted differentiated cells expressed astrocyte-like morphologies, and only few cells had oligodendrocyte-like appearance.
In addition, GFP+ cells with immature or undifferentiated appearance were observed in varying numbers, often forming chains of cells associated with small-sized blood vessels. As reported previously (9), these cells express the progenitor markers nestin and vimentin, and in a few cases also NG2. They are, in most cases, not detectable at longer survival times, suggesting that they constitute a pool of undifferentiated RN33B cells that with time either undergo differentiation or die (9).
Axonal Projections.
To determine whether the grafted pyramidal-like GFP+ neurons had established axonal connections with their primary targets, we injected the retrograde tracer FG into the ventro-lateral and ventro-basal thalamic nuclei (in animals with cortical transplants; Fig. 2A) or into the hippocampal CA3 region (in animals with hippocampal transplants; Fig. 2B). The ability of the GFP+ neurons to extend long distance projecting axons is suggested by the presence of GFP+ fibers along the internal capsule and hippocampal commissures, and networks of beaded GFP+ terminals in the ipsilateral thalamus and contralateral hippocampus, as observed in animals with cortical and hippocampal cell grafts, respectively (9). Consistent with these observations, a significant proportion of the GFP+ pyramidal-like cells, ≈20% in cortex and 35% in hippocampus, were found to contain the FG label (Fig. 3 and Table 1). In the fronto-parietal cortex, the grafted GFP+ cells labeled by FG from the thalamus were found in the same layers as the FG labeled, GFP-negative host cells, and were thus intermingled with the intrinsic FG-labeled cortico-thalamic neurons. In the hippocampus, the FG/GFP double-labeled cells occurred both in the CA3 pyramidal layer and in the dentate hilus, intermingled with host pyramidal neurons traced retrogradely from the contralateral hippocampus.
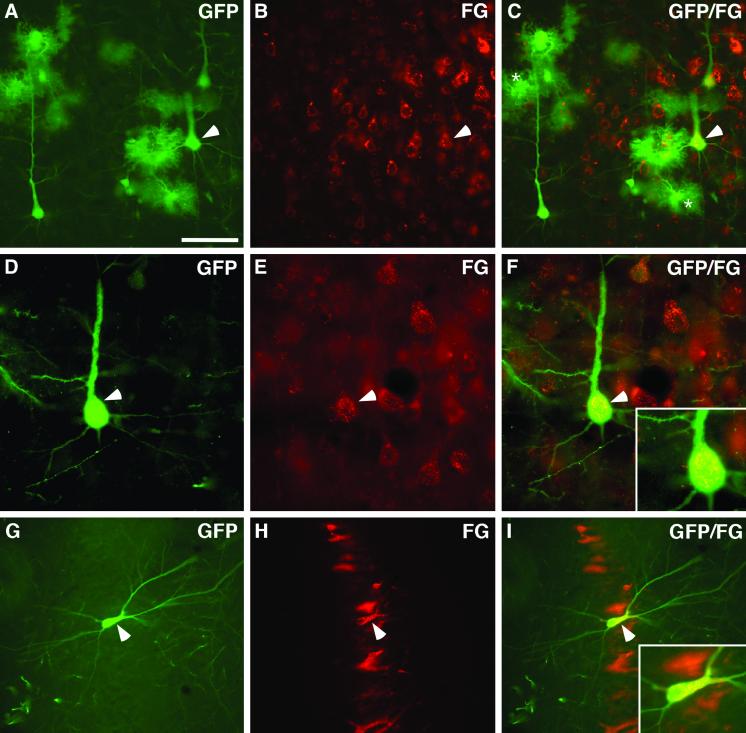
Fig 3.
Double immunofluorescent stainings of GFP and FG in cortex and hippocampus. (A–C) A significant number of the GFP+ cells (A, green), found in deeper cortical layers, were double-labeled with FG (B, red; merged image in C). Arrowhead in C shows a clear example of a double-labeled cell. (D–F) High magnification of a GFP-expressing cell (D, arrowhead) with axonally retrogradelly transported FG granules (E, arrowhead) distributed in the cell body (F, merged image, arrowhead) and in the proximal part of the apical dendrite (F, see Inset). (G–I) GFP/FG double-labeled grafted cell (G, GFP, green; H, FG, red; merged image in I) located in the pyramidal layer of the CA3 region and fulfilling the morphological criteria of a pyramidal neuron. (Scale bar = 80 μm in A, 35 μm in B, and 100 μm in C.)
Table 1.
Numbers of GFP+ neurons with pyramidal morphology in cortex and hippocampus retrogradely labeled with FG
| Rat no. | No. of GFP-labeled cells | No. of GFP/FG double-labeled cells | % of FG- labeled cells |
|---|---|---|---|
| Cortex | |||
| 1 | 67 | 14 | 21 |
| 2 | 38 | 14 | 37 |
| 3 | 81 | 11 | 14 |
| 4 | 112 | 13 | 12 |
| Average | 75 | 13 | 21 |
| Hippocampus | |||
| 1 | 31 | 13 | 42 |
| 2 | 17 | 4 | 24 |
| 3 | 22 | 8 | 36 |
| 4 | 30 | 14 | 47 |
| Average | 25 | 10 | 37 |
Cells with pyramidal neuronal morphology were counted in every sixth section throughout the region containing both GFP+ cells and FG-labeling, in the pyramidal layer of the CA3 region in the hippocampus and deeper layers in the cortex. Morphological criteria were angular cell bodies and apical and basal branching dendrites.
Physiological Properties and Functional Integration.
In this analysis, we only included grafted pyramidal-like cells, which showed co-localization of GFP autofluorescence and biocytin staining (Fig. 4A). All recorded RN33B cells were located in deep cortical layers.
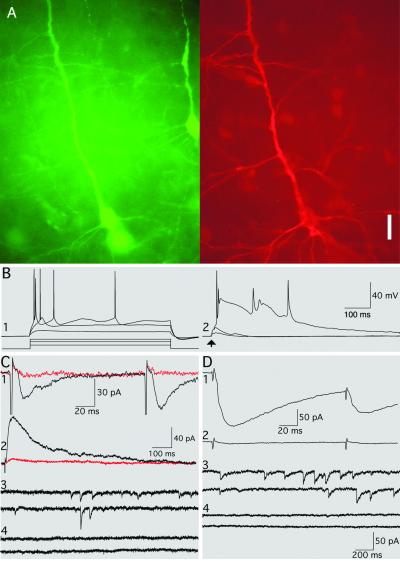
Fig 4.
Transplanted RN33B cells with neuronal morphology generate action potentials and are synaptically integrated into the host brain. (A) GFP+ cell (Left) filled with biocytin (Right). Note another transplanted GFP cell (Left) that is not filled with biocytin. (Scale bar = 25 μm.) (B) Current-clamp mode. Depolarization pulses induce action potentials (B1), whereas cortical stimulations induce EPSPs (B2) with superimposed action potentials in the double-labeled transplanted RN33B cell. Resting membrane potential = −66 mV; PTX is present in the aCSF. (C) Voltage-clamp mode. (C1) Paired-pulse facilitation of non-NMDA receptor-mediated EPSCs (black trace) recorded at holding potential of −70 mV from the transplanted double-labeled cell (same as in A). Addition of NBQX into the aCSF bocks EPSCs (red trace). (C2) NMDA receptor-mediated EPSC (black trace) at holding potential of +40 mV in the presence of NBQX in the aCSF. Addition of d-AP5 blocks EPSC (red trace). All traces are an average of three responses. (C3) Spontaneous/miniature EPSCs are blocked (C4) by NBQX and d-AP5 addition. PTX is present in the aCSF. (D) Voltage-clamp mode with holding potential of −70 mV. (D1) Paired-pulse depression of IPSCs recorded from the transplanted double-labeled cell. (D2) IPSCs are blocked by addition of PTX to the aCSF. All traces are an average of three responses. (D3) Spontaneous/miniature IPSCs are blocked (D4) by PTX addition. NBQX and d-AP5 are present in the aCSF. Duration of the depolarizing pulse in B is 1 s. Calibration for C 3 and 4 is the same as for D 3 and 4.
First, we tested the ability of these cells to generate action potentials. In all recorded RN33B cells, depolarizing current pulses elicited action potentials, which exhibited pronounced frequency accommodation and afterhyperpolarizations (Fig. 4B1). These properties of transplanted RN33B cells were similar to those of control cells, i.e., cortical pyramidal neurons in layers IV–VI recorded from the same group of animals (data not shown). The average resting membrane potential (72.3 ± 1.6 mV, n = 12) and input resistance (106 ± 13 MOhm, n = 12) of RN33B cells did not differ from those of host control neurons (73.6 ± 1.2 mV, n = 20; 113 ± 15 MOhm, n = 11, respectively). Spike amplitudes, as measured between depolarization pulse and spike peaks, were also not different between the host (72.0 ± 3.6 mV) and transplanted RN33B (67.8 ± 3.8 mV) cells.
Next we explored whether transplanted RN33B cells received excitatory synaptic inputs from host neurons. In support of this hypothesis, electrical stimulation of the surrounding cortex evoked EPSPs in the RN33B cells (Fig. 4B2). Because GABAA receptor-mediated inhibition was blocked by PTX, EPSPs were often manifested as so-called paroxysmal depolarizing shifts (PDSs; for example, see ref. 15) with superimposed, partially inactivated action potentials (Fig. 4B2).
We then investigated whether the excitatory synapses on the transplanted RN33B cells could express short-term plasticity, and whether they used the neurotransmitter glutamate and contained non-NMDA and NMDA receptors. All recorded cells exhibited paired-pulse facilitation of EPSCs, a form of short-term synaptic plasticity (16), at holding membrane potential of −70 mV (Fig. 4C1, black trace). These EPSCs were blocked by the selective non-NMDA receptor antagonist NBQX (Fig. 4C1, red trace). In the presence of NBQX, NMDA receptor-mediated EPSCs could still be induced on stimulation at holding potential of +40 mV (Fig. 4C2, black trace) and were effectively blocked by the selective NMDA receptor antagonist d-AP5 (Fig. 4C2, red trace). We also found that these excitatory synapses were active even without stimulation by recording spontaneous EPSCs (Fig. 4C3), which were blocked by addition of NBQX and d-AP5 (Fig. 4C4).
Finally, we examined whether transplanted RN33B cells also had inhibitory GABAergic synaptic inputs from host neurons. In the presence of NBQX and d-AP5 in the aCSF, paired stimulations induced IPSCs, which exhibited paired-pulse depression (Fig. 4D1). These IPSCs were effectively blocked by addition of PTX to the aCSF (Fig. 4D2). Spontaneous IPSCs (Fig. 4D3) were also blocked (Fig. 4D4) by PTX application, providing further evidence for the presence of GABAergic inhibitory synapses on the grafted RN33B cells.
All excitatory and inhibitory synaptic responses of the RN33B cells closely resembled those of host control pyramidal neurons recorded in the same animals and cortical layers (data not shown).
Discussion
Previous studies have shown that RN33B cells, grafted into neonatal rat cortex and hippocampus, can generate cells with morphological characteristics of intrinsic pyramidal neurons, including elaborated apical and basal dendritic arbors, richly equipped with spines, and vertical alignment within appropriate cortical and hippocampal layers (6–8). We demonstrate here that a large fraction of the graft-derived neurons in both cortex and hippocampus establish axonal projections with the two principal targets of the intrinsic pyramidal cells, i.e., the ventral thalamic nuclei and contralateral CA3 region, respectively. Most importantly, however, we show that the graft-derived, pyramidal-like cortical cells exhibit physiological properties of mature intrinsic pyramidal neurons and become functionally integrated into host neural circuitry. These morphological and electrophysiological observations are quite remarkable considering the fact that the generation of pyramidal neurons, both in cortex and hippocampus, is a prenatal process in the rat (17). Thus, at the time when the cells were implanted (P1–P2), cortical and hippocampal neurogenesis of the host pyramidal cells has been completed and the major efferent projections have already been established.
This study relies on the unambiguous identification of and electrophysiological recording from the transplanted RN33B cells. High resolution of the imaging equipment, and strong and stable GFP expression in combination with retroactive biocytin labeling make us absolutely confident that all recordings made from the grafted RN33B cells are genuine.
All grafted RN33B cells with pyramidal-like neuronal morphology, which were recorded in current-clamp mode 4–7 weeks after transplantation, were able to generate action potentials. The amplitude of action potentials, as well as input resistance and resting membrane potential of these cells closely resembled those of surrounding host cortical pyramidal neurons, indicating that at this time point, the transplanted RN33B cells had reached a degree of maturation similar to that of host neurons. In line with our observations, van Praag et al. (18) have reported that new granule cells formed from endogenous progenitors in the subgranular zone of the rat dentate gyrus were able to generate action potentials in response to depolarizing pulses at 4 weeks of age. In the study of Auerbach et al. (19), neuronal precursors from cultured (for 6 days) embryonic tissue transplanted into the hippocampus of 18-day-old rat embryos did not exhibit action potentials earlier than 4 weeks after implantation, and only two cells with neuronal morphology could generate spikes at this time point. Taken together, these data indicate that a time period of at least 4 weeks is needed to allow progenitors to develop a physiologically mature neuronal phenotype.
The generation of EPSPs and PDSs in the RN33B cells induced by stimulation of host tissue strongly suggests that surrounding neurons make excitatory synapses on the transplanted cells. It seems less likely that this response was caused by self-innervation, because action potentials generated by depolarizing pulses in the transplanted cells never induced EPSPs or PDSs in the same cell (for example, see ref. 20).
Whole-cell recordings of the transplanted RN33B cells in the voltage-clamp mode showed that they expressed paired-pulse facilitation of EPSCs, which was similar to that recorded in host pyramidal neurons, and is a characteristic feature of central excitatory synapses (for example, see ref. 16). These EPSCs were blocked by the selective non-NMDA receptor antagonist NBQX, supporting the glutamatergic nature of these synapses. Moreover, excitatory synapses on the transplanted cells contained functional NMDA receptors, which were blocked by the NMDA receptor antagonist d-AP5. Together with the observation of spontaneous EPSCs in the transplanted RN33B cells, our data indicate that excitatory synapses on these cells are functional, express short-term plasticity and have a pharmacological profile similar to that of host pyramidal neurons.
The transplanted RN33B cells exhibited both stimulation-induced and spontaneous IPSCs, which were blocked by PTX, a common GABAA receptor antagonist. This finding implies that host neurons make not only excitatory but also inhibitory, GABAergic synapses on the grafted cells. When paired stimulations were applied, the IPSCs expressed paired-pulse depression. This effect is thought to be mediated by activation of presynaptic GABAB receptors (refs. 21 and 22, but see ref. 23), resulting in a feedback inhibition of GABA release. This observation, therefore, may suggest that the inhibitory synapses formed by host neurons on the RN33B cells also contain presynaptic GABAB receptors. Taken together, inhibitory synapses on the transplanted RN33B cells closely resemble those present on host cortical pyramidal neurons.
Our data show the ability of in vitro expanded neural stem/progenitor cells to develop into functional neurons and become synaptically integrated into host cortical circuitry after grafting into the neocortex of neonatal rats. Whether similar integration would occur in adult brain remains to be elucidated. However, at the stage of development when RN33B cells were transplanted, cortical neurogenesis is complete (17), whereas synaptogenesis continues well into the third postnatal week in this region (24). Although the RN33B cells have a high intrinsic propensity to form neurons, both in vitro and in vivo (4, 5, 7), the extent of neurogenesis and the types of neurons formed differ greatly between different brain regions (see ref. 9). The ability of the grafted progenitors to differentiate into diverse, functionally mature neuronal phenotypes and form appropriate long-distance axonal connections is thus likely to depend on signals coming from the local tissue environment. The factors governing differentiation and functional integration of transplanted stem/progenitor cells remain largely unknown. Interestingly, in adult hosts subjected to focal excitotoxic lesions, Shihabuddin et al. (25) and Lundberg et al. (9) have observed that the grafted RN33B cells differentiate into fully mature neurons only in areas where the intrinsic neurons are intact or partially spared, and that cells that migrate into neuron-depleted (i.e., gliotic) areas remain undifferentiated. These data strongly suggest that neuronal differentiation, and perhaps also functional integration, of the grafted RN33B cells are controlled by the presence of as yet unidentified local cues, and that direct cell-to-cell interactions, rather than diffusible molecules, are involved.
The demonstration that grafted stem/progenitor cell lines can produce functionally integrated neurons, even after host neurogenesis is complete and in brain areas outside the classical neurogenic regions, suggest that such cells could be useful for neuronal replacement and brain repair.
Acknowledgments
We thank Birgit Haraldsson, Ulla Jarl, Anneli Josefsson, Monica Lundahl, Anna-Karin Oldén, and Bengt Mattsson for excellent technical assistance. This study was supported by the Swedish Research Council (04X-3874 and 33X-08666), the Medical Faculty at the University of Lund, and the Elsa and Thorsten Segerfalk, Crafoord, Knut and Alice Wallenberg, and Söderberg Foundations.
Abbreviations
FG, Fluorogold
aCSF, artificial cerebrospinal fluid
EPSC, excitatory postsynaptic current
EPSP, excitatory postsynaptic potential
IPSC, inhibitory postsynaptic current
GABA, γ-aminobutyric acid
NMDA, N-methyl-d-aspartate
NBQX, 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline
d-AP5, d(−)-2-amino-5-phosphonopentanoic acid
PTX, picrotoxin
References
- 1.Gage F. H. (2000) Science 287, 1433-1438. [DOI] [PubMed] [Google Scholar]
- 2.Svendsen C. & Caldwell, M. (2000) Prog. Brain Res. 127, 13-34. [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Serrano A., Rubio, F., Navorro, B., Bueno, C. & Villa, A. (2001) Curr. Gene Ther. 1, 279-299. [DOI] [PubMed] [Google Scholar]
- 4.Whittemore S. R. & White, L. A. (1993) Brain Res. 615, 27-40. [DOI] [PubMed] [Google Scholar]
- 5.Onifer S. M., Whittemore, S. R. & Holets, V. R. (1993) Exp. Neurol. 122, 130-142. [DOI] [PubMed] [Google Scholar]
- 6.Shihabuddin L. S., Hertz, J. A., Holets, V. R. & Whittemore, S. R. (1995) J. Neurosci. 15, 6666-6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shihabuddin L. S., Brunschwig, J. P., Holets, V. R., Bunge, M. B. & Whittemore, S. R. (1996) J. Neurocytol. 25, 101-111. [DOI] [PubMed] [Google Scholar]
- 8.Lundberg C., Field, P. M., Ajayi, Y. O., Raisman, G. & Bjorklund, A. (1996) Brain Res. 737, 295-300. [DOI] [PubMed] [Google Scholar]
- 9.Lundberg C., Englund, U., Trono, D., Bjorklund, A. & Wictorin, K. (2002) Exp. Neurol. 175, 370-387. [DOI] [PubMed] [Google Scholar]
- 10.Zufferey R., Nagy, D., Mandel, R. J., Naldini, L. & Trono, D. (1997) Nat. Biotechnol. 15, 871-875. [DOI] [PubMed] [Google Scholar]
- 11.Zufferey R., Donello, J. E., Trono, D. & Hope, T. J. (1999) J. Virol. 73, 2886-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Englund U., Ericson, C., Rosenblad, C., Mandel, R. J., Trono, D., Wictorin, K. & Lundberg, C. (2000) NeuroReport 11, 3973-3977. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham M. G. & McKay, R. D. (1993) J. Neurosci. Methods 47, 105-114. [DOI] [PubMed] [Google Scholar]
- 14.Schmued L. C. & Fallon, J. H. (1986) Brain Res. 377, 147-154. [DOI] [PubMed] [Google Scholar]
- 15.Jones R. S. & Lambert, J. D. (1990) Neuroscience 34, 657-670. [DOI] [PubMed] [Google Scholar]
- 16.Zucker R. S. & Regehr, W. G. (2002) Annu. Rev. Physiol. 64, 355-405. [DOI] [PubMed] [Google Scholar]
- 17.Bayer S. & Altman, J. (1995) in The Rat Nervous System, ed. Paxinos, G. (Academic, San Diego), pp. 1041–1078.
- 18.van Praag H., Schinder, A. F., Christie, B. R., Toni, N., Palmer, T. D. & Gage, F. H. (2002) Nature 415, 1030-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Auerbach J. M., Eiden, M. V. & McKay, R. D. (2000) Eur. J. Neurosci. 12, 1696-1704. [DOI] [PubMed] [Google Scholar]
- 20.Lessmann V. & Heumann, R. (1998) Neuroscience 86, 399-413. [DOI] [PubMed] [Google Scholar]
- 21.Davies C. H. & Collingridge, G. L. (1993) J. Physiol. 472, 245-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pitler T. A. & Alger, B. E. (1994) J. Neurophysiol. 72, 2317-2327. [DOI] [PubMed] [Google Scholar]
- 23.Jensen K., Lambert, J. D. & Jensen, M. S. (1999) J. Neurophysiol. 82, 42-49. [DOI] [PubMed] [Google Scholar]
- 24.Sutor B. & Luhmann, H. J. (1995) Perspect. Dev. Neurobiol. 2, 409-419. [PubMed] [Google Scholar]
- 25.Shihabuddin L. S., Holets, V. R. & Whittemore, S. R. (1996) Exp. Neurol. 139, 61-72. [DOI] [PubMed] [Google Scholar]