Abstract
Neuropeptide Y (NPY) is widely expressed throughout the nervous system and is known to reduce excitatory (but also inhibitory) synaptic transmission in many CNS areas, leading to the proposal that it is an endogenous antiepileptic agent. In the neocortex, where NPY is present in γ-aminobutyric acid (GABA)ergic interneurons, its effects on inhibitory and excitatory synaptic activities have not been completely explored. Here we report that NPY application elicits a long-lasting decrease in evoked excitatory postsynaptic current amplitude and a delayed, long-lasting increase in the amplitude of evoked monosynaptic inhibitory postsynaptic current (IPSC) in layer V pyramidal neurons of rat neocortex. The novel, late, NPY-mediated increase of inhibitory synaptic transmission is caused by modulation of Ca2+-dependent GABA release onto pyramidal neurons, as it was accompanied by an increase in Ca2+-dependent miniature IPSC frequency. NPY decreased evoked monosynaptic IPSCs in GABAergic interneurons, indicating that this neuropeptide has differential effects on different neuronal subtypes in the neocortex. Each of these NPY actions would decrease excitability in cortical circuits, a result that has important implications for both physiological neocortical operations as well as pathophysiological epileptiform activities.
The 36-aa neuropeptide Y (NPY) is one of the most abundantly expressed and widely distributed neuropeptides in the central and peripheral nervous system. NPY exerts various biological actions, such as modulation of anxiety, feeding behavior, memory consolidation, and blood pressure regulation (1, 2). All these effects are mediated by its interaction with Y-receptors, which belong to the G protein-coupled receptor superfamily (3, 4). In the neocortex and hippocampus, NPY is expressed in γ-aminobutyric acid (GABA)ergic interneurons (5–7), although, like many other peptides, its synthesis, storage, and release properties are different from those of “classical” neurotransmitters. Most recently, results of several studies have indicated that NPY modulates neuronal excitability in many areas of the CNS, and have emphasized its potential role as an endogenous antiepileptic agent (8). Indeed, it has been found that NPY expression increases in those brain areas undergoing seizure activity (9–12), that NPY receptor binding properties and expression are altered by seizures in various animal models of epilepsy (13, 14), and that NPY-deficient mice are more susceptible to seizure-induced death (15). Moreover, exogenous NPY application can decrease the frequency of cortical and hippocampal epileptiform discharges (16–19). The mechanism underlying the NPY-mediated reduction of epileptiform discharges is attributed to a G protein-dependent decrease in calcium channel functionality leading to a reduced release of glutamate from presynaptic terminals, as has been reported in the hippocampus (20–22). In line with this result, NPY has been found to reduce presynaptic release of glutamate (23) and GABA (24, 25) from neurons in different brain areas.
Because it is stored in large dense core granules, NPY is likely released during sustained high-frequency activity (5–40 Hz; ref. 26), such as that occurring during epileptiform seizures. Although NPY expression is well documented in the neocortex, little is known about its modulatory effects on synaptic transmission, and in particular on GABAergic inhibition, in neocortical neurons. Here we report that exogenously applied NPY reduces glutamatergic neurotransmission onto layer V pyramidal neocortical neurons, as is the case in hippocampal pyramidal cells (21, 22). Additionally, however, exogenously applied NPY has two previously undescribed effects: a long-lasting increase in Ca2+-dependent inhibitory synaptic transmission onto neocortical pyramidal neurons, as well as prolonged decreases in the amplitude of evoked monosynaptic inhibitory postsynaptic currents (IPSCs) in interneurons. Taken together, these differential actions would contribute to powerful anticonvulsant effects of NPY in neocortex.
Methods
In Vitro Slice Preparation and Electrophysiology.
Techniques for preparing neocortical slices and recording from visualized neurons were essentially as described (27, 28). P13–P21 Sprague–Dawley rats were killed with pentobarbital (50 mg/kg); brains rapidly removed and immersed in “cutting” solution containing (in mM) 234 sucrose, 11 glucose, 24 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 10 MgSO4, and 0.5 CaCl2; and gassed with 95% O2/5% CO2 (4°C). A block of sensorimotor cortex [HL (hindlimb area) and Par 1 (parietal cortex area 1); see ref. 29] was fastened to the stage of a vibratome with cyanoacrylate glue, submerged in the cutting solution, and 300-μm-thick coronal slices were cut. Slices were then incubated in oxygenated artificial cerebrospinal fluid (ACSF) containing (in mM) 126 NaCl, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 2 MgSO4, 2 CaCl2, and 10 glucose, pH 7.4, initially at 32°C for 1 h, and subsequently at room temperature, before being transferred to the recording chamber. Recordings were obtained at 32°C from layer V pyramidal neurons and interneurons in the sensorimotor cortex. Neurons were visually identified with infrared video microscopy. Firing behavior in current–clamp and the presence or absence of a large emerging apical dendrite were used to distinguish between pyramidal neurons and interneurons, respectively (e.g., Figs. 1A and 4A). These selection criteria have been validated in other experiments in our laboratory that used intracellular biocytin labeling (30, 31). For experiments on IPSCs, patch–clamp electrodes (tip resistance = 2–5 MΩ) were filled with an intracellular solution containing (in mM) K gluconate 70, KCl 70, NaCl 2, Hepes 10, EGTA 4, MgATP 4, and Na2GTP 0.3, pH 7.3, corrected with KOH; 290 milliosmolal. The estimated ECl was ≈−16 mV based on the Nernst equation, with no correction for liquid junction potentials. Under these recording conditions, activation of GABAA receptors resulted in inward currents at a holding potential (Vh) of −70 mV. Series resistance was continuously monitored during each recording, and data from cells showing changes >15% of the initial value were rejected. α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor-mediated excitatory postsynaptic currents (EPSCs) were recorded by using an intracellular solution containing (in mM) 120 cesium gluconate, 1 MgCl2, 1 CaCl2, 11 KCl, 10 Hepes, 2 NaATP, 0.3 NaGTP, 1 QX-314, and 11 EGTA; pH 7.3 corrected with Cs-OH; 290 milliosmolal. Drugs were delivered by using a local perfusion system (25, 27) composed of multiple fine tubes ending in a common outlet tube, positioned in proximity (∼250 μM) to the recorded neuron. IPSCs were isolated in 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 100 μM dl-2-amino-5-posphonovaleric acid (dl-APV), both in the bath and local perfusate. Miniature (m)-IPSCs were recorded in the presence of 1 μM tetrodotoxin (TTX). AMPA receptor-mediated EPSCs were isolated in 50 μM picrotoxin, 50 μM d-APV, and 50–75 nM 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline (NBQX), to prevent epileptiform activity (27). Extracellular stimuli, consisting of constant current pulses 50–130 μs in duration and 100–500 μA in amplitude, were delivered at low frequencies (0.1 Hz) by way of a concentric bipolar electrode (CB-XRC75, Frederick Haer & Co., Bowdoinham, ME) with a 75-μm tip diameter, positioned intracortically close to the recorded neuron. Signals were amplified with an Axopatch 200A or a Multiclamp 700A patch-clamp amplifier (Axon Instruments, Foster City, CA), sampled at 20 kHz, filtered at 10 kHz, and stored on a computer. Data were analyzed with pclamp (Axon Instruments) and origin (Microcal Software, Northampton, MA) software. Locally written software (detector, winscanselect, and thinscan; J. R. Huguenard, Stanford University School of Medicine) was used to analyze mIPSCs. Results are presented as means ± SEM. Data were statistically compared by using the Student's t test. Differences were considered significant if P < 0.05.
Fig 1.
Local NPY application causes delayed and long-lasting potentiation of IPSCs and long-lasting depression of EPSCs in pyramidal neurons. (A Left) Infrared videomicroscopic image of a pyramidal neuron soma and proximal dendrite. Patch electrode seen entering from right. (A Right) A depolarizing current pulse evokes an initial burst and a train of action potentials in the same cell. Injected current: 300 pA; 600 ms. (B) A 10-min NPY (1 μM) application to the neuron in A results in a delayed, long-lasting potentiation of the evoked IPSC amplitudes. (Upper) Averages of 10–15 IPSC traces in control, during NPY application, and at two time points during washout. (Lower) Time course of the increase in IPSC amplitude in the same cell. Each dot shows amplitude of a single IPSC. Series resistance was stable throughout the experiment (Lower). (C) Control application of ACSF to another cell elicited no change in IPSC amplitudes. (Upper) Averages of 10–15 IPSC traces at the indicated time points. (Lower) Time course of IPSC amplitude fluctuations, as in B. (D) Summary plot of IPSC amplitude vs. time in eight neurons exposed to NPY (○) and seven neurons exposed to the control ACSF (•). (E) AMPA receptor-mediated EPSCs are decreased by NPY application. Summary plot of nine NPY-treated (○) and six ACSF-treated (•) neurons. (Inset) Representative traces during control (1), NPY application (2), and 20 min of washout (3). [Bars (Inset) = 30 ms; 20 pA.] Each symbol in D and E represents normalized average of responses evoked at 0.1 Hz over 2 and 1 min, respectively.
Fig 4.
NPY generates a long-lasting depression of evoked IPSCs on neocortical interneurons. (A Left) Infrared image of an interneuron indicated by adjacent patch electrode seen entering from the right. Note the round shape of cell body and the lack of the apical dendrite, in contrast to the adjacent large cell with a pyramidal shape and a thick apical dendrite. (A Right) In the same cell, a depolarizing current pulse evokes typical FS behavior. Current pulses: −300 and 300 pA, 600 ms. (B Upper) Averages of 10–15 IPSC traces in control, during NPY application and at two time points during washout. (Lower) Time course of IPSC amplitudes in the same cell and a plot of series resistance over the same time period. Each symbol in B shows the amplitude of a single IPSC (•) and normalized values for series resistance (Rs) over time (○). (C) Summary plot of normalized IPSC amplitude vs. time in nine interneurons exposed to NPY (○) and five interneurons exposed to the control ACSF (•). Horizontal bar: NPY perfusion. Each symbol shows normalized IPSC amplitude averaged over 2 min.
Results
NPY Exerts a Differential Action on IPSCs and EPSCs in Pyramidal Neurons.
To study the effects of NPY on synaptic transmission onto pyramidal neurons, we performed whole-cell recordings from layer V pyramids, visually identified as large cells with an apical dendrite extending toward the pial surface (Fig. 1A Left). These neurons had a characteristic adapting firing behavior during injection of depolarizing current pulses (Fig. 1A Right). Extracellular stimulation in the presence of glutamate receptor blockers 2-amino-5-posphonovaleric acid (APV; 100 μM) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 20 μM) evoked in pyramidal cells monosynaptic GABAergic IPSCs that were blocked by 10 μM gabazine (data not shown). The effects of a local perfusion of NPY (1 μM; 10 min) on these IPSCs were assessed. During NPY application, a slight decrease, increase (Fig. 1B), or no effect could be detected, but a persistent increase in inhibitory synaptic transmission invariably appeared during NPY washout (Fig. 1D). The increase in evoked IPSC amplitude persisted as long as the recording remained stable, >1 h. The frequency of spontaneous IPSCs (sIPSCs) recorded between evoked IPSCs was variable but not significantly affected during or after NPY application compared with control ACSF perfusions, suggesting that neither mIPSC (see below) nor impulse-related IPSC frequency was significantly affected by the peptide. The alterations in evoked IPSCs could not be attributed to changes in the quality of recordings, because series resistance and holding current remained stable in the records selected for analysis (see Methods; n = 8; Fig. 1B). Focally applied NPY had no effect on membrane conductance at resting membrane potential, as judged by the absence of a shift in holding current. To rule out the possibility that the NPY-dependent long-lasting increase in IPSC amplitude was simply the result of the stimulation protocol or local application methodology, similar experiments were performed in which the extracellular perfusate contained ACSF plus the glutamate receptor blockers but no NPY. No effect on IPSC amplitudes was detected in these ACSF-perfused neurons (n = 7; Fig. 1 C and D).
To test whether the late NPY effect on IPSCs was due to limited diffusion of the peptide into the slice, we varied the duration of the NPY perfusion, using more prolonged applications (30 min). During the 30-min perfusions, no statistically significant change of evoked IPSC amplitudes was observed in pyramidal neurons (P > 0.2 at the 30th minute of NPY perfusion, compared with control; paired t test, n = 4; data not shown), but a long-lasting potentiation of evoked IPSC amplitudes appeared during NPY washout (P < 0.005 after 20 min in washout, compared with control; paired t test, n = 4; data not shown). Thus, the delay to the enhancement of evoked IPSCs was longer with 30-min perfusions than that found with 10-min NPY perfusions when the increase in IPSC amplitudes began within ≈10 min after the NPY application (Fig. 1D). Moreover, preliminary experiments, in which NPY was perfused for just 1 or 2 min, resulted in a long-lasting IPSC potentiation, appearing soon after NPY was washed out (data not shown). These results indicate that the delayed effect of NPY on GABAergic neurotransmission onto pyramidal neurons cannot be related to a slow action of the peptide or delayed penetration into the slice.
This enhancement of GABAergic neurotransmission represents a novel action of NPY, which has commonly been associated with a decrease in synaptic function (21–25). We therefore tested the effect of NPY on glutamatergic neurotransmission in neocortical pyramidal neurons to determine whether it would have depressant actions similar to those reported in hippocampal pyramidal neurons (21, 22). AMPA-mediated EPSCs were isolated in the presence of the GABAA receptor blocker picrotoxin (50 μM), the N-methyl-d-aspartate receptor blocker APV (50 μM), and low concentrations of the AMPA receptor blocker 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline (NBQX; 50–75 nM) to prevent epileptiform activity (27). As expected, NPY application elicited a decrease in amplitudes of evoked EPSCs in pyramidal cells. This alteration in evoked EPSCs was persistent for many minutes after cessation of NPY application, but, unlike the effect on evoked IPSCs, depression of EPSCs usually began during NPY perfusion (n = 9; compare Fig. 1 D and E). Control perfusion of ACSF did not change glutamatergic neurotransmission (n = 7; Fig. 1E). These data are consistent with a reported action of NPY on glutamatergic neurotransmission in cortical structures (21, 22). Thus, NPY has differential actions on GABAergic and glutamatergic neurotransmission in neocortical pyramidal neurons, with the former being augmented and the latter decreased.
mIPSCs Are Not Affected by NPY Treatment.
To test whether the late increase in evoked GABAergic IPSCs might be due to increased release of GABA from presynaptic terminals or to an enhanced postsynaptic sensitivity, mIPSCs were recorded in the presence of 1 μM TTX. Neither the frequency nor the amplitude of mIPSCs was significantly changed by the application or washout of NPY, during the time period over which the neuropeptide had produced an increase of evoked IPSCs in other experiments (compare Figs. 1 B and D and 2). Compared with control, normalized mIPSC frequency was 0.86 ± 0.1 in the presence of NPY, 0.7 ± 0.2 after 10 min of washout and 0.75 ± 0.2 after 20 min of washout (n = 6, P > 0.05 in all cases; Fig. 2C). Mean normalized mIPSC amplitudes were 0.95 ± 0.03 in the presence of NPY, 0.97 ± 0.1 during the first 10 min in washout, and 0.99 ± 0.08 after 20 min of washout in the same six neurons (P > 0.05 in all cases; Fig. 2C). These results indicate that NPY does not modulate miniature inhibitory synaptic transmission.
Fig 2.
mIPSCs recorded in 1 μM TTX are not affected by NPY treatment. (A) Representative traces of mIPSCs recorded in 1 μM TTX before (A1, control) and during (A2) NPY application and after 10 and 20 min of washout (A3 and A4). (B) mIPSC frequency and amplitudes were not affected by 1 μM NPY perfusion in the neuron of A. Symbols show average frequency (○) and amplitude (•) calculated in 30-sec bins. (C) Bar graphs of mIPSC frequency and amplitude from eight neurons. Changes during NPY application or wash were not significant.
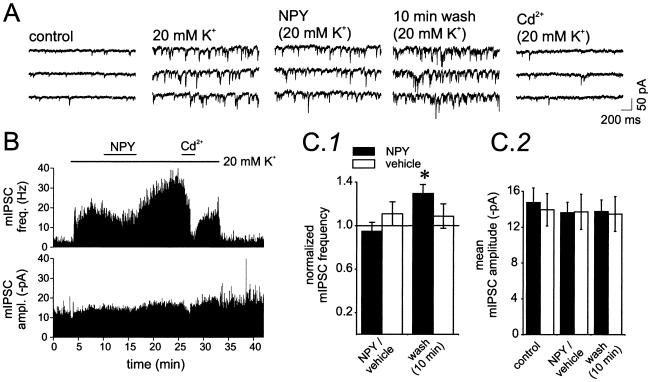
mIPSCs Recorded in 20 mM of KCl Are Sensitive to NPY.
Previous results in hippocampal pyramidal neurons have suggested that mIPSCs recorded in TTX and in normal [K+]o are largely Ca2+-independent, but that those obtained in elevated [K+]o are Ca2+-dependent (32). Further, the effect of NPY on neurotransmitter release has been shown to occur only through Ca2+-dependent mechanisms in the hippocampus (21) and in the thalamus (25). We therefore tested the possibility that the effect of NPY on the amplitude of evoked action potential-dependent IPSCs was due to enhanced Ca2+-dependent release of GABA onto pyramidal neurons. Application of elevated [K+]o presumably generates a steady-state depolarization of presynaptic terminals, resulting in persistent influx of calcium, making it possible to analyze the effects of NPY on Ca2+-dependent synaptic transmission. mIPSC frequency, but not amplitude, was strongly increased when [K+]o was raised from 2.5 to 20 mM (Fig. 3 A and B), consistent with an increase in Ca2+-dependent release (32). During NPY application under these conditions, there was a slight but statistically insignificant decrease of mIPSC frequency (95 ± 8% of control; n = 6, P > 0.05; Fig. 3C1). However, later during NPY washout, mIPSC frequency was significantly increased (130 ± 8% of control; n = 6, P < 0.05; Fig. 3A 10-min washout and Fig. 3 B and C1), an effect that was similar in its delayed onset to the increase in evoked IPSC amplitudes after NPY perfusion (e.g., Fig. 1 B and D). Addition of the Ca2+ channel blocker Cd2+ (200 μM) in 20 mM [K+]o ACSF caused a reduction of mIPSC frequency to values near those in control (2.5 mM K+) solution (Fig. 3B; mean mIPSC frequency was 8.5 ± 2.4 Hz in control and 6.5 ± 1.2 Hz in the presence of Cd2+, in 20 mM of KCl-containing ACSF; n = 5; P > 0.4), indicating that NPY affected Ca2+-dependent release and that GABA release in control [K+]o is mostly Ca2+-independent. None of these manipulations (increased [K+]o, addition of NPY and Cd2+) affected mIPSC amplitudes (Fig. 3 B and C2). Such results are consistent with the conclusion that the NPY-dependent increase in GABAergic neurotransmission might have a presynaptic origin. To exclude a nonspecific effect of a prolonged application of 20 mM [K+]o, similar experiments were performed in which ACSF containing 20 mM of [K+]o (vehicle) was applied instead of NPY (Fig. 3C2, NPY/vehicle and washout, 10 min). In these control experiments, no significant effects on mIPSC frequency or amplitude were detected over the same time course in which the NPY-induced effect on mIPSC frequency was evident. These data indicate that NPY application results in an increased Ca2+-dependent release at GABAergic terminals contacting pyramidal neurons.
Fig 3.
mIPSCs recorded in 20 mM KCl are sensitive to NPY. (A) Representative traces of mIPSCs recorded in 1 μM TTX in normal ACSF (control, 2.5 mM K+), during extracellular perfusion of 20 mM K+, during NPY application (in 20 mM K+), after 10 min of NPY washout (in 20 mM K+), and during application of 200 μM Cd2+ (in 20 mM K+). (B) Time plot of mIPSC instantaneous frequency (Upper) and amplitude (Lower) in the neuron of A. (C) Bar graphs of mIPSC frequency (C1) and amplitude (C2) in NPY-treated neurons (solid bar; n = 6) and in neurons treated with 20 mM K+-containing ACSF without NPY (vehicle) (controls; open bars; n = 4). mIPSC frequency increased but only during NPY washout (P < 0.05). Vehicle treatment had no effect. (C2) mIPSC amplitudes were unaffected by either treatment.
NPY Reduces Evoked IPSCs in Neocortical Interneurons.
To determine whether inhibitory neurotransmission onto inhibitory interneurons might be similarly affected by NPY, we performed whole-cell recordings from layer V interneurons, which were visually identified as round multipolar cells lacking an apical dendrite (Fig. 4A). On the basis of their firing behavior after injection of current pulses, GABAergic interneurons fell into two groups: the fast-spiking (FS) and low-threshold-spiking (LTS) cells (7, 33–36). In contrast to the effects seen with pyramidal neurons, NPY elicited a robust and long-lasting decrease in the amplitude of evoked monosynaptic IPSCs in six FS and three LTS cells that were tested (Fig. 4 B and C; see Methods and Fig. 1). Significant changes in monosynaptic IPSC amplitudes did not occur in control experiments in which control ACSF was perfused instead of NPY (Fig. 4C, ACSF; total number of interneurons = 5), indicating that the long-lasting depression of IPSCs was mediated by neuropeptide Y, rather than a nonspecific effect of the perfusion or the stimulation pattern.
Discussion
These experiments provide evidence for significant effects of exogenously applied NPY on both excitatory and inhibitory synaptic transmission onto layer V neocortical neurons and on inhibition of interneurons. Consistent with the reported reduction of EPSCs in hippocampal pyramidal neurons (21, 22), NPY decreased AMPA receptor-mediated glutamatergic neurotransmission onto neocortical pyramidal neurons. Although results in hippocampus have indicated that NPY does not affect GABAergic neurotransmission onto pyramidal neurons (37), this neuropeptide can modulate GABAergic synaptic transmission in other brain areas (24, 25, 38). Here we report an action of NPY in the neocortex, namely induction of a long-lasting increase of GABAergic neurotransmission on neocortical pyramidal neurons. This effect on evoked IPSCs might be attributed to an increase in action potential-dependent neurotransmitter release from presynaptic terminals, an enhanced postsynaptic GABAA-receptor sensitivity, or both effects. A postsynaptic effect seems unlikely, because the amplitude of mIPSCs was unchanged by NPY perfusion in either normal ACSF or increased [K+]o (Fig. 2 B and C; Fig. 3 B and C2). We did not detect an NPY-mediated alteration in the frequency of mIPSCs recorded in pyramidal neurons in normal [K+]o. This finding is consistent with reports that NPY does not affect Ca2+-independent spontaneous synaptic activity in either hippocampus (21) or thalamus (25). In the presence of high [K+]o, Cd2+ decreased mIPSC frequency to values similar to those in control (2.5 mM [K+]o) solution, confirming the conclusion that mIPSCs in normal [K+]o are mostly Ca2+-independent (32). When presynaptic terminals were constantly depolarized by high [K+]o, NPY application increased mIPSC frequency with no effect on mIPSC amplitude, indicating that the peptide enhances Ca2+-dependent release of GABA from interneuronal presynaptic terminals. An alternative (or perhaps additional) mechanism for the NPY-mediated potentiation of inhibition on pyramidal neurons might derive from an increased excitability (e.g., depolarization) of interneurons by the neuropeptide, causing increased numbers of interneurons to discharge after the extracellular stimulus. The absence of increased sIPSC frequency in pyramidal cells would argue against a direct excitatory effect of NPY on interneurons, as would the lack of a shift in holding current when NPY was applied to FS or low-threshold-spiking cells. However, interneuronal membrane properties were not examined in detail in control and NPY-containing solutions to test this possibility directly.
The effect of NPY on both evoked monosynaptic IPSCs and Ca2+-dependent mIPSCs in pyramidal cells is delayed and long-lasting. During NPY application, individual cells varied in their responses, generating a slight decrease, an increase, or no effect. However, the persistent increase in inhibitory synaptic transmission invariably appeared during NPY washout. Ca2+-dependent mIPSC frequency showed similar changes during NPY perfusion, but even when a decrease was present during NPY perfusion, a consistent and significant potentiation occurred after the peptide was washed out (see Fig. 3C1). The reason for this delayed effect is unclear. The more rapid onset of NPY effects on EPSCs vs. IPSCs (compare Fig. 1 D and E) and the results of prolonged (30-min) applications rule out delayed penetration into the slice as an explanation for delayed onset. Also, in other experiments in our laboratory, the same perfusion system has been used to apply NPY to thalamic slices, resulting in relatively rapid responses and washout (25). Moreover, preliminary experiments in which NPY was applied for much briefer time periods (1–2 min) resulted in an increase of evoked IPSCs soon after NPY washout (unpublished observation). A shorter response latency is also apparent in studies of NPY effects on evoked glutamatergic neurotransmission in the hippocampus (22) and in the arcuate nucleus (23). The delayed effect of NPY on IPSCs could be caused either by slow activation of Y receptors or by a “rebound” effect after NPY washout. The lack of effect on IPSCs during 30-min perfusions of NPY argues against the former possibility, as do data showing that NPY applied for 1 or 2 min still elicits an increase of evoked IPSCs during washout. The robust increase in evoked IPSCs during NPY washout indicates that the peptide is able to induce a long-term potentiation of inhibition on pyramidal neurons on its removal from the slice.
One admittedly speculative explanation for the long delay in the onset of NPY effects on inhibitory neurotransmission would be a simultaneous activation of two or more Y receptor subtypes, whose effects offset one another, and whose time courses are different. During washout, the depressant effect might be reversed, whereas the potentiating action, likely due to the activation of long-lasting modifications in the presynaptic terminal, would persist.
The NPY-mediated increase in GABAergic neurotransmission recorded in pyramidal neurons is accompanied by a concurrent long-lasting decrease in evoked IPSC amplitudes in both FS and low-threshold-spiking layer V interneurons. One possible explanation for these results would be a target-specific difference in NPYergic modulation of terminals from single interneurons that are presynaptic to pyramidal cells vs. other interneurons (39). This selectivity of action might be mediated by different receptors and/or different intracellular coupling mechanisms. An alternative mechanism might involve differential effects of NPY on subsets of “interneuron-selective” vs. “pyramidal neuron-targeting” GABAergic cells (40, 41).
NPY-induced disinhibition of interneurons would increase their output and amplify the overall effects of the peptide on Ca2+-dependent release of GABA from inhibitory terminals on pyramidal neurons (e.g., Fig. 3), resulting in a powerful potentiation of their inhibitory responses. Interneuronal activity in the neocortex is important in generating and sustaining network oscillations underlying several brain functions (42), and inhibition of GABAergic responses on interneurons should have important consequences for the function of their target downstream pyramidal neurons. Polysynaptic IPSCs in layer V pyramidal neurons are significantly larger in amplitude than monosynaptic IPSCs (43). Therefore, an estimate of the net effects of NPY on inhibition in the cortical network will require further experiments to determine whether excitatory synaptic events onto interneurons are decreased, as they are in pyramidal cells, and whether this would, in part, oppose the facilitatory effects described here.
The mechanisms of action of NPY on excitation and inhibition of pyramidal neurons and on inhibition of interneurons have not been completely investigated. In the hippocampus, the NPY-mediated decrease of glutamate release onto pyramidal neurons has been attributed to the activation of Y2 receptors (22) but this might not be the case in neocortex where Y1 is a highly expressed receptor subtype, and Y2 receptors are much less prominent (44–47). Indeed, the antiepileptic effects of NPY in hippocampus and cortex seem to be mediated by different Y-receptor subtypes (16, 18). Moreover, Y5 receptor activation has been implicated in decreasing glutamate release in the hippocampus (48) and in reducing seizures in different rat models of epilepsy (49, 50). The various NPY-mediated effects on synaptic transmission in neocortical pyramidal neurons and interneurons suggest the involvement of multiple receptors and possibly different intracellular coupling mechanisms. In any case, our results in neocortex show that NPY can affect inhibitory as well as excitatory synaptic events. The combined effects of potentiation of inhibition and the depression of excitation on pyramidal neurons, together with the depression of inhibition on interneurons, and the presumed release of NPY by high-frequency seizure-related neuronal firing, make this peptide a powerful endogenous antiepileptic agent.
Acknowledgments
We thank Isabel Parada for excellent assistance during these experiments. This work was supported by National Institutes of Health Grant NS39579 from the National Institute of Neurological Disorders and Stroke.
Abbreviations
NPY, neuropeptide Y
GABA, γ-aminobutyric acid
IPSC, inhibitory postsynaptic current
ACSF, artificial cerebrospinal fluid
AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
EPSC, excitatory postsynaptic current
mIPSC, miniature IPSC
TTX, tetrodotoxin
FS, fast-spiking
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Wahlestedt C. & Reis, D. J. (1993) Annu. Rev. Pharmacol. Toxicol. 33, 309-352. [DOI] [PubMed] [Google Scholar]
- 2.Balasubramaniam A. A. (1997) Peptides 18, 445-457. [DOI] [PubMed] [Google Scholar]
- 3.Blomqvist A. G. & Herzog, H. (1997) Trends Neurosci. 20, 294-298. [DOI] [PubMed] [Google Scholar]
- 4.Michel M. C., Beck-Sickinger, A., Cox, H., Doods, H. N., Herzog, H., Larhammar, D., Quirion, R., Schwartz, T. & Westfall, T. (1998) Pharmacol. Rev. 50, 143-150. [PubMed] [Google Scholar]
- 5.Hendry S. H., Jones, E. G., De Felipe, J., Schmechel, D., Brandon, C. & Emson, P. C. (1984) Proc. Natl. Acad. Sci. USA 81, 6526-6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendry S. H., Jones, E. G. & Emson, P. C. (1984) J. Neurosci. 4, 2497-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cauli B., Audinat, E., Lambolez, B., Angulo, M. C., Ropert, N., Tsuzuki, K., Hestrin, S. & Rossier, J. (1997) J. Neurosci. 17, 3894-3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vezzani A., Sperk, G. & Colmers, W. F. (1999) Trends Neurosci. 22, 25-30. [DOI] [PubMed] [Google Scholar]
- 9.Bellmann R., Widmann, R., Olenik, C., Meyer, D. K., Maas, D., Marksteiner, J. & Sperk, G. (1991) J. Neurochem. 56, 525-530. [DOI] [PubMed] [Google Scholar]
- 10.Rizzi M., Monno, A., Samanin, R., Sperk, G. & Vezzani, A. (1993) Eur. J. Neurosci. 5, 1534-1538. [DOI] [PubMed] [Google Scholar]
- 11.Gruber B., Greber, S., Rupp, E. & Sperk, G. (1994) Hippocampus 4, 474-482. [DOI] [PubMed] [Google Scholar]
- 12.Sadamatsu M., Kanai, H., Masui, A., Serikawa, T., Yamada, J., Sasa, M. & Kato, N. (1995) Life Sci. 57, 523-531. [DOI] [PubMed] [Google Scholar]
- 13.Schwarzer C., Kofler, N. & Sperk, G. (1997) Mol. Pharmacol. 53, 6-13. [DOI] [PubMed] [Google Scholar]
- 14.Gobbi M., Gariboldi, M., Piwko, C., Hoyer, D., Sperk, G. & Vezzani, A. (1998) J. Neurochem. 70, 1615-1622. [DOI] [PubMed] [Google Scholar]
- 15.Baraban S. C., Hollopeter, G., Erickson, J. C., Schwartzkroin, P. A. & Palmiter, R. D. (1997) J. Neurosci. 17, 8927-8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klapstein G. J. & Colmers, W. F. (1997) J. Neurophysiol. 78, 1651-1661. [DOI] [PubMed] [Google Scholar]
- 17.Patrylo P. R., van den Pol, A. N., Spencer, D. D. & Williamson, A. (1999) J. Neurophysiol. 82, 478-483. [DOI] [PubMed] [Google Scholar]
- 18.Bijak M. (1999) Neurosci. Lett. 268, 115-118. [DOI] [PubMed] [Google Scholar]
- 19.Bijak M. (2000) Neuroscience 96, 487-494. [DOI] [PubMed] [Google Scholar]
- 20.Colmers W. F., Lukowiak, K. & Pittman, Q. J. (1988) J. Neurosci. 8, 3827-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McQuiston A. R. & Colmers, W. F. (1996) J. Neurophysiol. 76, 3159-3168. [DOI] [PubMed] [Google Scholar]
- 22.Qian J., Colmers, W. F. & Saggau, P. (1997) J. Neurosci. 17, 8169-8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhim H., Kinney, G. H., Emmerson, P. J. & Miller, R. J. (1997) J. Neurosci. 17, 2980-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen G. & van den Pol, A. N. (1996) J. Neurosci. 16, 7711-7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Q. Q., Huguenard, J. R. & Prince, D. A. (2001) J. Physiol. (London) 531, 81-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartfai T., Iverfeldt, K., Fisone, G. & Serfozo, P. (1988) Annu. Rev. Pharmacol. Toxicol. 28, 285-310. [DOI] [PubMed] [Google Scholar]
- 27.Kumar S. S., Bacci, A., Kharazia, V. N. & Huguenard, J. R. (2002) J. Neurosci. 22, 3005-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H. & Prince, D. A. (2002) J. Neurophysiol. 88, 2-12. [DOI] [PubMed] [Google Scholar]
- 29.Zilles K., (1985) The Cortex of the Rat (Springer, New York).
- 30.Xiang Z., Huguenard, J. R. & Prince, D. A. (1998) J. Physiol. (London) 506, 715-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiang Z., Huguenard, J. R. & Prince, D. A. (1998) Science 281, 985-988. [DOI] [PubMed] [Google Scholar]
- 32.Doze V. A., Cohen, G. A. & Madison, D. V. (1995) J. Neurophysiol. 74, 43-53. [DOI] [PubMed] [Google Scholar]
- 33.Kawaguchi Y. & Kubota, Y. (1993) J. Neurophysiol. 70, 387-396. [DOI] [PubMed] [Google Scholar]
- 34.Kawaguchi Y. & Kubota, Y. (1997) Cereb. Cortex 7, 476-486. [DOI] [PubMed] [Google Scholar]
- 35.Kawaguchi Y. & Kubota, Y. (1998) Neuroscience 85, 677-701. [DOI] [PubMed] [Google Scholar]
- 36.Xiang Z., Huguenard, J. R. & Prince, D. A. (2002) J. Neurophysiol. 88, 740-750. [DOI] [PubMed] [Google Scholar]
- 37.Klapstein G. J. & Colmers, W. F. (1993) Hippocampus 3, 103-111. [DOI] [PubMed] [Google Scholar]
- 38.Obrietan K. & van den Pol, A. N. (1996) J. Neurosci. 16, 3521-3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scanziani M., Gahwiler, B. H. & Charpak, S. (1998) Proc. Natl. Acad. Sci. USA 95, 12004-12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freund T. F. & Buzsaki, G. (1996) Hippocampus 6, 347-470. [DOI] [PubMed] [Google Scholar]
- 41.Acsady L., Gorcs, T. J. & Freund, T. F. (1996) Neuroscience 73, 317-334. [DOI] [PubMed] [Google Scholar]
- 42.McBain C. J. & Fishan, A. (2001) Nat. Rev. Neurosci. 2, 11-23. [DOI] [PubMed] [Google Scholar]
- 43.Salin P. A. & Prince, D. A. (1996) J. Neurophysiol. 75, 1589-1599. [DOI] [PubMed] [Google Scholar]
- 44.Larsen P. J., Sheikh, S. P., Jakobsen, C. R., Schwartz, T. W. & Mikkelsen, J. D. (1993) Eur. J. Neurosci. 5, 1622-1637. [DOI] [PubMed] [Google Scholar]
- 45.Dumont Y., Fournier, A., St. Pierre, S. & Quirion, R. (1993) J. Neurosci. 13, 73-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dumont Y., Fournier, A., St. Pierre, S. & Quirion, R. (1995) J. Pharmacol. Exp. Ther. 272, 673-680. [PubMed] [Google Scholar]
- 47.Dumont Y., Fournier, A., St. Pierre, S. & Quirion, R. (1996) Synapse 22, 139-158. [DOI] [PubMed] [Google Scholar]
- 48.Ho M. W. Y., Beck-Sickinger, A. & Colmers, W. F. (2000) J. Neurophysiol. 83, 723-734. [DOI] [PubMed] [Google Scholar]
- 49.Woldbye D. P., Larsen, P. J., Mikkelsen, J. D., Klemp, K., Madsen, T. M. & Bolwig, T. G. (1997) Nat. Med. 7, 761-764. [DOI] [PubMed] [Google Scholar]
- 50.Reibel S., Nadi, S., Benmaamar, R., Larmet, Y., Carnahan, J., Marescaux, C. & Depaulis, A. (2001) Peptides 22, 529-539. [DOI] [PubMed] [Google Scholar]