Abstract
Notch receptors are single transmembrane receptors that contain a large number of epidermal growth factor-like repeats (EGF repeats) in their extracellular domains. Mutations in the EGF repeats of the human Notch 3 receptor lead to the vascular dementia disease Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL). The vast majority of CADASIL mutations are missense mutations removing or inserting cysteine residues in the EGF repeats, but it is not yet clear whether these mutations primarily affect receptor trafficking, maturation, and/or signaling. To address this issue, we have generated and analyzed stable cell lines expressing either wild-type murine Notch 3 (mNotch 3) or the mutant mNotch 3R142C, which corresponds to the prevalent CADASIL form of Notch 3, Notch 3R141C in humans. We find that a lower proportion of mNotch 3R142C is expressed in the site 1-cleaved configuration, and that reduced amounts of mNotch 3R142C appear at the cell surface, as compared with wild-type mNotch 3. This observation is accompanied by a higher propensity for mNotch 3R142C to form intracellular aggregates, which may be a result of increased accumulation or slowed transport in the secretory pathway. In contrast to the impaired cell surface expression, mNotch 3R142C signals equally well in response to Delta 1 and Jagged 1 as wild-type mNotch 3. Taken together, these data suggest that trafficking and localization rather than signaling of mNotch 3 are affected in mNotch 3R142C.
Keywords: delta, serrate, protein transport, cysteine residue, neurological disease
Notch signaling is a system for cell–cell communication, which is evolutionarily highly conserved (1). The Notch receptor is a single transmembrane-spanning protein with a large extracellular domain (EC). The Notch receptor undergoes a complex series of proteolytic processing events, which are a prerequisite for signaling. The first cleavage of the receptor, the site 1 (S1) cleavage, occurs at the extracellular side and takes place constitutively in the Golgi apparatus by a furin-like convertase (2). At the cell surface, the receptor interacts with ligand, Jagged or Delta, which are transmembrane proteins expressed on juxtaposed cells. Ligand interaction leads to a second cleavage of the receptor close to the plasma membrane at the extracellular side, the S2 cleavage (3, 4). The S2 cleavage in turn results in the third and final cleavage, the S3 cleavage, which occurs within the plasma membrane and is controlled by presenilin (5). This cleavage liberates the intracellular domain (IC) of the Notch receptor, which translocates to the nucleus and acts in conjunction with the DNA-binding protein RBP-Jκ to regulate transcription of target genes, primarily the hairy/enhancer of split (HES) genes (1).
A conspicuous feature of Notch receptors is the presence of a large number of tandemly organized epidermal growth factor-like repeats (EGF repeats) in the EC. An EGF repeat is a common protein motif that invariably contains six conserved cysteine residues, which form three intra-EGF-repeat disulfide bonds. The importance of the structural integrity of Notch receptor EGF repeats is underscored by the mutational spectrum in the dementia syndrome Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) (6). CADASIL was originally discovered by Bogaert in 1955 (7) and is characterized by migraine, cognitive impairment, depression, recurring subcortical infarcts, and dementia. The cell type primarily affected in CADASIL brains is the vascular smooth muscle cells (vsmc) of small and middle-sized arteries. These cells degenerate, which results in severe arteriopathy and infarcts. The human Notch3 gene was mapped to chromosome 19q13.1–13-2 (8), where the gene causing CADASIL was also located, and Joutel et al. (9) reported in 1996 that Notch 3 mutations were found in CADASIL patients. The CADASIL-causing mutations are either missense mutations or small in-frame deletions in the EGF repeats of Notch 3 and affect the number of cysteine residues, leading to an unpaired cysteine residue. Thus, either a cysteine residue is deleted or altered to another amino acid residue or, conversely, mutations of noncysteine residues lead to introduction of novel cysteine residues (6).
Despite the consistent pattern of cysteine mutations, it is not yet known how CADASIL-mutated Notch 3 receptors lead to degeneration of vsmc, i.e., whether this is a consequence of altered receptor trafficking, maturation, and/or signaling (10). In this report, we address the cellular mechanism underlying CADASIL by comparing intracellular trafficking, localization, and signaling from wild-type mNotch 3 or a CADASIL-causing mutant mNotch 3 receptor, mNotch 3R142C, in cultured human embryonic kidney (HEK) 293 cells. We find that both wild-type and mNotch 3R142C receptors can signal in response to both Delta and Jagged ligands, but that the amount of S1-cleaved and cell surface-expressed mNotch 3 is lower for the mNotch 3R142C form of the receptor. We discuss the implications of these findings for the pathogenesis of CADASIL.
Materials and Methods
DNA Constructs.
The cDNAs encoding mNotch 3 and mNotch 3R142C were cloned in pcDNA3. These cDNAs were further modified to also encode a Gal4VP16 domain (GVP), generating the mNotch 3-GVP and mNotch 3R142C-GVP constructs, to study Notch 3 receptor activation in a sensitive manner from an upstream activation sequence (UAS)-luc reporter gene construct, as described (11, 12). Information about these and other DNA constructs and the cloning procedures used can be found in Supporting Text, which is published as supporting information on the PNAS web site, www.pnas.org.
Generation of Stable HEK 293 Cell Lines.
HEK 293 cells were transfected with wild-type mNotch 3, mNotch 3R142C, mNotch 3-GVP, mNotch 3R142C-GVP, or empty pcDNA3 vector according to the Lipofectamine PLUS method (12). Stable lines were generated by recovering and expanding individual colonies after culturing the cells for 2 weeks after transfection in the presence of G418 (1 mg/ml). Expression levels of the mNotch 3 derivatives were examined by Western blot.
Western Blot.
Western blot analysis was performed as described (13). See the supporting information on the PNAS web site for further information.
Biotinylation Experiment.
Cultures of the stable HEK 293 cell lines were incubated with 0.5 mg/ml sulfo-NHS-biotin (Pierce) for 2 h at +4°C, and protein lysates were subsequently prepared in RIPA buffer. Twenty microliters of each sample were removed and stored at −70°C. The remaining lysate (380 μl) was incubated with 100 μl of streptavidin-coated magnetic beads (Dynal, Oslo) overnight at +4°C. Precipitated proteins were recovered in 40 μl of 2× SDS/PAGE loading buffer and further processed by Western blot.
Immunocytochemistry.
Stable HEK 293 transfectants were subjected to immunocytochemistry according to a previously described protocol (14). Presence of the large intracellular aggregates (specific antibody staining) was scored against total number of cells (DAPI positive) by manually counting the cells in the microscope. Brefeldin A (Sigma) was used at 2 μg/ml working solution, and MG132 was added to 3 μM. The following primary antibodies were used: rabbit (Rb) α-Calnexin (1:200) (Becton Dickinson); Rb α-p58 (1:700) (Sigma); mouse (m) α-CTR433 (1:1) (M. Bornens); m α-GAL4 (1:33) (Santa Cruz Biotechnology); Rb α-VP16 (1:100) (CLONTECH); goat (gt) α-Notch 3IC, M20 (1:100) (Santa Cruz Biotechnology); Rb α-pericentrin (1:300) (Becton Dickinson); and m α-vimentin (1:200) (Boehringer Mannheim). The following Alexa secondary antibodies (Molecular Probes) were used at dilution 1:1,500: gt α-m 488, gt α-m 546, gt α-Rb 488, and gt α-Rb 546. Donkey (d) α-gt 488 and d α-gt 546 were used at dilution 1:500.
Ligand/Receptor Coculture Assay.
Stable HEK 293 cell lines were transiently transfected with 2 μg of MH100 or 6×RBP-luc/2 μg pcDNA3.1/and 0.4 μg CMV-β-gal plasmid (12, 15) by the Lipofectamine PLUS method. Two separate 10-cm dishes of HEK 293 cells were transiently transfected with either 2 μg of Notch 3 IC-GVP or Notch 3 ΔE-GVP together with 2 μg of MH100 and 0.4 μg of CMV-β-gal plasmid. Three hours posttransfection, the cells were trypsinized and 3 × 105 cells of each transfected cell population were mixed with either 3 × 105 3T3-human Jagged 1, 3T3-Babe (control), 293-human Delta 1 or 293-pcDNA3 (control) cells, respectively, in separate 35-mm dishes. Forty-eight hours posttransfection, the cocultures were examined for Notch 3 signaling activity by luciferase reporter gene activity, as described (12).
Results
Reduced Levels of S1-Cleaved Notch 3R142C Receptor.
The R141C substitution is a prevalent mutation in CADASIL patients (6), and we decided to compare the behavior of receptors carrying this mutation with wild-type Notch 3 receptors in cultured cells. These studies were conducted with murine (m) Notch 3, which is highly homologous to human Notch 3, but where position 142 corresponds to position 141 of the human sequence (see Fig. 1 for a schematic depiction of the mNotch 3 receptor and the location of the R142C mutation). Cell lines stably expressing either wild-type mNotch 3 or mNotch 3R142C were generated in HEK 293 cells, and three separate lines expressing either receptor were selected for analysis.
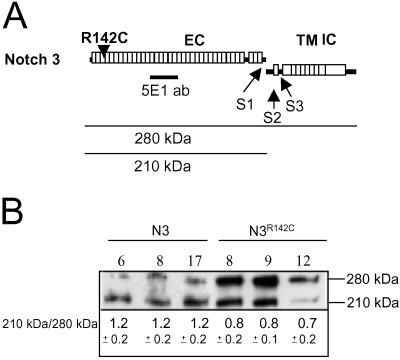
Fig 1.
Reduced expression of S1-cleaved Notch 3R142C receptor. (A) Schematic depiction of presumed processing events of the Notch 3 receptor. The full length (FL) Notch 3 receptor is processed at site 1 (S1) by a furin-like convertase during intracellular trafficking and is presented at the plasma membrane as a bipartite protein consisting of the EC and the transmembrane + intracellular domain (TMIC). The corresponding proteins migrate as 280- (FL), 210- (EC), and 97-kDa (TMIC) proteins. As a result of ligand activation, the receptor undergoes two consecutive cleavages (S2 and S3). The CADASIL-causing R142C mutation (R142C) is located in the third EGF repeat in the EC. (B) Total protein extracts from three different HEK 293 stable cell lines expressing wild-type mNotch 3 (clones 6, 8, and 17) and three lines expressing mNotch 3R142C (clones 8, 9, and 12) receptor were analyzed by Western blot using the α-Notch 3 EC antibody 5 E1. Note the increased levels of the 280-kDa band (FL receptor) in cells expressing the mNotch 3R142C receptor compared with wild-type mNotch 3-expressing cells. The Western blot is from one representative experiment, whereas the 210:280-kDa ratios are mean ± SD from three independent experiments.
We first analyzed the effects of the R142C mutation on S1 cleavage. Notch receptors are processed during trafficking to the cell surface by a furin-like convertase (S1 cleavage; see Fig. 1A), generating a bipartite receptor consisting of most of the EC linked via noncovalent binding to the rest of the protein, i.e., the transmembrane and IC domains. The data in Fig. 1B show that this is the case also for mNotch 3, which is in keeping with a recent report (10). Western blot analysis of protein extracts from cell lines expressing wild-type mNotch 3, using an antibody raised against the EC of mNotch 3 [5E1 (10)], revealed the presence of the unprocessed full length receptor (280 kDa) and the EC (210 kDa). Interestingly, however, the amount of S1-processed receptor was consistently reduced for mNotch 3R142C, i.e., less EC (210 kDa) was expressed in favor of the full length mNotch 3 receptor (280 kDa) (Fig. 1B). The ratio between the 210-kDa form and the 280-kDa form was 0.8, 0.8, and 0.7 for the three mNotch 3R142C receptor cell lines, whereas it was 1.2 for each of the three wild-type mNotch 3 cell lines (Fig. 1B).
Reduced Levels of the Notch 3R142C Receptor Reach the Cell Surface.
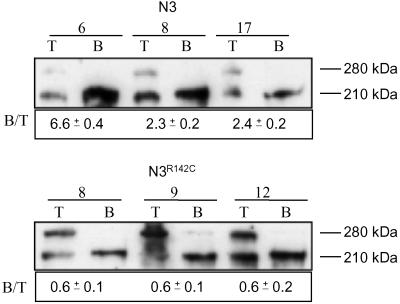
Given the reduction in the S1-cleaved mNotch 3R142C receptor, it may be anticipated that the amount of receptor reaching the plasma membrane was altered. To address this hypothesis, we performed cell-surface biotinylation experiments followed by streptavidin pull-down assays and Western blot analyses. In Notch 3 wild-type receptor-expressing cell lines, virtually all biotinylated receptors corresponded to the 210-kDa form (Fig. 2), indicating that only the S1-processed receptor reaches the cell surface, which is in keeping with previous observation (10). Similarly, the absolute majority of the biotinylated Notch 3R142C receptors were of the 210-kDa type (Fig. 2). However, the relative amounts of cell surface expression differed between wild-type Notch 3 and Notch 3R142C. The ratio of cell-surface-expressed, i.e., biotinylated, receptor vs. total amount of receptors was 3.8 for wild-type Notch 3 and 0.6 for Notch 3R142C (mean values) (Fig. 2). These data indicate that R142C-mutated Notch 3 receptors reach the cell surface almost exclusively in the S1-processed form, but at reduced levels.
Fig 2.
Reduced amounts of Notch 3R142C at the cell surface. Cell surface proteins of HEK 293 stable cell lines expressing either the mNotch 3 or the mNotch 3R142C receptor were biotinylated. Five percent of the protein extract was removed before the pull down with magnetic beads coupled to streptavidin. The remaining 95% of the extracted proteins was subjected to the streptavidin pull down (B) and followed by SDS/PAGE and Western blot analysis by using the 5E1 antibody along with 5% of total protein extract (T). Note that the ratio between the pulled-down receptor (B) and the total amount of expressed receptor (T) is lower for mNotch 3R142C compared with wild-type mNotch 3. The 210-kDa immunoreactive band that is present in all pulled-down fractions suggests that both the vast majority of wild-type and mNotch 3R142C receptors are expressed as S1-cleaved bipartite proteins at the cell surface. The Western blot is from one representative experiment, whereas the 210:280-kDa ratios are mean ± SD from three independent experiments.
Both Wild-Type and Notch 3R142C Receptors Respond to Induction by Jagged 1 and Delta 1 Ligands.
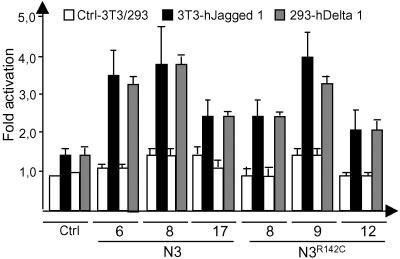
It has previously been shown that Notch 3 IC can serve as a weak activator of downstream gene expression (12, 14), but it has not been established whether a full length Notch 3 receptor can respond to known Notch ligands. To address this possibility, cell lines expressing wild-type or Notch 3R142C receptors were cocultured with cells expressing either human Delta 1 or Jagged 1 ligands. Both receptors responded to both ligands with similar efficacy, by using a hexamerized RBP-Jκ-binding site coupled to the luciferase gene as readout (Fig. 3). The rather low level of induction, both in terms of fold activation and absolute luciferase reporter gene activity, was expected based on the previously reported weak inductive capacity of the Notch 3 IC (12, 14).
Fig 3.
Both wild-type Notch 3 and Notch 3R142C receptors respond to Notch ligands. HEK 293 stable cell lines expressing either the wild-type mNotch 3 (Notch 3) or the mNotch 3R142C receptor were cocultured with 3T3 cells expressing either Jagged 1 (3T3-hJagged 1, black), HEK 293 cells expressing Delta 1 (293-hDelta 1, light gray), or the respective control line (Ctrl-3T3, white; Ctrl-293, white) and analyzed for 6× RBP–luciferase reporter gene activity. Similar levels of receptor activation were observed for the mNotch 3- and mNotch 3R142C-expressing cells. All transfections were performed in triplicate, and the experiment was repeated twice.
Introduction of a Notch 3R142C Mutation into a Notch 3 Gal4/VP16 Receptor Affects Receptor Maturation and Trafficking but Not Signaling.
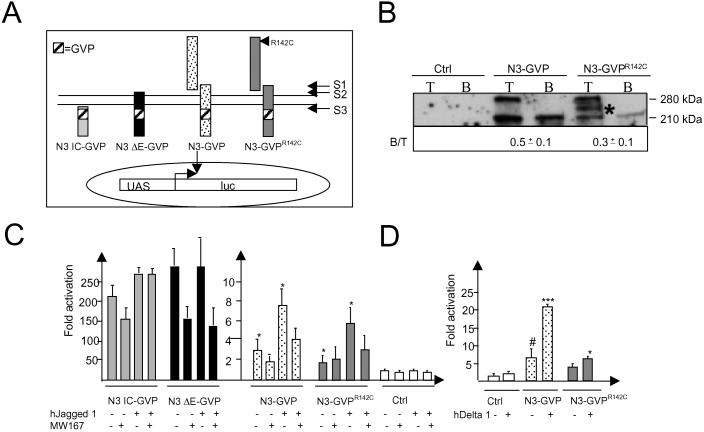
To examine the signaling capacity of wild-type and R142C-mutated mNotch 3 in an alternative and more sensitive way, we took advantage of a recently developed system in which Notch 3 receptor activation can be recorded in a specific manner by insertion of the GVP signaling domain into the IC (11, 13). After the final S3 cleavage, the Notch 3 IC containing GVP translocates to the nucleus, where it specifically activates transcription from a UAS promoter/reporter gene construct (see Fig. 4A). This system provides improved specificity, i.e., only signals from the engineered receptors but not from endogenous Notch receptors are recorded. Furthermore, the localization of the engineered receptors can be analyzed by using antibodies raised against the Gal4 or VP16 domains. Analysis of stable cell lines expressing the R142C mutation in a mNotch 3-GVP backbone, i.e., mNotch 3R142C-GVP, compared with mNotch 3-GVP showed an impaired S1 cleavage for mNotch 3R142C-GVP receptors (Fig. 4B). These data are in keeping with the results for the R142C mutation in a mNotch 3 lacking the GVP moiety (compare with Fig. 1B). We next analyzed by biotinylation whether mNotch 3R142C-GVP was present on the cell surface. Biotinylation experiments revealed that substantially less of mNotch 3R142C-GVP receptors appeared at the cell surface as compared with mNotch 3R142C (Fig. 4B). It thus appears that the R142C mutation leads to reduced S1 cleavage and transport to the cell surface also when introduced into the mNotch 3-GVP backbone, in keeping with data using a wild-type mNotch 3 receptor backbone.
Fig 4.
The Notch 3R142C-GVP receptor is impaired in S1-processing and appears in reduced amounts at the cell surface but retains signaling. (A) A GVP domain was inserted immediately C-terminal to the mNotch 3 S3-cleavage site of different mNotch 3 derivatives to enable specific recording of the engineered receptors from a UAS–luciferase reporter gene construct. (i) N3IC-GVP, the Notch 3 IC with GVP, ligand- and S3 cleavage-independent; (ii) N3 ΔE-GVP, an N-terminally truncated Notch 3 receptor, ligand-independent but S3-cleavage dependent; (iii) N3-GVP, ligand- and S3-cleavage dependent; (iv) N3R142C-GVP, the same as N3-GVP but with the R142C mutation. (B) Total protein (T) and cell surface biotinylated extracts (B) of HEK 293 stable cell lines expressing either mNotch 3-GVP or mNotch 3R142C-GVP were analyzed by Western blot by using the 5E1 antibody. As for the mNotch 3 receptors with a wild-type backbone (Fig. 2), we observed an impaired S1 cleavage as well as a decreased cell surface expression of mNotch 3R142C-GVP as compared with the corresponding mNotch 3-GVP receptors. The Western blot is from one representative experiment, and the B:T ratios are mean values from three independent experiments. *, Postlysis degradation product. The mNotch 3-GVP expressing cells were mixed with either 3T3-hJagged 1 cells (C), 293-hDelta 1 cells (D), or control (Ctrl) cells, and cultured in the presence or absence of the γ-secretase inhibitor MW167 (100 μM). The N3 IC-GVP and N3 ΔE-GVP transfected cells were included as controls for S3 cleavage and ligand-dependent activation, respectively. The experiment was performed in triplicate and repeated at least three times. *, P < 0,05; ***, P < 0.001 vs. Ctrl + Jagged 1 Delta 1; #, P < 0.05 vs. Ctrl.
Finally, we analyzed signaling using the UAS-luciferase reporter gene, which is specifically induced by the GVP-containing receptors. The same pattern was observed as for the wild-type backbones, i.e., both Delta 1 and Jagged 1 could activate signaling from both the mNotch 3-GVP and the mNotch 3R142C-GVP receptors (Fig. 4 C and D). The improved signal-to-noise ratio using the GVP system allowed us to test whether the ligand-induced Notch 3 signal required S3 cleavage and thus was presenilin dependent. We show that the ligand-induced signal was reduced by the γ-secretase inhibitor MW167 (Fig. 4C), which strongly suggests that the signal has to proceed via an S3 cleavage. As a control for MW167 activity, we show that signaling from membrane-bound constitutively active Notch 3 receptor, lacking most of the extracellular domain, mNotch 3 ΔE-GVP (Fig. 4A) (11), was sensitive to MW167, whereas signaling from the IC of mNotch 3, Notch 3 IC-GVP (Fig. 4A), was not (Fig. 4C). In conclusion, these data, using GVP-containing receptors, corroborate the data from wild-type receptor backbones and further underscore that the effects of the R142C mutation lie in decreased S1 cleavage and presence at the cell surface, rather than in an altered signaling capacity from the ligand-activated receptor.
Increased Intracellular Aggregation of Notch 3R142C and Notch 3-GVPR142C Receptors.
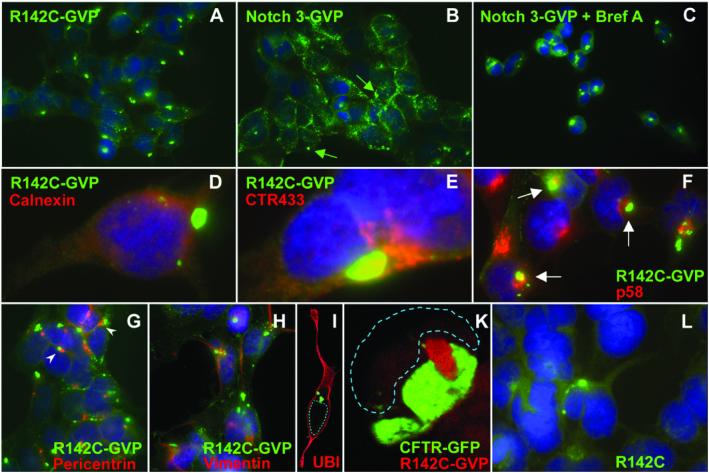
The observed reduction in S1 cleavage and in the amounts of mNotch 3R142C and mNotch 3-GVPR142C receptors at the cell surface may be a consequence of problems in transporting the receptors through the endoplasmic reticulum (ER) (16), in particular because S1 cleavage occurs in the Golgi compartment. Impaired ER transport of mutated proteins frequently results in ER stress and sometimes leads to secretion of the mutated protein into the cytoplasm (17, 18). To test whether R142C-mutated mNotch 3 receptors are more prone to intracellular aggregation, we analyzed their intracellular localization by immunocytochemistry in cells expressing R142C-mutated or wild-type receptors. We show that a large intracellular aggregate is formed in a majority (75%) of the mNotch 3R142C-GVP-expressing cells (Fig. 5A), whereas such an aggregate was seen in only 25% of the cells expressing mNotch 3-GVP (1,000 cells of each cell line counted; Fig. 5B), as visualized by an α-Gal4 antibody. Interestingly, treatment of the mNotch 3-GVP cells with Brefeldin A, which impairs vesicular transport between ER and Golgi (19), leads to a substantial increase in cells expressing intracellular aggregates, and the aggregates were indistinguishable from those seen in mNotch 3R142C-GVP-expressing cells (Fig. 5C).
Fig 5.
Introduction of the R142C mutation enhances the formation of cytoplasmic aggregates. The subcellular localization of the mNotch 3 receptors in stable HEK 293 cells was assayed by immunocytochemistry. mNotch 3R142C-GVP (R142C) expressing cells contain a single large cytoplasmic aggregate in 75% of the cells (A). In contrast, mNotch 3-GVP in most cells is distributed in patches at the cell surface, and the large cytoplasmic aggregate is found in only 25% of the cells (B). Brefeldin A treatment of the mNotch 3-GVP cells, however, increases the proportion of aggregate-containing cells (C). Costaining of the mNotch 3-containing aggregates with calnexin demonstrates that the aggregates do not colocalize with ER (D). Costaining with the Golgi-marker CTR433 revealed that the aggregates were located close to the Golgi, but with no apparent overlap (E). Similarly, no colocalization with the intermediate compartment was observed, as visualized by double staining with the p58 antibody (F). To investigate the relationship with aggresomes in more detail, double immunocytochemistry with antibodies recognizing pericentrin (G), vimentin (H), and ubiquitin (I) was performed. The aggregates are located close to the centrosomes (G) but are not caged with vimentin (H) nor do they contain ubiquitin (I). Coexpression of GFP-CFTRΔF508 (CFTR) in stable mNotch 3R142C-GVP cell lines shows that mNotch 3- and CFTR-containing aggregates are close to each other but form separate clusters (K). Single large cytoplasmic aggregates were detected at low frequency also in mNotch 3R142C cells (L). mNotch 3-containing proteins were detected by anti(α)-Gal4 antibodies (A–C and G), −VP16 antibodies (D–F and H), or with the M20 antibody raised against the intracellular domain of Notch 3 (L).
To establish the intracellular localization of the aggregate in more detail, we double-stained the aggregate with markers for ER (Calnexin), Golgi (CTR433), and the intermediate compartment (p58) (Fig. 5 D–F). The aggregates did not perfectly colocalize with any of these three markers but were often located in the vicinity of the Golgi complex. Proximity to the Golgi and the location near the nucleus was reminiscent of that of the recently discovered aggresome (20). Aggresomes are cytoplasmic structures formed in response to ER stress. They are high-molecular-weight complexes that contain ubiquitin, localize to the centrosomes, and are caged with vimentin. Expression of a specific mutation in the cystic fibrosis transmembrane conductance regulator (CFTR), GFP-CFTRΔF508, causes aggresome formation (20). The mNotch 3-containing aggregates were often localized close to centrosomes (Fig. 5G) but were not caged with vimentin (Fig. 5H) and did not contain ubiquitin (Fig. 5I). Coexpression of mNotch 3R142C-GVP with GFP-CFTRΔF508 showed that Notch 3 and CFTR-containing aggregates are close to each other but form separate clusters (Fig. 5K).
Formation of the large intracellular aggregates was not restricted to cells expressing mNotch 3-GVP or mNotch 3R142C-GVP but were also observed in cells expressing mNotch 3 receptors with a wild-type receptor backbone, although at a much lower frequency. Notably, the observation that an R142C mutation increased the frequency of receptor aggregation was still valid, i.e., four times more cells with aggregates were detected in mNotch 3R142C-expressing cells (4% of the cells) as compared with wild-type mNotch 3-expressing cells (1% of the cells) (1,000 cells of each cell line counted; Fig. 5L). We conclude from these experiments that the introduction of the R142C mutation increases the propensity to form cytoplasmic aggregates containing mNotch 3.
Discussion
The objective of this study was to address at what level a prevalent CADASIL mutation affects Notch 3 receptor function. Notch receptors undergo complex proteolytic processing events before they can activate downstream genes, which leaves a number of possibilities open for CADASIL mutations to interfere with Notch 3 function. Our data show that full-length mNotch 3 receptors can respond to ligand induction, and that the R142C CADASIL mutation does not affect signaling. In contrast, the first step in the proteolytic processing, S1 cleavage, is reduced for the R142C-containing receptors, and less mutant receptor is found at the cell surface.
The reduction in S1 cleavage could be explained in at least two ways. First, it is possible that the reduced cleavage is a consequence of R142C-containing receptors being less potent substrates for the furin-like convertase that executes the S1 cleavage in the Golgi complex (2). Alternatively, it is possible that transport of a mutated receptor through the ER is impaired or slowed down, which may result in fewer receptors becoming available for S1 cleavage. At this point, we cannot strictly distinguish between these possibilities, although we favor the latter view, because mutations in other genes structurally reminiscent of the Notch 3 CADASIL mutations lead to folding problems and/or protein aggregation in the ER. This is the case in Marfan's syndrome, where mutations altering the number of cysteine residues in the multimerized EGF repeats of elastin cause an accumulation of elastin in the ER (21). Furthermore, Brefeldin A treatment, which impairs vesicular transport between ER and Golgi and results in reduced S1 cleavage of the Notch 1 receptor (2), gives rise to an intracellular Notch 3 aggregate formation similar to that caused by the R142C mutation.
Irrespective of the exact reason for the observed reduction in S1 cleavage, it would be anticipated that it results in fewer receptors at the cell surface, because only S1-processed receptors appear at the plasma membrane (2, 22, 23). This is indeed the case for the R142C-mutated mNotch 3 receptors, as we observe 6.3- and 1.7-fold reductions for mNotch 3R142C and mNotch 3R142C-GVP, respectively, at the plasma membrane, compared with their nonmutated counterparts, i.e., mNotch 3 and mNotch 3-GVP.
We found mNotch 3-containing intracellular aggregates in the cytoplasm, which demonstrates that Notch 3 receptors are directed from their normal transport route through the ER and Golgi to the plasma membrane. In many cases, formation of such aggregates is a consequence of ER stress (16), further supporting the notion that transport in the ER is impaired for mutated mNotch 3 receptors. Interestingly, the insertion of both the R142C mutation and the GVP domain contributes to the frequency of aggregate formation. The GVP domain is a key contributor, because the frequency of aggregate formation increases 1–25% of the cells when wild-type mNotch 3 and mNotch 3-GVP receptors are compared. However, insertion of the R142C mutation also influences the frequency of aggregate formation, because the fraction of cells with mNotch 3-containing aggregates then increases by a factor three (25–75% when mNotch 3-GVP and mNotch 3R142C-GVP are compared and 1–4% when wild-type mNotch 3 and mNotch 3R142C are compared). It may be that the R142C mutation has an inhibitory effect on ER transport, leading to more receptors being secreted from the ER and that the GVP moiety enhances aggregate formation of secreted receptors (Gal4 DNA-binding regions form homodimers). In support of this view, exposure of cells expressing mNotch 3-GVP to Brefeldin A increased the proportion of cells containing mNotch 3 aggregates to a similar extent as the R142C mutation.
The exact nature of the intracellular mNotch 3-containing aggregates remains to be established, but they may have some features in common with the recently discovered aggresomes. This is based on mNotch 3-containing aggregates being located perinuclearly close to the centrosomes and colocalizing with the GFP-CFTR ΔF508 aggregates, although with no apparent intermingling of the two proteins. The mNotch 3-containing aggregates, however, are not caged by vimentin and do not contain ubiquitin, which is the situation with the GFP-CFTR ΔF508 aggresome.
In contrast to the impaired intracellular trafficking and maturation of the mutated mNotch 3 receptors, we find that the mNotch 3 receptor, in both the normal and the R142C-mutated form, can respond at comparable levels to both Delta and Jagged ligands. This is, to our knowledge, the first direct demonstration that full length Notch 3 can be functionally activated by ligand. The observation that ligand activation could be reduced by γ-secretase inhibitors supports the notion from experiments with truncated Notch 1 and Notch 3 receptors (11, 13) that ligand activation of Notch 3 requires presenilin-mediated S3-cleavage.
What may be the implications of our data for the understanding of CADASIL pathology? First, Jagged 1-induced activation of Notch 3 is of interest, because Jagged 1 is expressed in brain vsmc, where also Notch 3 receptors are expressed (10, 24). This observation makes Jagged 1 a candidate ligand to activate Notch 3 in vivo in the cell type primarily affected in CADASIL. Second, the observation that ligand-activated signaling is not affected by the introduction of a CADASIL mutation into Notch 3 may also shed light on the enigma that CADASIL has a late onset, despite the fact that the Notch 3 receptor is expressed at higher levels in the embryonic central nervous system and vascular system than in the adult (24, 25). ER stress, rather than altered signaling, would be more compatible with late onset of the disease. Radical alterations of Notch signaling, for example by knockout experiments of Notch receptors and ligands or introduction of constantly active signaling forms of the receptors (IC constructs), often result in severe embryonic defects (26–28). ER stress, on the other hand, for example in the form of increased NFκB or cytokine expression (16), may act over more extended time periods, which could lead to the gradual deterioration of the vsmc seen in CADASIL. In support of this view, ER stress plays a role in several other neurological diseases with slow progression, for example in Parkinson's and Huntington's diseases (29) and also in Marfan's syndrome, as discussed above.
It is important to note that the impaired receptor processing observed in this study is most likely not the only means by which R141C-mutated Notch 3 receptors (corresponding to R142C in mNotch 3) may contribute to CADASIL. In a recent study, Joutel et al. (10) observed that the EC of S1-processed Notch 3 receptors accumulates around vsmc in postmortem material from CADASIL patients, and that this is the case also in a patient carrying the R141C mutation. We could not detect any EC accumulation around the HEK 293 cells. This may be due to that EC accumulation either requires an intact cellular organization and takes place over a long period or, alternatively, being a specific phenomenon for Notch 3 expressed in vsmc. Alternatively, the apparent exocytotic activity of vsmc of CADASIL-affected individuals may explain the lack of intracellular Notch 3 aggregates in vivo. It is therefore possible that impaired receptor processing and EC accumulation together contribute to CADASIL.
Supplementary Material
Acknowledgments
We are grateful to Drs. Matthew Rand (Department of Cell Biology, Harvard Medical School), Anne Joutel (Faculté de Médecine Lariboisière, Paris), Jeff Sklar (Department of Medicine and Pathology, Brigham and Women's Hospital, Boston), Tom Kadesch (Department of Genetics, University of Pennsylvania School of Medicine, Philadelphia), Michel Bornens (Institute Curie, Paris), and Ron Kopito (Department of Biological Sciences, Stanford University, Stanford, CA) for kindly sharing reagents with us. This work was supported by Cancerfonden, the European Union (2808-B01-13XBC), Tore Nilssons Stiftelse (J.L.), and AMF Sjukförsäkrings Jubileumsstiftelse (J.L.). G.C. was supported by a National Health and Medical Research Council (Australia) CJ Martin Fellowship (No. 158043).
Abbreviations
EC, extracellular domain
IC, intracellular domain
EGF repeats, epidermal growth factor-like repeats
CADASIL, Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy
vsmc, vascular smooth muscle cells
GVP, Gal4VP16 domain
ER, endoplasmic reticulum
CFTR, cystic fibrosis transmembrane conductance regulator
S1, site 1
HEK, human embryonic kidney
References
- 1.Artavanis-Tsakonas S., Rand, M. D. & Lake, R. J. (1999) Science 284, 770-776. [DOI] [PubMed] [Google Scholar]
- 2.Logeat F., Bessia, C., Brou, C., LeBail, O., Jarriault, S., Seidah, N. G. & Israel, A. (1998) Proc. Natl. Acad. Sci. USA 95, 8108-8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brou C., Logeat, F., Gupta, N., Bessia, C., LeBail, O., Doedens, J. R., Cumano, A., Roux, P., Black, R. A. & Israel, A. (2000) Mol. Cell 5, 207-216. [DOI] [PubMed] [Google Scholar]
- 4.Mumm J. S., Schroeter, E. H., Saxena, M. T., Griesemer, A., Tian, X., Pan, D. J., Ray, W. J. & Kopan, R. (2000) Mol. Cell 2, 197-206. [DOI] [PubMed] [Google Scholar]
- 5.De Strooper B., Annaert, W., Cupers, P., Saftig, P., Craessaerts, K., Mumm, J. S., Schroeter, E. H., Schrijvers, V., Wolfe, M. S., Ray, W. J., Goate, A. & Kopan, R. (1999) Nature 398, 518-522. [DOI] [PubMed] [Google Scholar]
- 6.Joutel A., Vahedi, K., Corpechot, C., Troesch, A., Chabriat, H., Vayssiere, C., Cruaud, C., Maciazek, J., Weissenbach, J., Bousser, M. G., Bach, J. F. & Tournier-Lasserve, E. (1997) Lancet 350, 1511-1515. [DOI] [PubMed] [Google Scholar]
- 7.Bogaert V. (1955) Med. Hellen. 24, 961-972. [Google Scholar]
- 8.Larsson C., Lardelli, M., White, I. & Lendahl, U. (1994) Genomics 24, 253-258. [DOI] [PubMed] [Google Scholar]
- 9.Joutel A., Corpechot, C., Ducros, A., Vahedi, K., Chabriat, H., Mouton, P., Alamowitch, S., Domenga, V., Cecillion, M., Marechal, E., et al. (1996) Nature 383, 707-710. [DOI] [PubMed] [Google Scholar]
- 10.Joutel A., Andreux, F., Gaulis, S., Domenga, V., Cecillon, M., Battail, N., Piga, N., Chapon, F., Godfrain, C. & Tournier-Lasserve, E. (2000) J. Clin. Invest. 105, 597-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taniguchi Y., Karlström, H., Lundkvist, J., Mizutani, T., Otaka, A., Vestling, M., Bernstein, A., Donoviel, D., Lendahl, U. & Honjo, T. (2002) Proc. Natl. Acad. Sci. USA 99, 4014-4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beatus P., Lundkvist, J., Oberg, C. & Lendahl, U. (1999) Development (Cambridge, U.K.) 126, 3925-3935. [DOI] [PubMed] [Google Scholar]
- 13.Karlstrom H., Bergman, A., Lendahl, U., Naslund, J. & Lundkvist, J. (2002) J. Biol. Chem. 277, 6763-6766. [DOI] [PubMed] [Google Scholar]
- 14.Beatus P., Lundkvist, J., Oberg, C., Pedersen, K. & Lendahl, U. (2001) Mech. Dev. 104, 3-20. [DOI] [PubMed] [Google Scholar]
- 15.Kuroda K., Tani, S., Tamura, K., Minoguchi, S., Kurooka, H. & Honjo, T. (1999) J. Biol. Chem. 274, 7238-7244. [DOI] [PubMed] [Google Scholar]
- 16.Aridor M. & Balch, W. E. (1999) Nat. Med. 5, 745-751. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman R. J. (1999) Genes Dev. 13, 1211-1233. [DOI] [PubMed] [Google Scholar]
- 18.Kopito R. R. & Ron, D. (2000) Nat. Cell Biol. 2, E207-E209. [DOI] [PubMed] [Google Scholar]
- 19.Pelham H. R. (1991) Cell 67, 449-451. [DOI] [PubMed] [Google Scholar]
- 20.Johnston J. A., Ward, C, L. & Kopito, R, R. (1998) J. Cell Biol. 143, 1883-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Godfrey M., Olson, S., Burgio, R. G., Martini, A., Valli, M., Cetta, G., Hori, H. & Hollister, D. W. (1990) Am. J. Hum. Genet. 46, 661-671. [PMC free article] [PubMed] [Google Scholar]
- 22.Blaumueller C. M., Qi, H., Zagouras, P. & Artavanis-Tsakonas, S. (1997) Cell 90, 281-291. [DOI] [PubMed] [Google Scholar]
- 23.Haritunians T., Boulter, J., Hicks, C., Buhrman, J., DiSibio, G., Shawber, C., Weinmaster, G., Nofziger, D. & Schanen, C. (2002) Circ. Res. 90, 506-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prakash N., Hansson, E., Betsholtz, C., Mitsiadis, T. & Lendahl, U. (2002) Exp. Cell Res. 278, 31-44. [DOI] [PubMed] [Google Scholar]
- 25.Lardelli M., Dahlstrand, J. & Lendahl, U. (1994) Mech. Dev. 46, 123-136. [DOI] [PubMed] [Google Scholar]
- 26.Conlon R. A., Reaume, A. G. & Rossant, J. (1995) Development (Cambridge, U.K.) 121, 1533-1545. [DOI] [PubMed] [Google Scholar]
- 27.Hamada Y., Kadokawa, Y., Okabe, M., Ikawa, M., Coleman, J. R. & Tsujimoto, Y. (1999) Development (Cambridge, U.K.) 126, 3415-3424. [DOI] [PubMed] [Google Scholar]
- 28.Lardelli M., Williams, R., Mitsiadis, T. & Lendahl, U. (1996) Mech. Dev. 59, 177-190. [DOI] [PubMed] [Google Scholar]
- 29.Spinner N. B. (2000) J. Clin. Invest. 105, 561-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.